Abstract
Objectives
Human immunodeficiency virus type-1 (HIV-1) induces a series of alterations in the host cell that modify the intracellular environment in favor of viral replication, survival and spread. This research examined the impact of HIV-1 infection on autophagy in HIV-1 infected cells.
Methods
Protein extracts of HIV-1 infected and control CD4+ T-lymphocytes and U937 cells were semi-quantified by western blot. The autophagy-related protein Beclin 1, a Bcl-2 associated protein, and the 16 kD microtubule-associated protein (MAP) light chain three (LC3) which is essential for autophagy were quantified and validated using the intracellular protein GAPDH as an internal standard. Beclin 1 mRNA was quantified by real-time reverse transcriptase-polymerase chain reaction. Autophagosomes were assessed by visualization under confocal microscopy following intracellular staining of the LC3 protein.
Results
Following infection of human peripheral blood CD4+ T-cells or U937 cells with HIV-1 for 48 h, the autophagy protein Beclin 1 and LC3 II, which is essential for autophagy, were found to be markedly decreased. Beclin 1 mRNA expression was also reduced. Autophagosomes were reduced in HIV-1-infected cells. The reduction of autophagic protein expression and autophagosomes in HIV-1-infected cells could be overcome by amino acid starvation or rapamycin.
Conclusions
These data demonstrate that HIV-1 infection can down-regulate autophagy in infected cells during acute infection, and provide new insights into HIV-1-induced cell death and disease-related pathogenesis.
Keywords: autophagosome, autophagy, beclin 1, CD4+ cells, HIV-1, immunity, light chain three (LC3)
Introduction
Autophagy is an intracellular biological process also known as the type II programmed cell death pathway. Autophagy is involved in numerous human diseases including cancer, muscular disorders and neurodegeneration, major histocompatability complex (MHC) antigen presentation, and innate immunity against certain bacteria and viruses [1–3]. Three types of autophagy have beenwell described: chaperone-mediated autophagy, microautophagy and macroautophagy. Macroautophagy represents the typical autophagic process [4]. The hallmark of autophagy is a double-membraned autophagosome that engulfs bulk cytoplasm and cytoplasmic organelles such as mitochondria and endoplasmic reticulum [5]. Autophagosomes ultimately fuse with lysosomes thereby generating single-membraned autophagolysosomes and degrading their contents. Autophagy is evolutionarily conserved in eukaryotes from yeast to mammals. Beclin 1, the mammalian orthologue of yeast Atg6, localizes to the trans-golgi network (TGN), mitochondria and endoplasmic reticulum as a subunit of the mammalian class III phosphatidylinositol 3-kinase (PI 3-kinase) Vps34 and mainly engages hVps34 in the autophagic pathway and in autophagosome formation [5,6]. Microtubule-associated protein (MAP) light chain 3 (LC3) is a human homologue of yeast Apg8/Aut7/Cvt5 (Atg8). MAP-LC3 is cleaved by a cysteine protease to produce LC3-I (18 kD), which is located in the cytosolic fraction. LC3-I is converted to LC3-II (16 kD) through the actions of E1- and E2-like enzymes during autophagy. LC3-II is covalently attached to phosphatidylethanolamine on its C terminus, and it binds tightly to autophagosome membranes. Therefore, LC3-II is considered the functional form of LC3 and has been used as a specific marker of autophagy [1,7–9].
Recently, Espert et al. reported that HIV proteins can induce autophagy in bystander CD4+ T lymphocytes through contact of Env with CXCR4, leading to apoptotic cell death [10]. Based on the role of apoptosis in HIV-1 infection and the close interaction between apoptosis and autophagy, we hypothesized that HIV-1 infection may affect the autophagy pathway. Our findings indicate that the expression of Beclin 1 and the formation of autophagic vacuoles are significantly reduced during HIV-1 infection. Moreover, the reduction of Beclin 1 and autophagosome formation can be overcome by starvation of these cells.
Methods
Cell preparation and HIV-1 infection
U937 cells were maintained in RPMI 1640 medium with 10% fetal bovine serum (FBS). CD4+ T lymphocytes maintained in 50 IU/ml interleukin (IL)-2 (R&D, Minneapolis, Minnesota, USA) were purified by using a human CD4+ T Cell negative Isolation Kit II (Miltenyi Biotec, Auburn, California, USA) from healthy HIV-seronegative peripheral blood mononuclear cells (PBMC) following a 3-day treatment with 5 µg/ml phytohemagglutinin (PHA).
HIV-1MN (originally contributed by Robert Gallo through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institutes of Health, Bethesda, Maryland, USA) was propagated in H9 cells. The inactivated HIV-1MN was prepared by treating virus stocks with 1 mmol/l Aldrithiol-2 (AT-2) for 1 h at 37° C (AT-2 HIV-1)MN, as previously described [11,12].
Samples of 5 × 106 U937 or PHA-treated CD4+ T-cells in six-well plates were exposed to infectious HIV-1 at a multiplicity of infection (moi) of 5, AT-2-treated virus or supernatant from uninfected U937 cells at 37° C with 5% CO2 for 4 h. Cells were washed and incubated with RPMI 1640 with 10% FBS. HIV-1 gag p24 protein in culture supernatant of HIV-1 or inactivated AT-2 HIV-1-treated cells was measured by enzyme-linked immunosorbent assay according to the manufacturer’s protocol (Beckman Coulter, Miami, Florida, USA).
To identify HIV-1 infected cells, cells were fixed with 4% paraformaldehyde (PFA). TSA Kit #2* with horseradish peroxidase-goat antimouse IgG and Alexa Fluor 488 tyramide (Invitrogen, Carlsbad, California, USA) was used according to the manufacturer’s instructions. Mouse anti-HIV-1p24 monoclonal antibodies (ImmunoDiagnostics, Woburn, Massachusetts, USA) were used as the primary antibodies. Following staining, cells were examined under a fluorescent microscope.
Western blot analysis
Cytoplasmic proteins were separated on a 10 or 12% gradient Tris–glycine gel (Invitrogen) and blotted to polyvinylidine difluoride membrane. A WesternBreeze chemiluminescent western blot immunodetection kit (Invitrogen) was used according to the manufacturer’s protocol. Band intensity on exposed film was semi-quantified using ImageJ software (National Institutes of Health, Bethesda, Maryland, USA). Beclin 1 (BCN1), GAPDH, and MAP-LC3 antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, California, USA).
Intracellular staining of LC3 protein and visualization of autophagosome
The PFA-fixed cells were blocked and permeabilized. Rabbit antihuman LC3 monoclonal antibodies and Cy3-conjugated goat antirabbit monoclonal antibodies were used for detecting autophagosomes in the cells. The stained cells was mounted on a cover-slip and analyzed on an Olympus Disk Scan Confocal Microscope system (Olympus America Inc., Center Valley, Pennsylvania, USA). The number and volume of fluorescent-stained LC3+ particles per cell were determined and used as an assessment of autophagosomes.
Results
Autophagy is down-regulated in HIV-1-infected cells
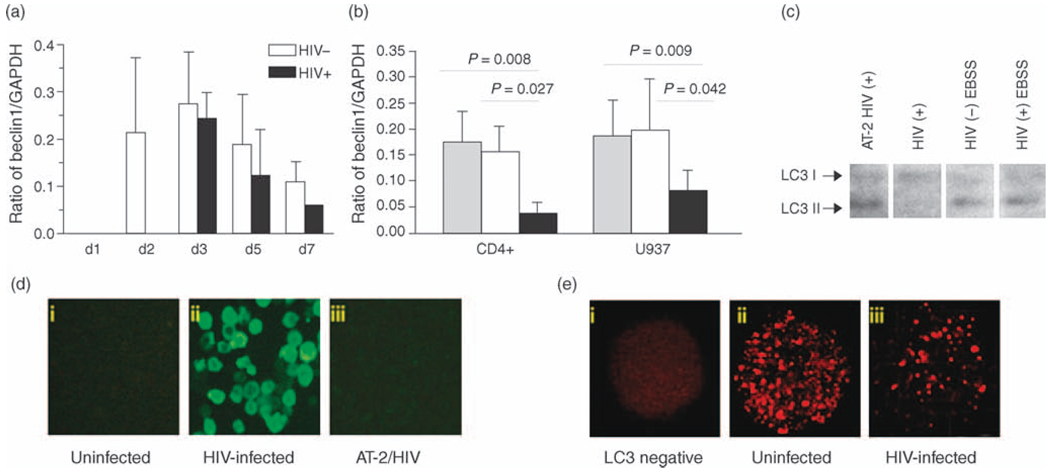
As autophagy is known to be inhibited by serum-rich medium, autophagy formation in U937 cells and CD4+ cells was assessed in complete RPMI medium at a high density of 5 × 106 cells/ml. Using this system, Beclin1 protein in untreated cells could be detected within 2 days. The level of protein is expressed as the ratio of the Beclin 1 band to that of GAPDH. Dynamic changes of Beclin 1 protein expression were observed in U937 cells over time as the cells underwent increasing nutrient stress (Fig. 1a). With HIV-1 infection, comparable levels of autophagy were not observed until day 4 in culture, and up to day 7 the HIV-1-infected cells never achieved the levels of Beclin 1 observed in the uninfected cultures at day 2.
Fig. 1. Autophagy proteins and autophagosomes in HIV-1 infected and uninfected cells.
(a) Ratio of Beclin 1: GAPDH at different days 1, 2, 3, 5 and 7 (d1, 2, 3, 5 and 7). The bars depict cells treated with media only (white bars) and HIV-1 (black bars). Results are representative of five independent experiments. (b) Western blot of proteins extracted from CD4+ T-lymphocytes and U937 cells collected after 48 h of HIV-1 infection. White bars are cells treated with media only, gray bars treated with AT-2 HIV-1 and black bars infected with HIV-1. (c) LC3 protein in U937 cells detected by western blot assay. (d) HIV-1 infected cells were identified by p24 TSA staining. (i) Uninfected cells. (ii) Positive staining cells are in green. (iii) AT-2 HIV-1 treated cells. (e) Autophagosome vacuoles (AV) are stained as red particles. (i) AV negative staining. (ii) AV positive staining in HIV-1 negative cells. (iii) HIV-1 infected cells. Results are representative of five independent experiments.
To examine the impact of HIV-1 infection on autophagy, U937 cells and PHA-treated CD4+ T lymphocytes were infected with HIV-1. Aldrithiol-2 (AT-2) inactivated HIV-1 (AT-2 HIV-1) was used as a control for HIV-1 infection. To ensure comparable conditions, infected and uninfected cells were treated identically at each step, including when changing culture media. Beclin 1 protein was detected by western blotting and the autographic film was analyzed and semi-quantitated with ImageJ software. The data from five independent experiments showed that the Beclin 1 expression levelwas significantly decreased in both U937 (P=0.042 and CD4+ T-cells (P=0.027) infected with HIV-1 for 48 h compared to the cells treated with media only and AT-2 treated HIV-1 (P=0.008 for CD4+ cells and P=0.009 for U937 cells, respectively (Fig. 1b). Beclin 1 mRNA expression levels of the cells exposed to infectious HIV-1 were also significantly decreased in comparison with that of untreated cells (data not shown). The mean reduction of mRNA expression was 28.5% in CD4+ cells (P=0.006) and 20.5% in U937 cells (P=0.024), respectively. The reduction of Beclin 1 caused by HIV-1 infection was confirmed further by detection of intracellular p24. In order to identify HIV-1-infected cells, a highly sensitive fluorescent signal tyramide-based staining system was employed to detect intracellular p24 [13]. Using this detection system, approximately 80% of cells demonstrated HIV-1 infection on days 2 and 7 (Fig. 1d).
LC3 fluorescent staining was used to visualize andmonitor autophagic vacuole formation during HIV-1 infection using a disk scanning confocal microscopy system that enabled analysis in three-dimensional scanning. The total number of LC3 positive (LC3+) particles and the volume of the total number of the particles in each cell were determined and used as the indicators of autophagosome formation. Following 48 h HIV-1 infection, LC3+ staining autophagocytic vacuoles were significantly reduced as measured by the volume and the number of LC3+ intracellular particles (Fig. 1e). In comparison with the uninfected cells, the mean (±SD) of LC3+ particles in infected U937 cells went from 687 ± 70 to 123 ± 74 (P < 0.001) and for CD4+ cells from 280 ± 60 to 53 ± 19 (P < 0.01), respectively. The volume of the particles observed was also significantly reduced in both cell types (P < 0.001 for U937 cells and P < 0.01 for CD4+ T cells, respectively) (data not shown). Similarly, LC3 II was markedly reduced in HIV-1-infected cells as determined by western blot (Fig. 1c).
Autophagy is induced in HIV-1-treated cells by amino acid starvation and rapamycin
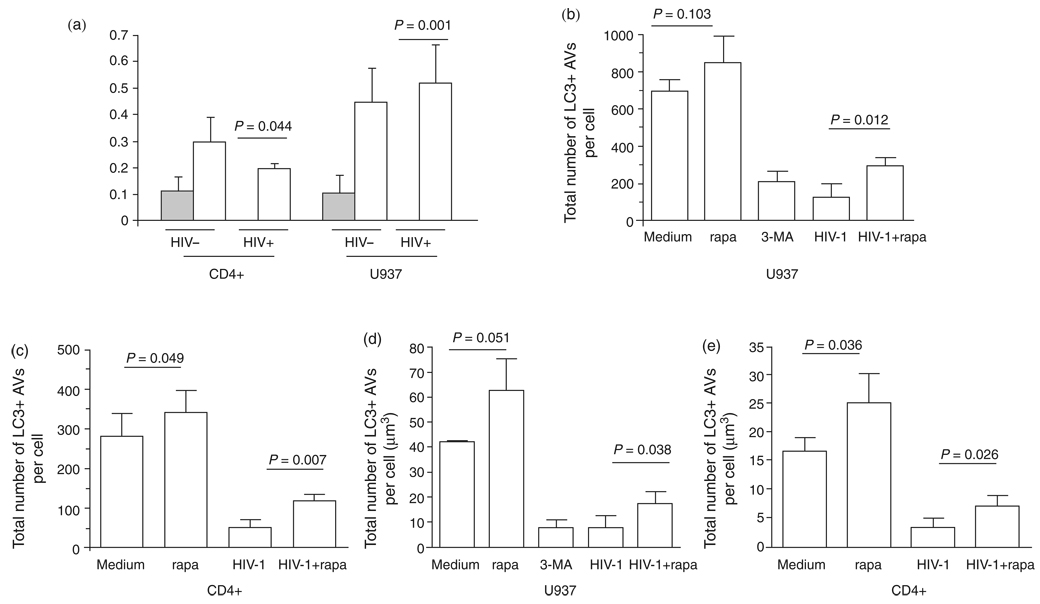
During starvation, the induction of autophagy contributes to the maintenance of cellular homeostasis that helps maintain an amino acid pool for gluconeogenesis and for the synthesis of essential proteins. Starvation and rapamycin are widely used autophagic inducers that works through inhibition of mammalian target of rapamycin (mTOR), which regulates a wide array of cellular functions, including apoptosis and autophagy [14,15]. To assess whether the inhibition of Beclin 1 expression and autophagosome formation were reversible, cells were treated in serum-free Earle’s balanced salts solution (EBSS) or with 200 nmol/l rapamycin at 37° C with 5% CO2 for 3 h. After induction, Beclin 1 protein was significantly increased in HIV-1-infected U937 cells (P=0.001) and for CD4+ T lymphocytes (P=0.044) (Fig. 2a) as were the number of autophagosomes per cell seen in CD4+ T cells (P=0.007) and U937 cells (P=0.012). Similarly, the volume of autophagosomes per cell was also increased in CD4+ T cells (P=0.026) and U937 cells (P=0.038) (Fig. 2b–e). These data demonstrate that the inhibition of autophagy by HIV-1 infection is reversible.
Fig. 2. Induction of autophagy. Cells were incubated for 48 h following treatment with media only or infectious HIV-1 and then half of the cells were starved for 3 h.
(a) Beclin 1 protein expression levels of the cells undergoing starvation are represented by open bars and those without starvation by shaded bars. Beclin 1 and GAPDH proteins were probed by western blot. (b–e) Autophagosome number and volume were detected by fluorescent LC3 staining after autophagy induction. Results are representative of three independent experiments.
Discussion
Our data indicate that HIV-1 infection down-regulates the expression of the autophagy protein Beclin 1 and the formation of autophagic vacuoles in U937 cells and CD4+ T lymphocytes. It is broadly reported that HIV-1 infection induces apoptosis through various viral products including envelope proteins, Vpr, Tat, Nef and protease. Among these apoptotic viral proteins, envelope protein gp120 induces apoptosis through the CD4/CXCR4–mTOR–p53 axis [16]. In this pathway, activated mTOR, plays a central role in gp120-induced apoptosis. mTOR is a downstream effector of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway and is an important kinase in many cellular processes including autophagy and apoptosis. mTOR negatively controls autophagy and positively controls apoptosis. As activated mTOR inhibits autophagy, it is possible that during active HIV-1 infection envelope proteins interact with mTOR leading to a down-regulation of autophagy. Previous studies have demonstrated that gp120 activates both PKCepsilon and its upstream effector PI3K/Akt which inhibits autophagy through activating mTOR [17]. Theoretically, factors that induce apoptosis could also inhibit autophagy due to the close relationship between autophagy and apoptosis [18]. As amino acid starvation of the cells inhibits mTOR, the findings that starvation and rapamycin increase Beclin 1 expression and autophagic vacuole formation further support our hypothesis that HIV-1 causes the down-regulation of autophagy through mTOR activation.
As a general rule of viruses, the alterations of cellular processes induced by viral infection favors viral replication and spread. Single-stranded RNA viruses including poliovirus block the degradation of autophagosome membranes and use the membranes to anchor theirRNA replication complexes [19,20]. Viruses that do not use autophagosomal membranes for their replication appear to down-regulate the formation of autophagosomes in order to enhance viral replication. HIV-1 would appear to fall into this latter category. It is likely that the down-regulation of autophagy by HIV-1 is part of an elaborate strategy used by the virus to avoid immunologic control.
In conclusion, our findings suggest HIV-1 has developed mechanisms for the down-regulation of autophagy that are probably part of a strategy designed to enhance viral replication and to evade the immune system. Additional studies of the relationship of autophagy with HIV-1 replication and survival are needed to understand the role of autophagy in HIV-1 pathogenesis.
Acknowledgements
We thank Brendan Brinkman at the UCSD Neuroscience Microscopy Shared Facility for his assistance.
Sponsorship: this study was supported in part by grants from the National Institute of Allergy and Infectious Diseases [AI-68632, AI-39004 and AI-36214 (Virology Core, University of California, San Diego, Center for AIDS Research)].
References
- 1.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paludan C, Schmid D, Landthaler M, Vockerodt M, Kube D, Tuschl T, et al. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- 3.Dengjel J, Schoor O, Fischer R, Reich M, Kraus M, Muller M, et al. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc Natl Acad Sci USA. 2005;102:7922–7927. doi: 10.1073/pnas.0501190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12 Suppl 2:1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inbal B, Bialik S, Sabanay I, Shani G, Kimchi A. DAP kinase and DRP-1 mediate membrane blebbing and the formation of autophagic vesicles during programmed cell death. J Cell Biol. 2002;157:455–468. doi: 10.1083/jcb.200109094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, et al. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng X, Overmeyer JH, Maltese WA. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J Cell Sci. 2006;119:259–270. doi: 10.1242/jcs.02735. [DOI] [PubMed] [Google Scholar]
- 8.Kouno T, Mizuguchi M, Tanida I, Ueno T, Kanematsu T, Mori Y, et al. Solution structure of microtubule-associated protein light chain 3 and identification of its functional subdomains. J Biol Chem. 2005;280:24610–24617. doi: 10.1074/jbc.M413565200. [DOI] [PubMed] [Google Scholar]
- 9.Kabeya Y, Mizushima N, Yamamoto A, Oshitani-Okamoto S, Ohsumi Y, Yoshimori T. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J Cell Sci. 2004;117:2805–2812. doi: 10.1242/jcs.01131. [DOI] [PubMed] [Google Scholar]
- 10.Espert L, Denizot M, Grimaldi M, Robert-Hebmann V, Gay B, Varbanov M, et al. Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. J Clin Invest. 2006;116:2161–2172. doi: 10.1172/JCI26185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossio JL, Esser MT, Suryanarayana K, Schneider DK, Bess JW, Jr, Vasquez GM, et al. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J Virol. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbeuval JP, Grivel JC, Boasso A, Hardy AW, Chougnet C, Dolan MJ, et al. CD4+ T-cell death induced by infectious and noninfectious HIV-1: role of type 1 interferon-dependent. TRAIL/DR5-mediated apoptosis. Blood. 2005;106:3524–3531. doi: 10.1182/blood-2005-03-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Gijlswijk RP, Zijlmans HJ, Wiegant J, Bobrow MN, Erickson TJ, Adler KE, et al. Fluorochrome-labeled tyramides: use in immunocytochemistry and fluorescence in situ hybridization. J Histochem Cytochem. 1997;45:375–382. doi: 10.1177/002215549704500305. [DOI] [PubMed] [Google Scholar]
- 14.Lu B, Capan E, Li C. Autophagy induction and autophagic cell death in effector T cells. Autophagy. 2007;3:158–159. doi: 10.4161/auto.3637. [DOI] [PubMed] [Google Scholar]
- 15.Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–262. doi: 10.1016/s0092-8674(00)00117-3. [DOI] [PubMed] [Google Scholar]
- 16.Perfettini JL, Castedo M, Roumier T, Andreau KR, Nardacci R, Piacentini M, et al. Mechanisms of apoptosis induction by the HIV-1 envelope. Cell Death Differ. 2005 Suppl 1:916–923. doi: 10.1038/sj.cdd.4401584. [DOI] [PubMed] [Google Scholar]
- 17.Misse D, Gajardo J, Oblet C, Religa A, Riquet N, Mathieu D, et al. Soluble HIV-1 gp120 enhances HIV-1 replication in nondividing CD4+ T cells, mediated via cell signaling and Tat cofactor overexpression. AIDS. 2005;19:897–905. doi: 10.1097/01.aids.0000171403.07995.92. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi H, Kondo Y, Fujiwara K, Kanzawa T, Aoki H, Mills GB, et al. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005;65:3336–3346. doi: 10.1158/0008-5472.CAN-04-3640. [DOI] [PubMed] [Google Scholar]
- 19.Jackson WT, Giddings TH, Jr, Taylor MP, Mulinyawe S, Rabinovitch M, Kopito RR, et al. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 2005;3:e156. doi: 10.1371/journal.pbio.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munz C. Viral evasion of autophagy. Cell Host & Microbe. 2007;1:9–11. doi: 10.1016/j.chom.2007.02.005. [DOI] [PubMed] [Google Scholar]