Abstract
X-chromosome linked inhibitor of apoptosis (XIAP) mRNA has been proposed to bear a stress-activated internal ribosome entry site (IRES) that stimulates translation under conditions that inhibit cap-dependent initiation. However, several reports have indicated that the strong activity of the XIAP IRES in certain bicistronic reporter assay systems stems from production of unintended monocistronic transcripts through splicing or cryptic promoter activity. Here we extend these findings by providing evidence that the XIAP IRES similarly provokes the production of monocistronic mRNA encompassing the 3′ cistron in the βgal/CAT bicistronic reporter plasmid that was originally used to identify and characterize this putative IRES, through cryptic promoter activity.
Keywords: translation, bicistronic reporter, internal initiation, cap independent
INTRODUCTION
Translation of most eukaryotic mRNAs initiates through a cap-dependent mechanism in which the 43S preinitiation complex is recruited to the 5′ end of the mRNA via interactions with the cap-binding complex eIF4F. The 43S complex then scans the mRNA until a translation initiation codon in an appropriate sequence context is encountered, and the 60S subunit is recruited. However, some mRNAs are translated through alternative cap-independent mechanisms that employ specialized RNA elements termed internal ribosome entry sites (IRESs) to recruit ribosomes (for review, see Holcik et al. 2000a; Gebauer and Hentze 2004; Holcik and Sonenberg 2005). IRES elements were originally discovered in picornaviruses (Jang et al. 1988; Pelletier and Sonenberg 1988) and have since been described in the 5′ untranslated regions (UTRs) of a variety of viral and cellular mRNAs (for review, see Stoneley and Willis 2004; Kieft 2008). Cellular mRNAs proposed to bear IRES elements include some that encode proteins involved in stress responses such as hypoxia, apoptosis, and the unfolded protein response (for review, see Stoneley and Willis 2004; Holcik and Sonenberg 2005). The ability of certain viral IRES elements to drive cap-independent internal initiation has been firmly established by a variety of experimental approaches, including the elegant demonstration that the encephalomyocarditis virus (EMCV) IRES can direct translation of a circular mRNA template (Chen and Sarnow 1995). In contrast, the case for internal initiation driven by some of the proposed cellular IRES elements is less compelling and rests primarily on the activity of these sequences in bicistronic reporter assays (for review, see Kozak 2001). In such assays the putative IRES is tested for its ability to activate expression of a 3′ cistron when inserted between two reporter open reading frames that are driven from a common upstream promoter. However, as emphasized by Kozak (2001), a positive result in this test does not by itself demonstrate IRES activity: the possibility that the putative IRES contains cryptic splicing signals and/or promoters that direct the production of unintended monocistronic transcripts of the 3′ cistron must be rigorously excluded. Indeed, several putative cellular IRES sequences appear to induce 3′ cistron expression primarily through such alternative nontranslational mechanisms (Han and Zhang 2002; Van Eden et al. 2004; Liu et al. 2005; Wang et al. 2005; Bert et al. 2006; Baranick et al. 2008).
One such contested IRES is that proposed to reside in the 5′ UTR of the mRNA encoding the mammalian X-linked inhibitor of apoptosis (XIAP), the product of the BIRC4 gene (Holcik et al. 1999). Translation of XIAP mRNA is enhanced by stimuli including serum deprivation and γ-irradiation, an effect that has been attributed to IRES activation (Holcik et al. 1999, 2000b). The putative IRES strongly stimulates 3′ cistron expression in bicistronic reporter assays (Holcik et al. 1999). However, two groups reported that it does so by directing production of spliced monocistronic reporter mRNA bearing the 3′ cistron through use of a splice acceptor located at the 3′ end of the IRES sequence (Van Eden et al. 2004; Baranick et al. 2008), and a third group reported that activity in another bicistronic reporter system is due to a cryptic promoter within the IRES (Bert et al. 2006). Taken in combination, these studies provide strong evidence that the XIAP IRES lacks significant internal initiation activity in some bicistronic vector systems. However, these studies did not examine the activity of this IRES in the context of the β-galactosidase/CAT bicistronic reporter plasmid that was originally used to identify and characterize the element (Fig. 1A, pßgal/CAT; Holcik et al. 1999). Holcik et al. (2005) have argued that spurious splicing does not occur in this system and suggested that it reliably measures internal initiation of translation driven by the XIAP element.
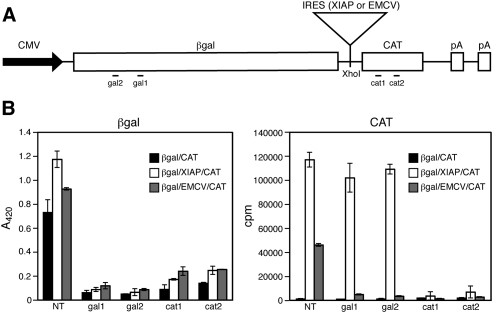
FIGURE 1.
Effects of siRNAs on reporter gene expression. (A) Schematic representation of the bicistronic reporter constructs used in this study. The locations of the sequences targeted by the gal1, gal2, cat1, and cat2 siRNAs are indicated. (B) Activity assays. β-galactosidase and chloramphenicol acetyltransferase assays were performed on lysates of HeLa cells transfected with the indicated bicistronic reporter constructs and siRNAs. The averages of triplicate replicates obtained within a representative experiment are shown ± the standard deviation. (NT) Nontargeting control siRNA.
The quantitative real-time PCR data presented by Holcik et al. (2005) clearly document that the most abundant transcripts produced by the β-galactosidase/XIAP/CAT construct contain both β-galactosidase and CAT sequences, as intended. However, the analysis did not exclude the possibility that less abundant monocistronic CAT mRNAs are also produced, giving rise to the observed 3′ cistron expression. Here we re-examine the mechanism by which the XIAP IRES activates 3′ cistron expression in this vector system, using stringent tests originally developed by the Lloyd laboratory (Van Eden et al. 2004).
RESULTS AND DISCUSSION
As expected on the basis of previous work, βgal/CAT constructs bearing the EMCV or XIAP IRES elements (pßgal/EMCV/CAT and pßgal/XIAP/CAT) directed the accumulation of high levels of CAT activity encoded by the 3′ cistron relative to the control vector lacking an IRES in transfected HeLa cells (Fig. 1B). To determine if CAT activity arises via translation of the intended bicistronic mRNAs, we examined the effects of siRNAs targeting β-galactosidase and CAT coding sequences (Fig. 1B). As expected, siRNAs targeting either cistron severely reduced the expression of both reporter proteins from pßgal/EMCV/CAT relative to the nontargeted (NT) siRNA control (Fig. 1B). These data confirm that the EMCV IRES drives 3′ cistron expression from the intended bicistronic mRNA. However, a very different result was obtained with pßgal/XIAP/CAT bearing the XIAP IRES. In this case, the siRNAs targeting β-galactosidase coding sequences failed to inhibit CAT expression, although the CAT siRNAs were highly effective. In contrast, β-galactosidase expression was efficiently knocked down by siRNAs targeting either cistron. These data argue that although β-galactosidase is translated from bicistronic RNA, CAT activity arises from one or more additional mRNAs that lack β-galactosidase sequences (i.e., monocistronic CAT transcripts).
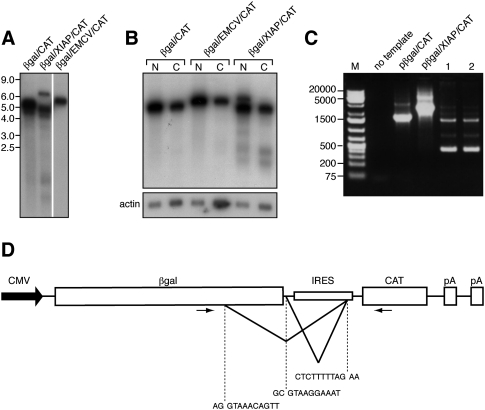
Northern blot analysis demonstrated that pßgal/CAT and pßgal/EMCV/CAT both gave rise to a single transcript of the mobility predicted for intact biscistronic mRNA (∼5.3 and 5.9 kb, respectively) when analyzed with a CAT probe (Fig. 2A). In contrast, pßgal/XIAP/CAT generated multiple CAT-reactive RNA species: a band with the mobility predicted for intact bicistronic mRNA (∼6.3 kb), a more abundant broader band or bands migrating at ∼4–5 kb, and two much less abundant species of ∼1.5 and 2 kb (Figs. 2A,B, 3). The 6.3-kb transcript also hybridized to probes for β-galactosidase and XIAP IRES sequences, as expected for intact bicistronic mRNA (Fig. 3). In contrast, the band or bands at 4–5 kb hybridized to β-galactosidase and CAT sequences but did not react with the IRES probe, and the 1.5–2-kb species reacted with only the CAT probe (Fig. 3). These data suggested that the 4–5-kb RNAs correspond to spliced transcripts that contain both β-galactosidase and CAT sequences and lack most or all of the IRES, while the 1.5–2-kb transcripts likely represent unspliced monocistronic CAT mRNAs. The 6.3 kb transcript was largely confined to the nucleus, as befits an unspliced pre-mRNA, while the 4–5-kb and 1.5–2-kb RNAs were recovered in both the nuclear and cytoplasmic fractions, as were the full-length transcripts arising from pßgal/CAT and pßgal/EMCV/CAT (Fig. 2B). Thus, the most abundant cytoplasmic RNAs produced by the pßgal/XIAP/CAT vector appeared to lack most or all of the XIAP IRES.
FIGURE 2.
pßgal/XIAP/CAT produces multiple RNA species. (A) Northern blot of poly(A)+ RNA extracted from HeLa cells transfected with the indicated bicistronic reporter plasmids and probed for CAT sequences. (B) Northern blot of poly(A)+ RNA isolated from the nuclear (N) and cytoplasmic (C) fractions of HeLa cells transfected with pßgal/XIAP/CAT, probed with a CAT probe. After exposure, the blot was stripped and reprobed with an actin-specific labeled oligonucleotide. (C) Amplimers obtained by RT-PCR analysis of RNA arising from pßgal/XIAP/CAT. PCR templates were plasmid DNA (pßgal/CAT, pßgal/XIAP/CAT) or cDNA prepared from RNA extracted from transfected cells (lanes 1,2). cDNAs were generated using oligo dT (lane 1) or a CAT-specific oligonucleotide (lane 2) as the primer. Products were separated on a 1% agarose gel. (M) GeneRuler 1-kb Plus DNA ladder (Fermentas). (D) Schematic diagram of the bicistronic reporter construct showing the splice donor and acceptor sequences used to generate the spliced RNAs detected in C, as well as relative positions of the PCR primers used (primers are not drawn precisely to scale). Splicing out the larger intron (detected by the 450-nt PCR product) gives rise to a 4-kb transcript, while splicing of the smaller intron (detected by the 1500-nt PCR product) gives rise to a 5-kb RNA.
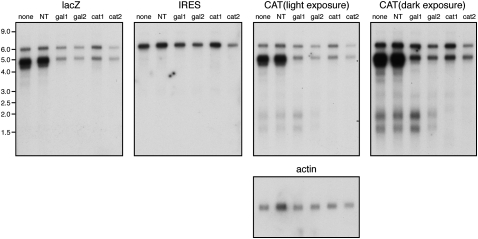
FIGURE 3.
Further characterization of transcripts produced by pßgal/XIAP/CAT. HeLa cells were transfected with pßgal/XIAP/CAT in the presence of various siRNAs, and the RNA species produced were analyzed by Northern blotting using the indicated probes. Duplicate gels were prepared: the membrane that was originally hybridized to the CAT probe was stripped and reprobed for β-actin RNA, while the membrane that was originally hybrized to the XIAP IRES probe was subsequently hybridized to a lacZ-specific probe. (NT) Nontargeted control siRNA.
We confirmed the production of spliced transcripts bearing both β-galactosidase and CAT sequences via reverse-transcriptase (RT)-PCR analysis of RNA harvested from transfected cells. Using a 3′ primer that anneals to CAT coding sequences and a 5′ primer located within β-galactosidase coding sequences (Fig. 2D), we detected several RT-PCR products consistent with such spliced mRNAs, the most prominent being ∼1500 and 450 nucleotides (nt) in length (Fig. 2C, lanes 1,2). The 2741-nt amplimer predicted for unspliced RNA was not prominently detected in these experiments, although this product was observed when plasmid DNA was used as the template. The nucleotide sequences of the ∼1500-nt and 450-nt amplimers revealed that they arise through alternative splicing events that join one of two splice donor sites located within the β-galactosidase insert to the previously described splice acceptor located at the 3′ end of the XIAP IRES (Fig. 2D; Van Eden et al. 2004). One of the splice donors is located within β-galactosidase coding sequences, while the other is immediately downstream from the β-galactosidase stop codon. Use of the of the upstream donor site accounts for production of an ∼4-kb mRNA, which encodes a truncated form of β-galactosidase, while use of the downstream donor gives rise to an ∼5-kb mRNA, which bears an intact β-galactosidase open reading frame. These data argue that 5-kb spliced mRNA is the source of the β-galactosidase activity specified by pßgal/XIAP/CAT. Both spliced RNAs bear an intact CAT open reading frame and lack all but 30 nt of the 1007-nt XIAP IRES element. Although the ∼450-nt PCR product derived from the 4-kb mRNA was more abundant than the ∼1500-nt product arising from the 5-kb transcript, this probably does not reflect the relative abundance of the two spliced RNAs, as smaller amplicons are likely to be preferentially amplified during PCR. Both spliced species retain the β-galactosidase sequences targeted by the gal1 and gal2 siRNAs that fail to inhibit CAT expression (Fig. 1B). Thus, although these spliced RNAs retain the CAT open reading frame, they are not the source of the CAT activity specified by pßgal/XIAP/CAT. Of note, both of these transcripts retain the regions complementary to the real-time PCR primers used in the analysis of Holcik et al. (2005). Thus, we agree with their conclusion that the most abundant transcripts arising from this plasmid bear both lacZ and CAT sequences.
As expected on the basis of the preceding data, the 4–5-kb spliced bicistronic RNAs were strongly depleted by siRNAs targeting either β-galactosidase or CAT coding sequences, while the 1.5–2.0-kb RNAs were depleted only by the CAT siRNA (Fig. 3). The full-length ∼6.3-kb transcript appeared to be highly resistant to knockdown by all of the siRNAs tested, presumably because it is confined to the nucleus. Taken in combination with the data presented in Figures 1 and 2, these results argue that the 4–5-kb and 1.5–2.0-kb transcripts are translated to yield β-galactosidase and CAT, respectively. As noted above, one of the two spliced 4–5-kb transcripts is predicted to encode intact β-galactosidase (1047 amino acids), while the other would give rise to a truncated protein of 716 amino acids. Cells transfected with pßgal/XIAP/CAT displayed only full-length β-galactosidase by Western blot analysis using a rabbit polyclonal antibody (data not shown), raising the possibility that the predicted truncated product is either not produced or is rapidly degraded.
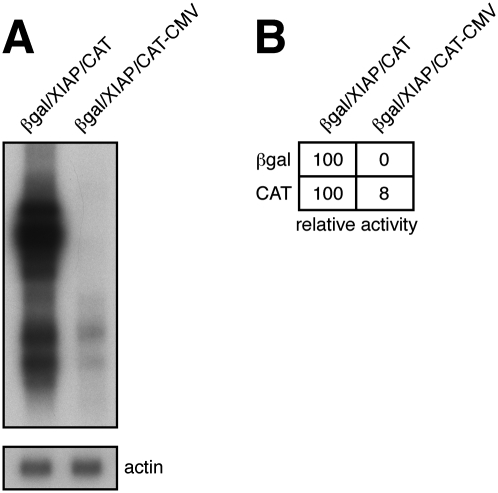
These data strongly suggest that the XIAP element induces efficient 3′ cistron expression in the βgal/CAT bicistronic reporter system by provoking production of monocistronic 1.5–2.0-kb CAT mRNAs. Such mRNAs could arise via splicing of the 6.3-kb biscistronic mRNA or through activation of the previously documented cryptic promoter present within the IRES sequence by the CMV and SV40 enhancer elements present in the reporter plasmid. (A copy of the SV40 enhancer is located ∼1.2 kb downstream from the proximal polyA site diagrammed in Figure 1A.) Deleting the cytomegalovirus (CMV) promoter/enhancer from pßgal/XIAP/CAT (pßgal/XIAP/CAT–CMV) reduced CAT expression ∼10-fold (n = 7) and the levels of the 1.5–2.0-kb CAT mRNAs approximately sixfold (n = 2); however, CAT activity remained well above that of the no IRES control (Fig. 4). In contrast, the same deletion completely abolished β-galactosidase expression and production of the 6.3-kb and 4–5-kb transcripts. These data confirm that the 1.5–2.0-kb transcripts serve as sources of CAT activity and document that they are not derived by splicing of the 6.3-kb bicistronic mRNA. The simplest explanation is that they are driven from the cryptic promoter in the XIAP IRES sequence, which we propose is activated by both the CMV and SV40 enhancer elements present on the βgal/XIAP/CAT plasmid. While we have yet to determine why two monocistronic CAT transcripts are produced, it seems likely that they arise through alternative use of the two tandem polyadenylation signals present in the flanking vector DNA (Fig. 1A).
FIGURE 4.
Effects of deleting the CMV promoter from pßgal/XIAP/CAT. (A) Northern blot analysis of transcripts arising from pßgal/XIAP/CAT and pßgal/XIAP/CAT-CMV (which lacks the CMV promoter), using a CAT probe. In this experiment, the levels of 2.0/1.5 RNAs arising from pßgal/XIAP/CAT-CMV were approximately fivefold lower than those obtained with pßgal/XIAP/CAT. (B) The corresponding β-galactosidase and chloramphenicol acetyltransferase activities are indicated.
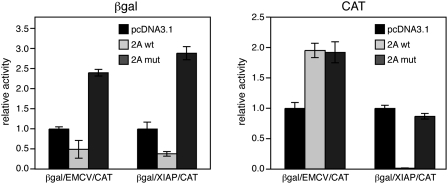
Taken in combination, the preceding results argue that CAT expression from βgal/XIAP/CAT occurs via cap-dependent translation of monocistronic mRNA arising from one or more cryptic promoters located within the XIAP insert. As a further test of this hypothesis, we tested the effects of inhibiting cap-dependent translation through expression of the human rhinovirus 2A protease, which cleaves the eIF4G subunit of eIF4F (Haghighat et al. 1996). As expected, wild-type 2A protease impaired expression of the 5′ β-galactosidase cistron of both βgal/XIAP/CAT and βgal/EMCV/CAT, while an inactive mutant bearing the C106Al mutation (Petz et al. 2007) did not; indeed, the mutant protease appeared to enhance expression (Fig. 5). Also as expected, expression of the 3′ CAT cistron of βgal/EMCV/CAT was completely resistant to the 2A protease, but CAT expression from βgal/XIAP/CAT was strongly inhibited. These data indicate that expression driven from the XIAP element is cap dependent.
FIGURE 5.
Effects of rhinovirus2A protease on 5′ and 3′ cistron expression. HeLa cells were transfected with pßgal/EMCV/CAT or pßgal/XIAP/CAT along with p2Awt, p2Amut, or pcDNA 3.1 control. (A) β-galactosidase activity. (B) Chloramphenicol acetyltransferase activity. For each bicistronic plasmid, the data were normalized to yield a value of 1 for the pcDNA 3.1 control.
The original rationale for proposing that XIAP mRNA contains an IRES was that early studies indicated that the mRNA 5′ UTR is unusually long and contains upstream start codons and extensive predicted secondary structure (Holcik et al. 1999; Lagace et al. 2001), features that are predicted to hinder cap-dependent scanning. However, as noted by others (Van Eden et al. 2004; Baranick et al. 2008), extensive cDNA sequencing data have subsequently documented that this putative 5′ UTR corresponds to the 3′ portion of a large intron that is spliced out of mature XIAP mRNA, using the same splice acceptor site that is used in bicistronic reporter plasmids (including βgal/XIAP/CAT). Thus, most, if not all, endogenous XIAP mRNAs lack the proposed IRES sequence and instead bear a 227-nt 5′ UTR, which is devoid of AUG codons. These observations are incompatible with the hypothesis that translation of XIAP mRNA is regulated by the proposed IRES and suggest that such regulation is likely achieved through other mechanisms.
As reviewed in the Introduction, previous studies have documented that several putative cellular IRES elements drive 3′ cistron expression by providing cryptic splicing signals or promoters that give rise to unintended monocistronic mRNAs (Han and Zhang 2002; Van Eden et al. 2004; Liu et al. 2005; Wang et al. 2005; Bert et al. 2006; Baranick et al. 2008). Both of these mechanisms have been previously reported for the XIAP IRES (Van Eden et al. 2004; Bert et al. 2006; Baranick et al. 2008), and our results confirm these findings. However, unlike the situation with some of the other vector systems (Van Eden et al. 2004; Baranick et al. 2008), the cryptic splicing events that we observe give rise to biscistronic mRNAs (Fig. 2D) and hence do not account for the expression of the 3′ cistron. Rather, 3′ cistron expression appears to rely solely on cryptic promoter activity in the βgal/XIAP/CAT system. Van Eden et al. (2004) have proposed two tests that should be satisfied to validate a candidate IRES in bicistronic reporter assays: siRNAs targeting the 5′ cistron should knock down expression of the 3′ cistron, and spurious splicing should be stringently excluded using RT-PCR assays. We suggest two additional tests to exclude cryptic promoter activity. First, because rare RNAs arising from a cryptic promoter will not be detected by standard RT-PCR, very heavily overexposed Northern blots or 5′ RACE assays should be used to exclude the presence of minor transcripts encompassing the 3′ cistron. Second, the putative IRES should be tested for promoter activity in a vector that provides a strong external enhancer such as the SV40 element (as the cryptic promoters residing in putative IRESs often do not function in the absence of an enhancer; Bert et al. 2006).
MATERIALS AND METHODS
Plasmids and probes
The βgal/CAT, βgal/EMCV/CAT, and βgal/XIAP/CAT constructs were obtained from Martin Holcik, University of Ottawa, and the rhinovirus 2A protease vectors p2A and p2Amut (Petz et al. 2007) were obtained from Joachim Seipelt, Medical University of Vienna. pßgal/XIAP/CAT-CMV (lacking the CMV promoter) was constructed by digesting pßgal/XIAP/CAT with NruI and HindIII and religating the ends after treatment with T4 DNA polymerase. Probes for lacZ and CAT sequences were derived by PCR amplification of the corresponding regions of pßgal/XIAP/CAT using the following primers:
lacZ forward: 5′-TCGTTGCCGGTCTGGGAG-3′;
lacZ reverse: 5′-CGCAGCACCATCACCGC-3′;
CAT forward: 5′-ATCACTGGATATACCACCGTTG-3′; and
CAT reverse: 5′-CTGGTGAAACTCACCCAGG-3′.
The XIAP IRES was isolated by gel electrophoresis after cleaving pßgal/XIAP/CAT with XhoI. Probes were radiolabeled with 32P by random priming. γ-actin mRNA was detected using an oligonucleotide (5′-GACGACGAGCGCGGCGATATCATCATCCATG-3′), which was 5′-end-labeled using polynucleotide kinase and [32P]ATP.
RT-PCR
Total cellular RNA from HeLa cells transfected with βgal/XIAP/CAT was reverse transcribed using Superscript III (Invitrogen). Reactions were primed with oligo dT or a primer specific to the CAT gene (5′-TTACGCCCCGCCCTG-3′). PCR was performed with the following primers:
Forward: 5′-TACCTGTTCCGTCATAGCG-3′; and
Reverse: 5′- CCATATCACCAGCTCACCG-3′.
The resulting amplimers were resolved on a 1% agarose gel.
siRNA transfection
siRNA duplexes targeting the following sequences were obtained from Qiagen:
cat1: 5′-TGGAGTGAATACCACGACGATT-3′;
cat2: 5′-AGACCGTTCAGCTGGATATTA-3′;
gal1: 5′-GACGTCTCGTTGCTGCATAAA-3′; and
gal2: 5′-GTTGTTACTCGCTCACATTTA-3′.
The NT control siRNA was purchased from Dharmacon. HeLa cells in 24 well plates were transfected with the bicistronic reporter plasmid along with siRNA using Lipofectamine 2000 (Invitrogen). Medium was changed the next morning, and the following day lysates were prepared using 1× Reporter Lysis Buffer (Promega). β-galactosidase and chloramphenicol acetyltransferase assays were then performed on portions of the lysates using standard procedures. In experiments where the effects of rhinovirus 2A protease were examined, cells were cotransfected with 600 ng of the bicistronic reporter plasmid and 200 ng of the 2A protease expression vectors, and cells lysates were analyzed for β-galactosidase and chloramphenicol acetyltransferase 40 h later. In experiments where samples were analyzed for RNA and enzymatic activity, the transfections were scaled up to 60 mm dishes containing 12 mm coverslips. After transfection and incubation, the coverslips were transferred to a 24 well plate, and lysates were prepared for enzymatic activity analyses. Total cellular RNA was extracted from the remainder of the dish using TRIzol (Invitrogen).
Cytoplasmic RNA was prepared by isotonic lysis in the presence of 0.5% NP40 according to the protocol described in the RNeasy Mini Handbook (Qiagen), and nuclear RNA was purified from the associated nuclear pellets using TRIzol (Invitrogen). Polyadenylated RNA was purified from both fractions using an Oligotex mRNA mini kit (Qiagen) according to the manufacturer's instructions.
Northern blot analysis
RNA samples were electrophoresed through a 1.2% agarose-formaldehyde gel and transferred to a Genescreen membrane (NEN). All hybridizations were done using ExpressHyb (Clontech) according to the user's manual.
ACKNOWLEDGMENTS
We thank Martin Holcik and Joachim Seipelt for gifts of plasmids and Rob Maranchuk for technical assistance. This work was supported by an operating grant from the Canadian Institutes for Health Research (FRN 37995). J.R.S. is a Canada Research Chair in Molecular Virology.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.1557809.
REFERENCES
- Baranick BT, Lemp NA, Nagashima J, Hiraoka K, Kasahara N, Logg CR. Splicing mediates the activity of four putative cellular internal ribosome entry sites. Proc Natl Acad Sci. 2008;105:4733–4738. doi: 10.1073/pnas.0710650105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bert AG, Grepin R, Vadas MA, Goodall GJ. Assessing IRES activity in the HIF-1α and other cellular 5′ UTRs. RNA. 2006;12:1074–1083. doi: 10.1261/rna.2320506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Sarnow P. Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science. 1995;268:415–417. doi: 10.1126/science.7536344. [DOI] [PubMed] [Google Scholar]
- Gebauer F, Hentze MW. Molecular mechanisms of translational control. Nat Rev Mol Cell Biol. 2004;5:827–835. doi: 10.1038/nrm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighat A, Svitkin Y, Novoa I, Kuechler E, Skern T, Sonenberg N. The eIF4G–eIF4E complex is the target for direct cleavage by the rhinovirus 2A proteinase. J Virol. 1996;70:8444–8450. doi: 10.1128/jvi.70.12.8444-8450.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Zhang JT. Regulation of gene expression by internal ribosome entry sites or cryptic promoters: The eIF4G story. Mol Cell Biol. 2002;22:7372–7384. doi: 10.1128/MCB.22.21.7372-7384.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat Rev Mol Cell Biol. 2005;6:318–327. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- Holcik M, Lefebvre C, Yeh C, Chow T, Korneluk RG. A new internal-ribosome-entry-site motif potentiates XIAP-mediated cytoprotection. Nat Cell Biol. 1999;1:190–192. doi: 10.1038/11109. [DOI] [PubMed] [Google Scholar]
- Holcik M, Sonenberg N, Korneluk RG. Internal ribosome initiation of translation and the control of cell death. Trends Genet. 2000a;16:469–473. doi: 10.1016/s0168-9525(00)02106-5. [DOI] [PubMed] [Google Scholar]
- Holcik M, Yeh C, Korneluk RG, Chow T. Translational upregulation of X-linked inhibitor of apoptosis (XIAP) increases resistance to radiation-induced cell death. Oncogene. 2000b;19:4174–4177. doi: 10.1038/sj.onc.1203765. [DOI] [PubMed] [Google Scholar]
- Holcik M, Graber T, Lewis SM, Lefebvre CA, Lacasse E, Baird S. Spurious splicing within the XIAP 5′ UTR occurs in the Rluc/Fluc but not the βgal/CAT bicistronic reporter system. RNA. 2005;11:1605–1609. doi: 10.1261/rna.2158605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang SK, Krausslich HG, Nicklin MJ, Duke GM, Palmenberg AC, Wimmer E. A segment of the 5′ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988;62:2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieft JS. Viral IRES RNA structures and ribosome interactions. Trends Biochem Sci. 2008;33:274–283. doi: 10.1016/j.tibs.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. New ways of initiating translation in eukaryotes? Mol Cell Biol. 2001;21:1899–1907. doi: 10.1128/MCB.21.6.1899-1907.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace M, Xuan JY, Young SS, McRoberts C, Maier J, Rajcan-Separovic E, Korneluk RG. Genomic organization of the X-linked inhibitor of apoptosis and identification of a novel testis-specific transcript. Genomics. 2001;77:181–188. doi: 10.1006/geno.2001.6635. [DOI] [PubMed] [Google Scholar]
- Liu Z, Dong Z, Han B, Yang Y, Liu Y, Zhang JT. Regulation of expression by promoters versus internal ribosome entry site in the 5′-untranslated sequence of the human cyclin-dependent kinase inhibitor p27kip1. Nucleic Acids Res. 2005;33:3763–3771. doi: 10.1093/nar/gki680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J, Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988;334:320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Petz M, Kozina D, Huber H, Siwiec T, Seipelt J, Sommergruber W, Mikulits W. The leader region of Laminin B1 mRNA confers cap-independent translation. Nucleic Acids Res. 2007;35:2473–2482. doi: 10.1093/nar/gkm096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoneley M, Willis AE. Cellular internal ribosome entry segments: Structures, trans-acting factors and regulation of gene expression. Oncogene. 2004;23:3200–3207. doi: 10.1038/sj.onc.1207551. [DOI] [PubMed] [Google Scholar]
- Van Eden ME, Byrd MP, Sherrill KW, Lloyd RE. Demonstrating internal ribosome entry sites in eukaryotic mRNAs using stringent RNA test procedures. RNA. 2004;10:720–730. doi: 10.1261/rna.5225204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Weaver M, Magnuson NS. Cryptic promoter activity in the DNA sequence corresponding to the pim-1 5′-UTR. Nucleic Acids Res. 2005;33:2248–2258. doi: 10.1093/nar/gki523. [DOI] [PMC free article] [PubMed] [Google Scholar]