Abstract
Mammary alveologenesis is abrogated in the absence of the transcription factors STAT5A/5B, which mediate cytokine signaling. To reveal the underlying causes for this developmental block, we studied mammary stem and progenitor cells. While loss of STAT5A/5B did not affect the stem cell population and its ability to form mammary ducts, luminal progenitors were greatly reduced and unable to form alveoli during pregnancy. Temporally controlled expression of transgenic STAT5A in mammary epithelium lacking STAT5A/5B restored the luminal progenitor population and rescued alveologenesis in a reversible fashion in vivo. Thus, STAT5A is necessary and sufficient for the establishment of luminal progenitor cells.
Keywords: STAT5, alveologenesis, mammary gland, progenitor, CD61, tetracycline
STAT5A and STAT5B, collectively referred to as STAT5A/5B, are two highly conserved transcription factors activated by various cytokines—including prolactin, growth hormone, and EGF—that play important roles in the development and function of mammary glands, hematopoietic cells, liver, and muscle (Hennighausen and Robinson 2008). Deletion of the Stat5a gene in the germline results in impaired mammary alveologenesis during pregnancy (Liu et al. 1997). However, in the absence of Stat5a, increased expression of STAT5B after several pregnancies partially rescued the lactation defect (Liu et al. 1998). Deletion of Stat5b had no effect on mammary epithelial cells (Udy et al. 1997; Teglund et al. 1998). In subsequent studies using germline deletion, as well as mammary epithelial-specific ablation, of both Stat5a and Stat5b genes, the role of STAT5A/5B during mammary gland development was investigated (Miyoshi et al. 2001; Cui et al. 2004). We discovered that the deletion of both Stat5a/5b genes in mammary epithelium resulted in a severe defect of alveologenesis, and that the presence of STAT5A/5B was essential for the proliferation, differentiation, and survival of mammary epithelial cells.
Mammary epithelium consists of two types of cells: basal myoepithelial cells and luminal cells, which form a ductal tree in virgins and alveoli during pregnancy. These events are coordinated by systemic hormones and cytokines (Hennighausen and Robinson 2005). Elaboration of mature epithelium from stem cells is thought to proceed in a hierarchical progression. Stem cells give rise to transient amplified progenitor cells capable of generating ductal and alveolar structures that become restricted to only ductal or alveolar fates and eventually give rise to differentiated lineages (Stingl 2009). In recent years, a combination of enzyme digestion and fluorescence-activated cell sorting (FACS) techniques have been developed to allow the isolation of these different cell populations from single-cell suspensions derived from mammary tissue of virgin female mice (Shackleton et al. 2006; Stingl et al. 2006). This knowledge enabled us to study the role of STAT5A/5B in a defined cell population of mammary epithelium.
STAT5A/5B control stem and progenitor cell fate in the hematopoietic system (Wang et al. 2009). In the absence of STAT5A/B, mice fail to develop T, B, and natural killer cells (Hoelbl et al. 2006; Yao et al. 2006). STAT5A/5B are also required for the maintenance and expansion of primitive stem and progenitor cells, both in normal and leukemic hematopoiesis (Li et al. 2007; Liu et al. 2008). These studies support our proposal that STAT5A/5B are critical for mammary cell lineage development from primitive stem/progenitor cells.
Several mechanisms might account for the lack of alveolar development in the absence of STAT5A/5B: (1) Stem cells are defective and fail to generate alveolar progenitor cells. (2) Although stem cells generate alveolar progenitor cells, progenitors cannot proliferate or survive. (3) Although stem cells give rise to alveolar progenitor cells that can proliferate and survive, progenitors do not generate daughter alveolar cells. (4) STAT5A/B play a role only in differentiated alveolar cells. To test these hypotheses, we isolated and analyzed epithelial stem and progenitor cell populations from mammary epithelium containing or lacking STAT5A/5B.
Results and Discussion
The mammary luminal progenitor cell population is reduced in the absence of STAT5A/5B
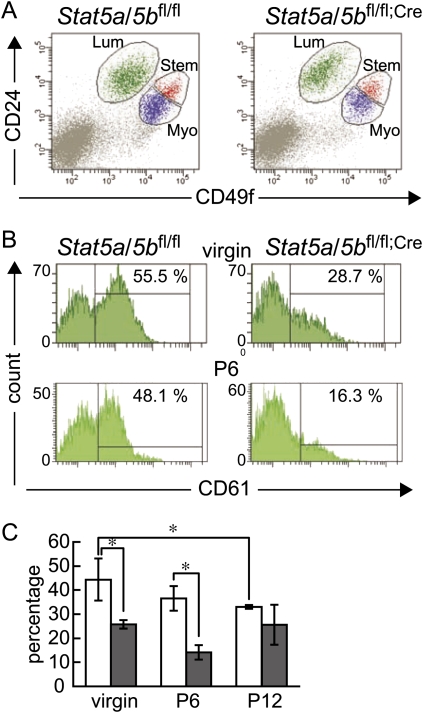
To ask which of the steps in the lineage progression of mammary stem cells to functional secretory epithelium is dependent on the presence of STAT5A/5B, we used conditional gene deletion with a MMTV-Cre transgenic mouse line that affects all epithelial cells of the newborn as determined with a lacZ reporter construct. Therefore, we consider the entire epithelium null for Stat5a/5b (Wagner et al. 1997, 2001; Buono et al. 2006). Our observation that Stat5a/5bfl/fl;Cre mice could not lactate even after five to six pregnancies further supports this. We prepared single-cell suspensions from mammary tissue of nulliparous mature female Stat5a/5bfl/fl and Stat5a/5bfl/fl;Cre mice and performed flow cytometry analysis using antibodies against CD24, CD49f, and CD61 (Stingl et al. 2006; Asselin-Labat et al. 2007). Both Stat5a/5bfl/fl and Stat5a/5bfl/fl;Cre virgin mice showed similar dot plot patterns that defined four cell populations: negative (CD24−CD49f−), luminal (CD24hiCD49flo), myoepithelial (CD24loCD49fhi), and the stem cell-enriched upper portion of myoepithelial cells (CD24midCD49fhi) (Fig. 1A). Percentages of each population in Stat5a/5bfl/fl mice versus Stat5a/5bfl/fl;Cre mice were 11.1% ± 0.97% versus 11.5% ± 1.33% in luminal, 19.0% ± 3.06% versus 11.3% ± 1.82% in myoepithelial, and 3.38% ± 0.33% versus 2.00% ± 0.40% in stem cell-enriched populations, respectively. Quantitative real-time RT–PCR analysis revealed that Stat5a and Stat5b mRNAs were not present in the luminal, myoepithelial, and stem cell-enriched fractions in mammary tissue of Stat5a/5bfl/fl;Cre mice (Supplemental Fig. 1). Stat5a/5b mRNAs were present in the CD24−CD49f− population in both Stat5a/5bfl/fl and Stat5a/5bfl/fl;Cre mice, suggesting that these were of stromal origin. These results corroborate the high deletion efficiency in this MMTV-Cre transgenic line (Wagner et al. 1997, 2001; Buono et al. 2006).
Figure 1.
Flow cytometry analyses of mammary cells from Stat5a/5bfl/fl mice (left panels) and Stat5a/5bfl/fl;Cre mice (right panels). (A) Dot plot pattern of luminal (Lum; CD24hiCD49flo), myoepithelial (Myo; CD24loCD49fhi) and stem cell-enriched (Stem; CD24midCD49fhi) populations in 12-wk-old virgin mice. (B) CD61 staining histograms of luminal cell population (CD24hiCD49flo) in the mammary gland of 12-wk-old virgin mice (top panels) and 6 d pregnant mice (P6, bottom panels). The CD61+ population was determined according to fluorescence minus one (FMO) control of the same mouse. (C) Bar graph depicting the percentage of CD61+ cells in luminal cell populations of Stat5a/5bfl/fl (blank bars) and Stat5a/5bfl/fl;Cre (filled bars) mammary glands in virgins, and on day 6 (P6) and day 12 (P12) of pregnancy. Values are means ± SD. (*) P < 0.05.
To assess the ability of mammary stem cells to form an entire functional mammary gland in the presence or absence of STAT5A/5B, the FACS-purified stem cell-enriched populations were transplanted into cleared fat pads of athymic nude mice. Mammary stem cells from Stat5a/5bfl/fl and Stat5a/5bfl/fl;Cre mice were able to generate ductal outgrowths with primary and secondary side branches (Supplemental Fig. 2A,B). This is in agreement with the results obtained by transplantation of small pieces of mammary tissue from Stat5a/5bΔN/ΔN, another mouse line with a partially disrupted Stat5a/5b locus (Miyoshi et al. 2001). Limiting dilution analyses revealed no difference in the ability of stem cells to generate epithelial outgrowths in the presence or absence of STAT5A/5B (Supplemental Table 1). These results demonstrate that STAT5A/5B are not important for the ability of mammary stem cells to reconstitute both luminal and myoepithelial cell lineages in ducts of virgin mice. To assess alveolar development and differentiation, stem cell-transplanted hosts were mated to induce pregnancy. While Stat5a/5bfl/fl stem cells were able to develop a full alveolar compartment at term (Supplemental Fig. 2C,E), the epithelial compartment in Stat5a/5bfl/fl;Cre stem cell transplants remained sparse (Supplemental Fig. 2D,F). Histological analyses demonstrated that outgrowths from Stat5a/5bfl/fl stem cells had alveoli that consisted of cells secreting milk (Supplemental Fig. 2G,I), while the Stat5a/5bfl/fl;Cre stem cells produced only a ductal tree with virgin-like characteristics (Supplemental Fig. 2H,J). These results validate earlier studies that alveolar development is inhibited in the absence of STAT5A/5B.
To determine potential causes for the absence of alveolar development, we analyzed luminal progenitor cells. The amount of CD61+ cells in the luminal cell population was profoundly reduced in Stat5a/5bfl/fl;Cre mice (44.4% ± 3.93% in Stat5a/5bfl/fl vs. 25.8% ± 0.81% in Stat5a/5bfl/fl;Cre) in virgins (Fig. 1B). Furthermore, the CD61+ population was not increased during pregnancy (Fig. 1B,C). This strongly suggests that the CD61+ luminal progenitor cells present in the Stat5a/5bfl/fl;Cre mammary gland are ductal luminal progenitor cells, and the reduction in the total number of CD61+ cells reflects an absence of alveolar precursors. Thus, impaired alveologenesis and lactation of Stat5a/5b-null mammary epithelium most likely are due to a reduced number of alveolar luminal progenitor cells already in the virgin state.
STAT5A is necessary and sufficient for the generation of alveolar luminal progenitor cells and mature alveoli
We next asked whether the deficit in CD61+ cells in the ducts of virgin glands and the failure to generate alveoli can be overcome by a temporally defined expression of STAT5A after the ductal system has been established at the end of puberty. Mice were generated that carried a Stat5a transgene under control of the tetracycline-inducible promoter (tet-op-Stat5a), as well as MMTV-rtTA (Gunther et al. 2002) and MMTV-Cre transgenes and two floxed Stat5a/5b alleles (Stat5a/5bfl/fl;Cre;iStat5). These experimental mice were fed with doxycyline-containing food to induce transgenic expression of Stat5a and were continuously mated. Mice being exposed to doxycycline during pregnancy developed functional mammary glands during pregnancy and were able to feed their pups. In order to demonstrate the dependence of mammary development and lactation on the expression of transgenic STAT5A induced by doxycycline, successfully nursing dams were switched to regular food after weaning of the second litter. Insufficient amounts of milk were present and pups of the third and fourth litters did not survive. Return to doxycycline food, and therefore expression of transgenic Stat5a, after the death of the fourth litter resulted in the survival of two more litters.
Mammary tissue samples from the Stat5a/5bfl/fl;Cre;iStat5 mice taken by biopsy in the morning after delivering the first, fourth, and sixth litters were analyzed by histology and immunofluorescence staining (Fig. 2A). Expanded alveoli lined with differentiated secretory cells were found after doxycycline treatment (Fig. 2B, panel a). The lumen contained proteinaceous material and lipid droplets. In the absence of doxycycline, the alveoli were small, secretory cells appeared undifferentiated, and the lumina were unexpanded and devoid of lipid droplets (Fig. 2B, panel b). Activated STAT5 was found in the majority of nuclei after doxycycline treatment, but was scarce in the absence of doxycycline (Fig. 2B, panels d,e). Epithelial differentiation was reversed when expression of exogenous STAT5A was abolished and could be induced again upon stimulation of STAT5A expression (Fig. 2B, panels c,f). Furthermore, staining of the sodium/potassium/cotransporter 1 (NKCC1), a protein expressed in undifferentiated ductal cells of virgin and early pregnancy stages (Shillingford et al. 2002), was seen in few luminal cells in the samples from the first and sixth lactation, but was strongly expressed in the absence of doxycycline after the fourth pregnancy (data not shown). These results show that the presence of only STAT5A during pregnancy leads to the formation of alveolar structures and functional differentiation of luminal cells. It clearly indicates that Stat5a/5b-deficient mammary epithelium contains stem cells that are capable of differentiation along the ductal but not alveolar luminal cell lineage, and luminal cell fate depends on STAT5A.
Figure 2.
Histology of mammary tissue of Stat5a/5bfl/fl;Cre;iStat5 mice in the presence or absence of doxycycline. (A) Schematic design of the experimental protocol. Mice were fed with doxycycline-containing food from mating to the end of the second lactation, and again after delivery of the fourth litter. (B) Mammary glands of Stat5a/5bfl/fl;Cre;iStat5 mice were biopsied on the day of delivering the first (panels a,d), fourth (panels b,e), and sixth (panels c,f) litters and were stained with hematoxylin and eosin (panels a–c) or immunostained with anti-phosphorylated STAT5 antibody (red) and anti-E-cadherin antibodies (green) (panels d–f). Black arrows indicate lumina. White arrows point to cells containing nuclear pSTAT5.
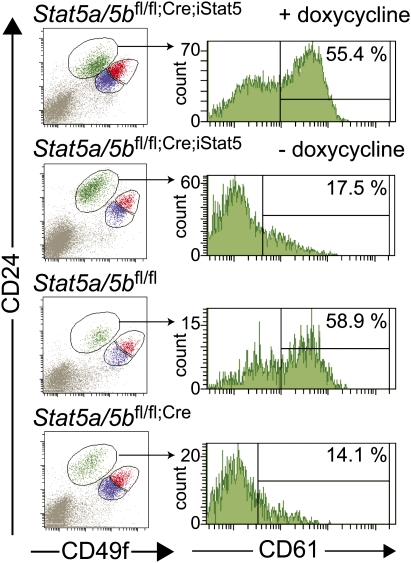
To evaluate the role of STAT5A in the generation of alveolar luminal progenitor cells, flow cytometry analyses were performed with mammary tissues of Stat5a/5bfl/fl;Cre;iStat5 mice 4 wk after weaning (Fig. 3). When fed with doxycycline food, the Stat5a/5bfl/fl;Cre;iStat5 mice were able to generate CD61+ luminal progenitor cells during pregnancy to a similar extent as Stat5a/bfl/fl mice. In contrast, the number of CD61+ luminal progenitor cells in the mammary glands of Stat5a/bfl/fl;Cre;iStat5 mice fed with normal diet was comparable with that of Stat5a/5bfl/fl;Cre mice. These results demonstrate that expression of STAT5A in the Stat5a/5b-deficient mammary epithelium was necessary and sufficient for the generation of luminal progenitor cells in the quiescent gland and alveologenesis during pregnancy.
Figure 3.
Flow cytometry analyses of mammary luminal progenitor cells in the presence or absence of STAT5A/5B. Dot plot patterns of CD24 and CD49f populations and histograms of CD61 staining of luminal cell population in the mammary tissue of mice with indicated genotype 4 wk after weaning. Mammary tissues of Stat5a/5bfl/fl;Cre;iStat5 mice were analyzed with or without feeding doxycycline.
We performed genome-wide microarray analyses to investigate changes in the gene expression profile of Stat5a/5bfl/fl and Stat5a/5bfl/fl;Cre mammary tissues on day 6 of pregnancy. We considered this a stage that would allow the identification of genes that are involved in the early stages of luminal cell proliferation and differentiation and are induced by STAT5. Besides β-casein, cyclin D1, and connexin 26 (Hennighausen and Robinson 2005), which are known STAT5 target genes, Gene Ontology analyses showed decreased expression of genes involved in regulation of the cell cycle, DNA metabolic processes, response to DNA damage stimuli, and chromosome organization pathways in Stat5a/5b-deleted samples (Supplemental Table 2). These genes may be either directly transcribed by STAT5 or represent targets of genomic regulatory networks activated by STAT5 in the early phase of pregnancy that is characterized by proliferation and rapid expansion of cells.
Impaired mammary development also occurs in Gata3- and Elf5-deficient mammary epithelium (Asselin-Labat et al. 2007; Oakes et al. 2008). To explore a possible link between these transcription factors and STAT5A, we measured the expression of these two genes in the stem and progenitor cell fractions. mRNA levels of Gata3 were similar in both cell populations, irrespective of the genotype (Supplemental Fig. 3). However, expression of Elf5 was barely detectable in the Stat5a/5bfl/fl;Cre cells (Supplemental Fig. 3).
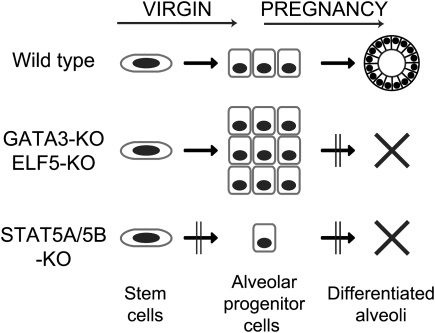
This demonstrates a distinct function for these transcription factors in the different cell lineages (Fig. 4). GATA3, which has been shown to control maintenance of luminal cells in ducts as well as alveoli (Kouros-Mehr et al. 2006; Asselin-Labat et al. 2007), is not regulated by STAT5A/5B. A higher proportion of CD61+ luminal progenitor cells in Gata3-deficient mammary tissues is observed in virgins and sustained through pregnancy, reflecting a block of differentiation to mature ductal and alveolar luminal cells (Asselin-Labat et al. 2007). Elf5-null mammary epithelium also contains a larger fraction of CD61+ luminal progenitor cells during pregnancy but not in virgins, indicating a block of differentiation to secretory alveolar cells (Oakes et al. 2008). We observed a decrease of CD61+ luminal progenitor cells in the Stat5a/5b-null mammary epithelium already in virgins and no increase of this cell population during pregnancy. Since ductal morphogenesis is not affected, this indicates that STAT5A/5B regulate the production or expansion of alveolar luminal progenitor cells (Fig. 4). The current lack of surface markers to distinguish ductal and luminal progenitor cells makes it impossible to discriminate the two possibilities. Our results indicate a failure of primitive stem/progenitor cells to generate alveolar luminal progenitor cells in the absence of STAT5A/5B.
Figure 4.
Schematic model depicting the role of STAT5A/5B and other transcription factors in the development of mammary epithelial cell lineages. (Top row) In the wild-type mammary gland, epithelial stem cells generate alveolar luminal progenitor cells that develop mature alveoli during pregnancy. (Middle) Alveolar progenitor cells fail to differentiate into secretory luminal cells in the absence of GATA3 or ELF5, leading to their accumulation in pregnancy and lack of alveolar development. (Bottom row) Loss of STAT5A/5B impairs development of alveolar precursor cells (this study) as well as maintenance of mature alveolar cells as shown previously.
We previously identified Elf5 as a gene highly up-regulated in lactating mammary tissue (Renou et al. 2003). Like STAT5A/5B, ELF5 is a mediator of the prolactin–receptor signaling pathway (Harris et al. 2006). ELF5 binding to the proximal Stat5a gene promoter in late pregnancy has been demonstrated, and loss of ELF5 in primary mammary epithelial cells resulted in a modest decrease in the overall levels of STAT5A (Choi et al. 2009). Real-time RT–PCR analyses demonstrated that the levels of Elf5 mRNA were lower in Stat5a/5bfl/fl;Cre compared with Stat5a/5bfl/fl, and that Elf5 mRNA was induced to a greater extent by prolactin in mammary tissue of Stat5a/5bfl/fl mice as compared with Stat5a/5bfl/fl;Cre mice (Supplemental Fig. 4), indicating cell- and stage-specific cross-regulation of their expression in the course of mammary development. In addition, we found 48 sites of TTCNNNGAA, the consensus STAT5-binding sequence, in the distal region of the Elf5 gene promoter. The closest one to the transcription start site is TTCAGTGAA at 3727 base pairs (bp) upstream. Among the 48 sites, three (at −111,201 and −96,996, at −65,696 and −62,683, and at −51,935 and −51,189 in mice and humans, respectively) plus their flanking sequences are well conserved between humans and mice. This indicates that prolactin-induced Elf5 expression is regulated by STAT5A/5B-dependent as well as STAT5-independent mechanisms. Together, induction of Elf5 by prolactin is partially regulated by STAT5A/5B for the proliferation and differentiation of mammary alveolar cells during pregnancy. However, these data are obtained from whole mammary tissue samples rather than enriched cell populations. We found that Stat5a/5b-null luminal progenitor cells did not express Elf5 (Supplemental Fig. 3). The presence of alveolar luminal progenitor cells in Elf5-null mammary epithelium in virgins puts STAT5A/5B above ELF5 in the gene hierarchy (Oakes et al. 2008). It further suggests that the absence of Elf5 expression in the Stat5a/5b-null CD61+ progenitor cells reflects the paucity of alveolar progenitor cells in the Stat5a/5b-null mammary epithelium.
The reversible generation of luminal progenitor cells and the rescue of alveologenesis by the transgenic expression of STAT5A prove that STAT5A is able to induce the development of the alveolar progenitor lineage in Stat5a/5b-null mammary stem cells during pregnancy, even if STAT5A/5B were not present during the ductal development period during puberty. Indeed, mammary alveologenesis was impaired by the germline deletion of Stat5a gene but not Stat5b (Liu et al. 1997; Udy et al. 1997; Teglund et al. 1998). Taken together with our previous findings that STAT5A/5B are critical for the survival and function of alveolar cells during pregnancy (Cui et al. 2004), this supports the notion that STAT5A is required not only for the proliferation or survival of alveolar cells, but also the generation of alveolar progenitors from stem cells. This study provides evidence for a mechanism by which the normal mammary epithelial cell hierarchy is established and maintained through the transcription factor STAT5A.
Materials and methods
Mouse and genotype analysis
All animals were handled and housed in accordance with the guidelines of the National Institutes of Health Animal Care and Use Committee. Generation of MMTV-Cre and MMTV-rtTA transgenic mice (kind gift from Dr. L. Chodosh) and Stat5a/bfl/fl mice was described previously (Wagner et al. 1997; Gunther et al. 2002; Cui et al. 2004). We mated Stat5a/bfl/fl mice with the MMTV-Cre transgenic mouse line A in order to generate Stat5a/bfl/fl;Cre mice. The presence of Cre recombinase itself did not affect the number of luminal progenitor cells (Supplemental Fig. 5).
Generation of iStat5 (MMTV-rtTA;tet-op-Stat5a) transgenic mice
Tetracycline-inducible STAT5A transgenic mice were generated by introducing a SacII site into Stat5a cDNA at the 5′ ATG start codon and a XbaI site at the 3′ TGA stop codon by PCR, using MSCV-IRES-Stat5a as a template and primer set 5′-ATACCGCGGATGGCGGGCTGGATTCA-3′ (forward) and 5′-ATATCTAGATCAGGACAGGGAGCTT-3′ (reverse). The purified SacII and XbaI fragment was subcloned into plasmid p43 (kindly provided by Dr. Priscilla A Furth), which is composed of the tet operator sequences followed by a CMV minimal promoter and the rabbit β-globin intron and poly(A) signal at the 3′ end. The NotI-released DNA fragments were injected into fertilized C57BL/6 eggs (The Jackson Laboratory) to generate transgenic mice according to standard procedures. For PCR analysis of the founders, a primer set 5′-CTGAGTTCGTCAATGCATCCA-3′ (forward) and 5′-GGTGATACAAGGGACATCTT-3′ (reverse) was designed to detect the unique area of the tet-op-Stat5a. The PCR protocol was 35 cycles of 30 sec at 94°C, 45 sec at 57°C, and 1 min at 72°C. An amplified product of 411 bp from tet-op-Stat5a was detected.
Preparation of single cells from mammary gland
Single cells from mammary tissue were prepared as described previously (Stingl et al. 2006) with minor modifications. Briefly, mammary tissue from virgin female Stat5a/bfl/fl mice and Stat5a/bfl/fl;Cre mice at 12–16 wk of age were digested for 8 h at 37°C in complete EpiCult-B medium (EpiCult-B medium with 5% fetal bovine serum, 10 ng/mL recombinant human epidermal growth factor, 10 ng/mL recombinant human basic fibroblast growth factor, 0.0004% Heparin) supplemented with 300 U/mL collagenase and 100 U/mL hyaluronidase. After lysis of red blood cells in NH4Cl, a single-cell suspension was obtained by sequential dissociation of the fragments with prewarmed 0.25% trypsin-EDTA for 1–2 min, followed by prewarmed 5 mg/mL dispase II plus 0.1 mg/mL DNase I (DNase; Sigma) for 2 min, and filtration through a 70-μm mesh. All reagents were from StemCell Technologies, Inc. unless otherwise specified.
FACS analysis and cell sorting
Prepared single cells were stained with biotinylated anti-CD45, anti-Ter119, and anti-CD31 antibody (StemCell Technologies); anti-CD24-fluorescein isothiocyanate (FITC, clone M1/69, BD Biosciences); anti-CD49f-R-phycoerythrin (R-PE, clone GoH3, BD Biosciences); anti-CD61-Alexafluor647 (clone 2C9.G2, BioLegend); and 7-amino-actinomycin D (7AAD, BD Biosciences), followed by pacific blue-conjugated streptavidin (Invitrogen). FACS analysis and cell sorting were performed using Diva version 6.1.1 software and FACSAria (BD Biosciences).
Histological analysis
Harvested mammary tissues were fixed in 10% formalin, dehydrated through ethanol and xylene, embedded in paraffin, and sectioned. For immunostaining, antigen unmasking was performed in a Decloaking chamber (Biocare Medical) using BORG Decloaker Solution (pH 9.5) (Biocare Medical) for 5 min at 125°C, 18–24 PSI. The sections were blocked for 30 min in TBS-T containing 3% goat serum. Primary antibodies were incubated overnight at 4°C (phosphorylated Stat5, Cell Signaling Technologies, #9314, 1:200; E-cadherin, BD Biosciences #610182, 1:200). AlexaFluor488- or AlexaFluor594-conjugated secondary antibodies (Invitrogen) were used at a dilution of 1:400 for 30 min at room temperature.
Acknowledgments
We are grateful to Dr. Lewis Chodosh for MMTV-rtTA transgenic mice, Dr. Priscilla Furth for plasmid p43, and all the members of the Laboratory of Genetics and Physiology for helpful discussions. This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digesitve and Kidney Diseases, NIH.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1840109.
Supplemental material is available at http://www.genesdev.org.
References
- Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC, Hartley L, Robb L, Grosveld FG, van der Wees J, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- Buono KD, Robinson GW, Martin C, Shi S, Stanley P, Tanigaki K, Honjo T, Hennighausen L. The canonical Notch/RBP-J signaling pathway controls the balance of cell lineages in mammary epithelium during pregnancy. Dev Biol. 2006;293:565–580. doi: 10.1016/j.ydbio.2006.02.043. [DOI] [PubMed] [Google Scholar]
- Choi YS, Chakrabarti R, Escamilla-Hernandez R, Sinha S. Elf5 conditional knockout mice reveal its role as a master regulator in mammary alveolar development: Failure of Stat5 activation and functional differentiation in the absence of Elf5. Dev Biol. 2009;329:227–241. doi: 10.1016/j.ydbio.2009.02.032. [DOI] [PubMed] [Google Scholar]
- Cui Y, Riedlinger G, Miyoshi K, Tang W, Li C, Deng CX, Robinson GW, Hennighausen L. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol. 2004;24:8037–8047. doi: 10.1128/MCB.24.18.8037-8047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther EJ, Belka GK, Wertheim GB, Wang J, Hartman JL, Boxer RB, Chodosh LA. A novel doxycycline-inducible system for the transgenic analysis of mammary gland biology. FASEB J. 2002;16:283–292. doi: 10.1096/fj.01-0551com. [DOI] [PubMed] [Google Scholar]
- Harris J, Stanford PM, Sutherland K, Oakes SR, Naylor MJ, Robertson FG, Blazek KD, Kazlauskas M, Hilton HN, Wittlin S, et al. Socs2 and elf5 mediate prolactin-induced mammary gland development. Mol Endocrinol. 2006;20:1177–1187. doi: 10.1210/me.2005-0473. [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev Mol Cell Biol. 2005;6:715–725. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Interpretation of cytokine signaling through the transcription factors STAT5A and STAT5B. Genes & Dev. 2008;22:711–721. doi: 10.1101/gad.1643908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelbl A, Kovacic B, Kerenyi MA, Simma O, Warsch W, Cui Y, Beug H, Hennighausen L, Moriggl R, Sexl V. Clarifying the role of Stat5 in lymphoid development and Abelson-induced transformation. Blood. 2006;107:4898–4906. doi: 10.1182/blood-2005-09-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouros-Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell. 2006;127:1041–1055. doi: 10.1016/j.cell.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Wang Z, Zhang Y, Kang Z, Haviernikova E, Cui Y, Hennighausen L, Moriggl R, Wang D, Tse W, et al. STAT5 requires the N-domain to maintain hematopoietic stem cell repopulating function and appropriate lymphoid–myeloid lineage output. Exp Hematol. 2007;35:1684–1694. doi: 10.1016/j.exphem.2007.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Robinson GW, Wagner KU, Garrett L, Wynshaw-Boris A, Hennighausen L. Stat5a is mandatory for adult mammary gland development and lactogenesis. Genes & Dev. 1997;11:179–186. doi: 10.1101/gad.11.2.179. [DOI] [PubMed] [Google Scholar]
- Liu X, Gallego MI, Smith GH, Robinson GW, Hennighausen L. Functional rescue of Stat5a-null mammary tissue through the activation of compensating signals including Stat5b. Cell Growth Differ. 1998;9:795–803. [PubMed] [Google Scholar]
- Liu F, Kunter G, Krem MM, Eades WC, Cain JA, Tomasson MH, Hennighausen L, Link DC. Csf3r mutations in mice confer a strong clonal HSC advantage via activation of Stat5. J Clin Invest. 2008;118:946–955. doi: 10.1172/JCI32704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K, Shillingford JM, Smith GH, Grimm SL, Wagner KU, Oka T, Rosen JM, Robinson GW, Hennighausen L. Signal transducer and activator of transcription (Stat) 5 controls the proliferation and differentiation of mammary alveolar epithelium. J Cell Biol. 2001;155:531–542. doi: 10.1083/jcb.200107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes SR, Naylor MJ, Asselin-Labat ML, Blazek KD, Gardiner-Garden M, Hilton HN, Kazlauskas M, Pritchard MA, Chodosh LA, Pfeffer PL, et al. The Ets transcription factor Elf5 specifies mammary alveolar cell fate. Genes & Dev. 2008;22:581–586. doi: 10.1101/gad.1614608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renou JP, Bierie B, Miyoshi K, Cui Y, Djiane J, Reichenstein M, Shani M, Hennighausen L. Identification of genes differentially expressed in mouse mammary epithelium transformed by an activated β-catenin. Oncogene. 2003;22:4594–4610. doi: 10.1038/sj.onc.1206596. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Shillingford JM, Miyoshi K, Flagella M, Shull GE, Hennighausen L. Mouse mammary epithelial cells express the Na-K-Cl cotransporter, NKCC1: Characterization, localization, and involvement in ductal development and morphogenesis. Mol Endocrinol. 2002;16:1309–1321. doi: 10.1210/mend.16.6.0857. [DOI] [PubMed] [Google Scholar]
- Stingl J. Detection and analysis of mammary gland stem cells. J Pathol. 2009;217:229–241. doi: 10.1002/path.2457. [DOI] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci. 1997;94:7239–7244. doi: 10.1073/pnas.94.14.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, Li M, Furth PA, Hennighausen L. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 1997;25:4323–4330. doi: 10.1093/nar/25.21.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KU, McAllister K, Ward T, Davis B, Wiseman R, Hennighausen L. Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Res. 2001;10:545–553. doi: 10.1023/a:1013063514007. [DOI] [PubMed] [Google Scholar]
- Wang Z, Li G, Tse W, Bunting KD. Conditional deletion of STAT5 in adult mouse hematopoietic stem cells causes loss of quiescence and permits efficient nonablative stem cell replacement. Blood. 2009;113:4856–4865. doi: 10.1182/blood-2008-09-181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, Cui Y, Watford WT, Bream JH, Yamaoka K, Hissong BD, Li D, Durum SK, Jiang Q, Bhandoola A, et al. Stat5a/b are essential for normal lymphoid development and differentiation. Proc Natl Acad Sci. 2006;103:1000–1005. doi: 10.1073/pnas.0507350103. [DOI] [PMC free article] [PubMed] [Google Scholar]