Abstract
We examined in HepG2 cells whether glucose-induced changes in AMP-activated protein kinase (AMPK) activity could be mediated by SIRT1, an NAD-dependent histone/protein deacetylase that has been linked to the increase in longevity caused by caloric restriction. Incubation with 25 vs. 5 mM glucose for 6 h concurrently diminished the phosphorylation of AMPK (Thr 172) and ACC (Ser 79), increased lactate release, and decreased the abundance and activity of SIRT1. In contrast, incubation with pyruvate (0.1 and 1 mM) for 2 h increased AMPK phosphorylation and SIRT1 abundance and activity. The putative SIRT1 activators resveratrol and quercetin also increased AMPK phosphorylation. None of the tested compounds (low or high glucose, pyruvate, and resveratrol) significantly altered the AMP/ATP ratio. Collectively, these findings raise the possibility that glucose-induced changes in AMPK are linked to alterations in SIRT1 abundance and activity and possibly cellular redox state.
Keywords: AMPK, redox state, SIRT1, resveratrol
Introduction
AMP-activated protein kinase (AMPK) belongs to a family of highly conserved serine kinases that are regulated by nutritional and metabolic stresses that alter cellular energy state [1–3]. When activated, AMPK protects the cell against ATP depletion by stimulating processes such as fatty acid oxidation that promote ATP generation and inhibiting others, such as protein and lipid synthesis, that require ATP but are not acutely necessary for survival [1, 4]. Although the activation of AMPK appears to be a direct consequence of an increase in the AMP to ATP ratio in many situations, studies in various tissues have shown that AMPK can be activated or inhibited by mechanisms that may not involve changes in adenine nucleotide levels [5, 6]. In one such study, Itani et al. [7] demonstrated that the incubation of rat extensor digitorum longus (EDL) muscle with a high glucose medium (25 vs. 6 or 0 mM) for 4 hours diminished AMPK activity without changing the whole-tissue concentrations of creatine phosphate or adenine nucleotides. The reduced AMPK activity correlated with an increased release of lactate by the EDL, raising the possibility that alterations in its redox state contributed to these changes.
Sirtuins are a family of redox-sensitive, NAD+-dependent deacetylases that regulate gene expression by controlling the acetylation status of lysine residues on histones, transcription factors, and transcriptional coactivators [8]. Sir2 and its mammalian homolog SIRT1 are induced in response to nutrient deprivation and are thought to mediate the effects of caloric restriction on longevity [9, 10]. Rodgers et al. [11] found an increase in SIRT1 abundance and activity in the livers of 24-hour fasted mice, demonstrating increased deacetylation and activation of PGC-1α (PPAR-gamma coactivator 1-α). Conversely, refeeding following a 24-hour fast decreased hepatic SIRT1 abundance, as did incubation of cultured Fao rat hepatocytes in a high glucose medium (10 vs. 0 mM). We have previously shown that refeeding following a 48-hour fast similarly reduces AMPK activity in rat liver [12], as does incubation of HepG2 cells with 25 vs. 5 mM glucose [3]. These findings suggest a link between SIRT1 and AMPK.
In the present study, the linkage between SIRT1 and AMPK was examined more directly. We determined in HepG2 cells whether 1) glucose- and pyruvate-induced changes in AMPK activity (phosphorylation) are associated with alterations in SIRT1 abundance and activity, 2) SIRT1 activation and inhibition by pharmacological agents produce parallel changes in AMPK, and 3) observed alterations in AMPK activity occur in the presence or absence of changes in cellular energy state. The results suggest concurrent regulation of SIRT1 and AMPK in the absence of a change in whole cell energy state.
Materials and Methods
Resveratrol was from Calbiochem (San Diego, CA). Pyruvate and nicotinamide were from Sigma (St. Louis, MO). Fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM), and PBS were from Gibco (Grand Island, NY). Rabbit monoclonal anti-phospho-Thr 172 AMPK and rabbit polyclonal anti-AMPKa subunit antibodies were from Cell Signaling. Anti-phospho-Ser 79 ACC1 was from Upstate Biotechnology (Lake Placid, NY). Rabbit polyclonal anti-SIRT1 (H-300) and HRP-conjugated secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell culture and treatments
HepG2 cells were cultured in DMEM containing 5 mM glucose supplemented with 10% FBS, 1% penicillin/streptomycin and subjected to assays after overnight serum and pyruvate depletion. C2C12 myocytes were cultured to 80% confluence in 5mM glucose DMEM containing 1% GlutaMAX, 1% penicillin/streptomycin and 10% FBS, then differentiated to 80% myotubes in 5 mM glucose DMEM containing 2% Horse Serum, 1% GlutaMAX, and 1% penicillin/streptomycin.
Immunoblotting analysis
Samples (50 ug protein, as determined by Bio-Rad assay) were separated by SDS-PAGE, transferred to PVDF membrane, blocked with 5% milk in TBST, and incubated with primary antibody overnight at 4°C. Bound antibodies were detected with HRP-linked secondary antibodies and visualized using enhanced chemiluminescence (Thermo Fisher) and autoradiography.
PGC-1α acetylation assay
PGC-1α lysine acetylation was determined in HepG2 cells transfected with an HA-tagged PGC-1α adenovirus, as described previously [13].
Other analyses
ATP, AMP, and ADP were measured spectrophotometrically as described previously [14]. Lactate release was determined spectrophotometrically using lactate dehydrogenase and NAD [15].
Experimental Animals
Male Sprague-Dawley rats (Charles River Breeding Laboratories, Wilmington, MA) were maintained on a 12-h light cycle and fed ad libitum. Experiments were performed following an 18–20 h fast. All research on these animals was reviewed and performed in accordance with the requirements of the IACUC at Boston University Medical Center.
Muscle incubation
Rats were anesthetized with sodium pentobarbital (6mg/100g body weight i.p.). EDL muscle were isolated as described previously [14]. Muscles were initially equilibrated in Krebs-Henseleit solution containing 5 mM glucose for 20 minutes, followed by incubation ± resveratrol in DMSO for 2 h.
Statistics
Results are expressed as means ± SE. Student’s t-test or ANOVA followed by Student-Newman-Keuls post hoc analysis were used to determine significance (p<.05).
Results
Effect of incubation with different glucose concentrations on the phosphorylation of AMPK and ACC, and SIRT1 protein abundance and activity
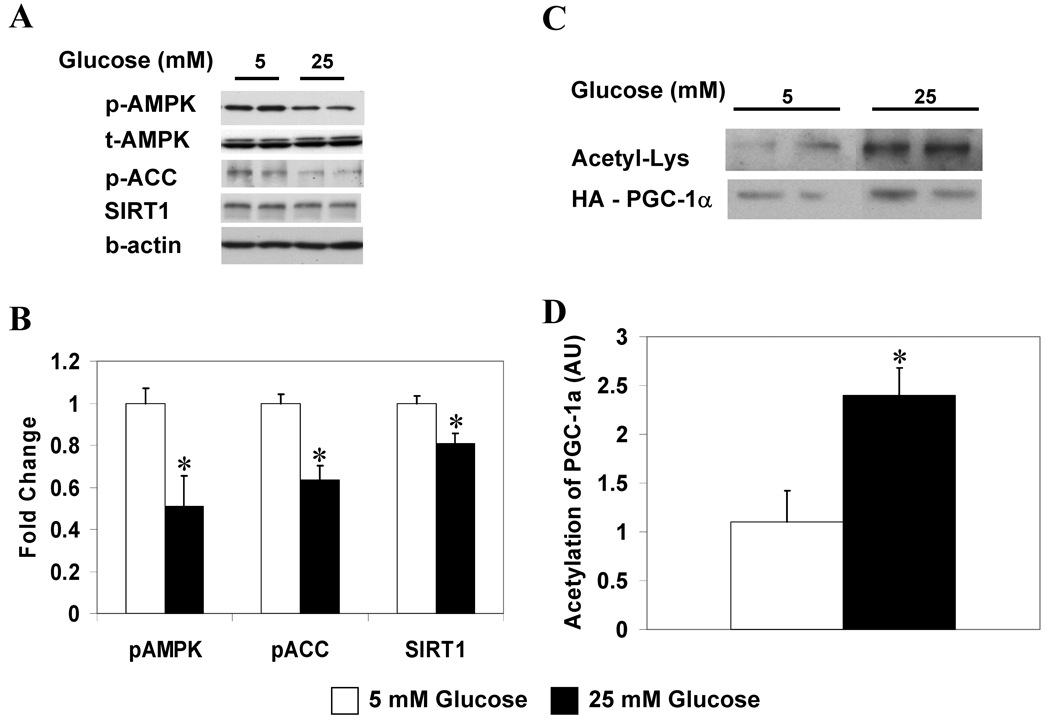
Using a high glucose-induced model of insulin resistance in HepG2 cells [3], we first confirmed the finding of Zang et al. [3] that incubation of HepG2 cells in a high glucose medium (25 vs. 5 mM) for 6 hours decreases the phosphorylation of AMPK and its downstream target acetyl-CoA carboxylase (ACC) without altering AMPK abundance (Fig 1A, B). We found that these alterations were not paralleled by changes in cellular energy state, as reflected by the AMP/ATP ratio (Table 1). On the other hand, they were associated with a decrease in SIRT1 protein abundance and a more than 2-fold increase in PGC-1α acetylation (Fig. 1A–D), suggesting that hyperglycemia decreased SIRT1 activity. In addition, the release of lactate was increased by 23% in the cells incubated with 25 vs. 5 mM glucose for 6 hours (n=3; p<0.01).
Figure 1. Increasing the ambient glucose concentration decreases the phosphorylation of AMPK and ACC, and the abundance and activity of SIRT1 in HepG2 cells.
HepG2 cells incubated in 5 or 25 mM glucose for 6 h. (A) Western blot analysis for p-AMPK (Thr 172), p-ACC (Ser 79), and SIRT1. (B) Quantification of representative blot shown in A, results are means ± SE (n=5); *,p<0.05. (C) PGC-1α acetylation after 6 h incubation in 5 or 25 mM glucose. (D) Quantification of Acetyl-Lys/HA-PGC-1α. Results are means ± SE, (n = 4); *, p<0.05.
Table 1.
The effect of variations in glucose, pyruvate, and resveratrol concentration on cellular energy state in HepG2 cells
| ATP | ADP | AMP | AMP/ATP | |
|---|---|---|---|---|
| 6 h incubation, nmol/mg protein | ||||
| 5 mM glucose | 9.4 ± 1.4 | 2.2 ± 0.2 | 0.39 ± 0.06 | 0.042 ± 0.009 |
| 25 mM glucose | 9.7 ± 0.8 | 3.10 ± 0.1 | 0.31 ± 0.03 | 0.035 ± 0.003 |
| 2 h incubation, nmol/mg protein | ||||
| 0 mM pyruvate | 11.9 ± 0.5 | 3.4 ± 0.2 | 0.37 ± 0.02 | 0.031 ± 0.001 |
| 1 mM pyruvate | 12.6 ± 0.2 | 3.6 ± 0.1 | 0.34 ± 0.02 | 0.027 ± 0.002 |
| 1 h incubation, nmol/mg protein | ||||
| 0 uM resveratrol | 11.3 ± 0.8 | 2.4 ± 0.2 | 0.28 ± 0.03 | 0.025 ± 0.002 |
| 50 uM resveratrol | 11.7 ± 1.1 | 2.6 ± 0.2 | 0.36 ± 0.06 | 0.033 ± 0.009 |
| 100 uM resveratrol | 11.9 ± 1.1 | 2.2 ± 0.2 | 0.39 ± 0.07 | 0.032 ± 0.005 |
Unless otherwise noted, incubations were carried out in 5 mM glucose DMEM. Adenine nucleotide levels were measured spectrophotometrically. Values are means ± SEM, n = 9 (n = 3/group on 3 independent experiments)
The effects of pyruvate on AMPK and ACC phosphorylation, and SIRT1 protein and activity
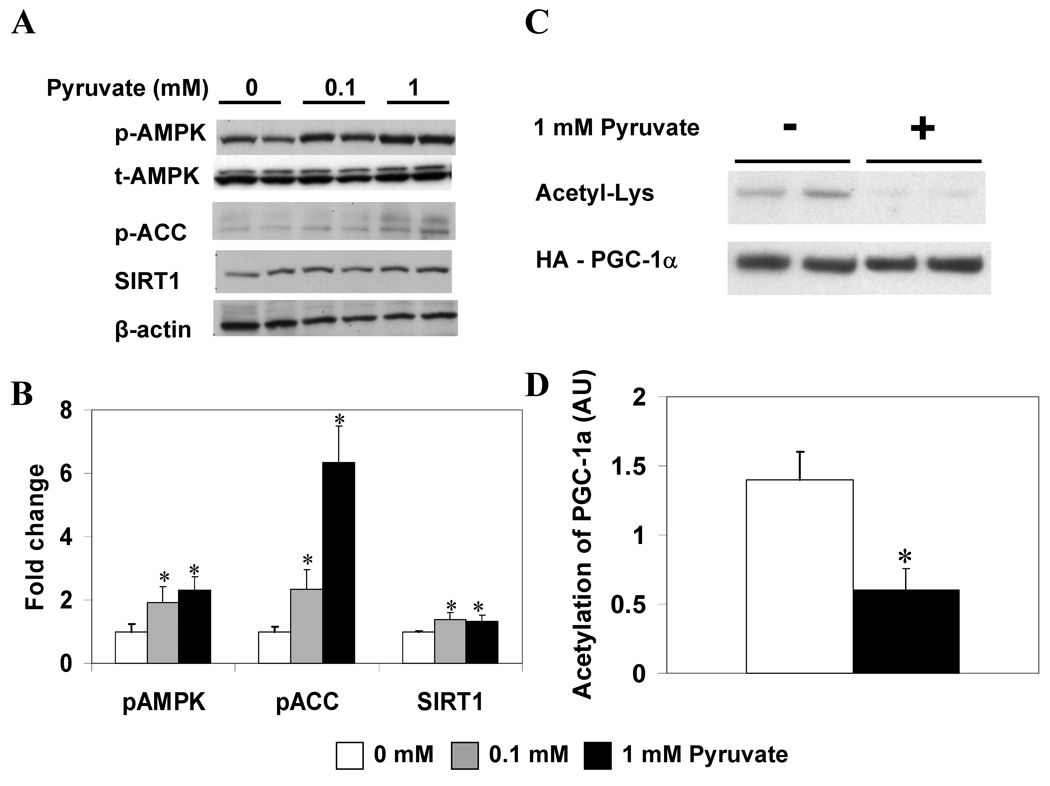
The effects of incubating HepG2 cells in a 5 mM glucose medium with increasing concentrations of pyruvate are shown in Figure 2. The addition of 0.1 or 1 mM pyruvate markedly enhanced the phosphorylation of AMPK, and even more so that of ACC at 2 hours (Fig. 2A, B). In cells incubated with 1 vs. 0 mM pyruvate, these changes were associated with a 30% increase in SIRT1 protein abundance (Fig. 2B) and a 60% decrease in the acetylation of PGC-1α (Fig. 2C, D). Incubation with 1 mM pyruvate did not increase the AMP/ATP ratio (Table 1), suggesting that the increase in AMPK activity occurred without an alteration of cellular energy state.
Figure 2. Increasing pyruvate concentration increases p-AMPK, p-ACC, and SIRT1 abundance and activity.
(A) Western blot analysis of HepG2 cells following 2 h incubation with the indicated concentrations of pyruvate. (B) Quantification of p-AMPK, p-ACC, and SIRT1 relative to β-actin. Results are means ± SE (n = 6); *, p<0.05 vs. 0 mM. (C) PGC-1α acetylation after 2 h incubation ± 1 mM pyruvate. (D) Quantification of Acetyl-Lys/HA-PGC-1α. Results are means ± SE, (n = 4); *, p<0.05.
Nicotinamide suppresses SIRT1 activity and phosphorylation of AMPK and ACC
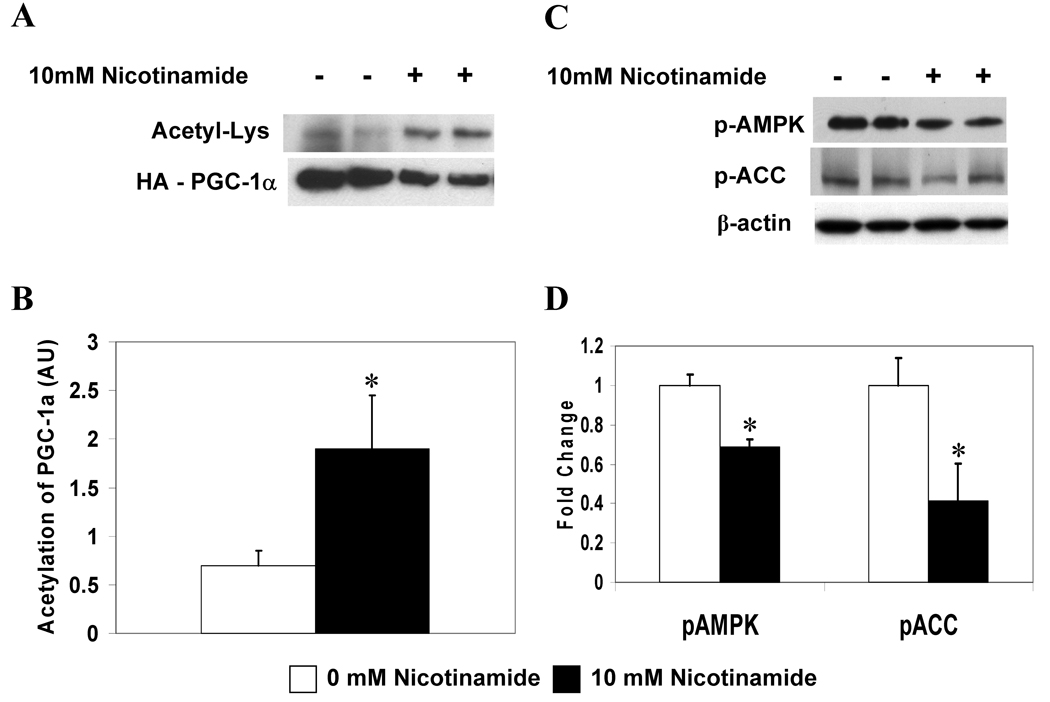
Nicotinamide and the reduced dinucleotide, NADH, are inhibitors of sirtuins [16], although their biological significance is incompletely understood. Rodgers et al. [11] have shown that nicotinamide induces PGC-1α acetylation in 293T cells, an effect that is overcome by SIRT1 overexpression. Here we demonstrate that HepG2 cells exposed to 10 mM nicotinamide for 6 hours show enhanced acetylation of PGC-1α (Fig. 4A, B) as well as diminished phosphorylation of AMPK and ACC (Fig. 4C, D). Thus, nicotinamide concurrently modulates the activities of SIRT1 and AMPK.
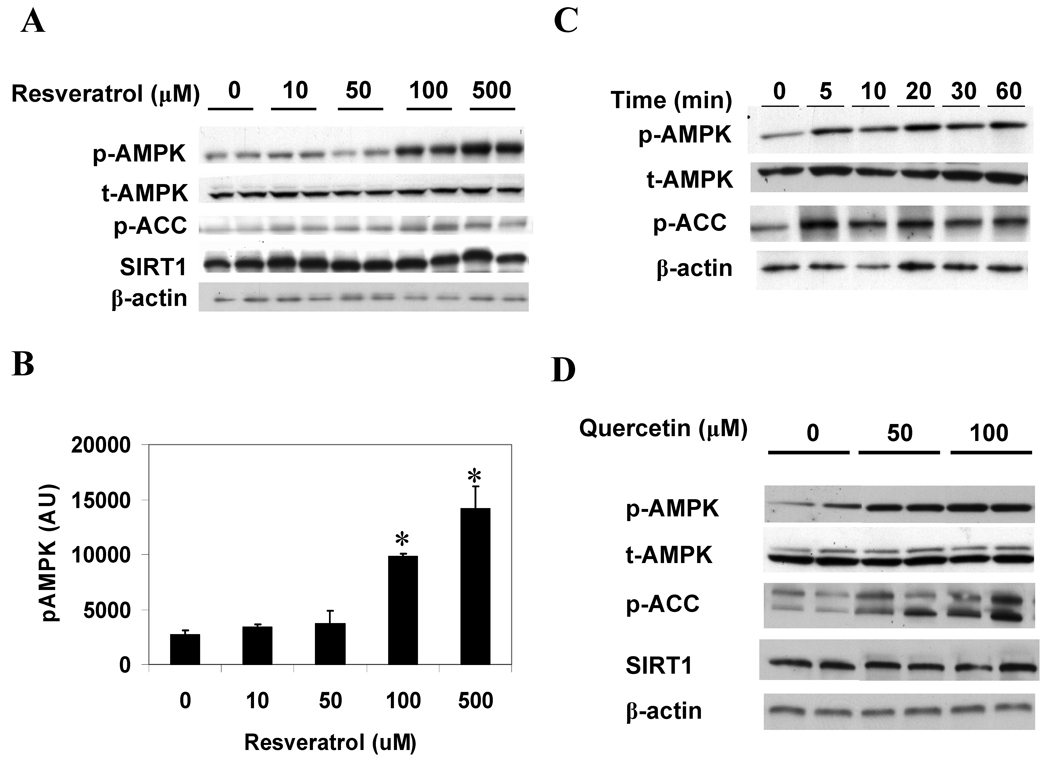
Figure 4. Resveratrol and quercetin increase the phosphorylation of AMPK and ACC in HepG2 cells.
(A, B) Dose response of p-AMPK and p-ACC to 1 h resveratrol incubation. Results are means ± SE (n = 2); *,p<0.05 vs. 0 µM resveratrol. (C) Time course of changes in p-AMPK and p-ACC caused by 100 µM resveratrol. (D) Western blot analysis showing enhanced p-AMPK and p-ACC following 1 h incubation with increasing concentrations of quercetin.
SIRT1 activators enhance the phosphorylation of AMPK in HepG2 and C2C12 cells, and in incubated rat EDL muscle
Resveratrol, a polyphenol found in foods such as grapes, red wine, and peanuts, has received increasing attention as a putative SIRT1 activator that can mimic the effects of caloric restriction on the aging process [17], the development of obesity and insulin resistance in fat-fed rodents [13, 18], and possibly the prevalence of atherosclerotic cardiovascular disease in humans [19]. Fig. 4A and B demonstrate that resveratrol increases the phosphorylation of AMPK in a dose dependent manner in HepG2 cells. The dose response of ACC phosphorylation was less apparent, although at all concentrations resveratrol significantly increased p-ACC (p<0.05). A time course experiment demonstrated that at a concentration of 100 µM, a marked increase in p-AMPK and p-ACC was evident within 5 minutes (Fig. 4C). No alteration in SIRT1 abundance was observed (Fig. 4A), in keeping with evidence suggesting that resveratrol binds to and activates SIRT1 by increasing its protein substrate affinity [20]. Similar effects of resveratrol were observed in C2C12 cells and incubated rat extensor digitorum longus (EDL) muscle, in which incubation with 50 and 100 µM resveratrol enhanced p-AMPK and p-ACC abundance (data not shown). Resveratrol had no effect on the cellular energy state of HepG2 cells after incubations of 1 hour (Table 1) or 15 min (data not shown). Quercetin, a putative SIRT1-activating flavonoid, also enhanced the phosphorylation of AMPK and ACC in a dose-dependent manner in HepG2 cells (Fig. 4D). As with resveratrol, no change in the abundance of SIRT1 was observed.
Discussion
The results indicate that incubation in a high glucose medium (25 vs. 5 mM) diminishes the activity of AMPK in HepG2 cells, whereas incubation with pyruvate has the opposite effect. The observed changes in AMPK activity were not associated with alterations in cellular AMP/ATP ratios, but were linked to concomitant changes in SIRT1 abundance and activity. In line with this, we found that AMPK phosphorylation is diminished during incubation with nicotinamide, a SIRT1 inhibitor, whereas resveratrol, a putative SIRT1 activator [17], enhanced the phosphorylation of AMPK without altering cellular energy state. Similar findings were obtained with another putative SIRT1 activator, quercetin [17], although energy state was not determined. The effects of resveratrol on p-AMPK were also observed in C2C12 myotubes and incubated rat EDL muscle, indicating that they are not tissue- or cell-specific. Overall these results provide strong correlative evidence for a linkage between SIRT1 and AMPK.
The observation that increasing the ambient glucose concentration decreases SIRT1 abundance is in agreement with the findings of Rodgers et al. [11] in Fao rat hepatocytes. Collectively, these findings raise the question of how changes in the ambient glucose concentration influence SIRT1. The sirtuins depend on the availability of NAD+ as both an activator and a substrate for deacetylase reactions. Hence, changes in the NAD+/NADH ratio within a cell, i.e. the cellular redox state, may influence SIRT1 activity. Results from the present study show that incubation in a high glucose medium increases the amount of lactate released by HepG2 cells. If as assumed this reflects changes in the cytoplasmic and nuclear free NAD+/NADH ratio [21], it raises the possibility that glucose-induced alterations in cellular redox state could influence the activity of SIRT1 and secondarily AMPK. This possibility is in line with the observation that in the fasted state, where both SIRT1 and AMPK are activated, hepatic pyruvate and NAD+ content are increased whereas lactate content is decreased [11]. Furthermore, incubation with pyruvate, which acutely would increase the NAD+/NADH ratio, has been shown to increase SIRT1 abundance in Fao cells [11], and in the present study it increased SIRT1 and AMPK activity in HepG2 cells. Furthermore, we found that incubation with quercetin, a SIRT1-activating compound that has been shown to increase the acetoacetate to β-hydroxybutyrate ratio [22] (an indicator of the mitochondrial NAD+/NADH ratio), also increased AMPK activity. Collectively these data suggest a correlation between redox state and the activities of SIRT1 and AMPK; however, direct measures of cellular redox state, such as the lactate/pyruvate ratio and cellular NAD and NADH, are needed to establish this more definitively. Still unanswered is the basis for the changes in SIRT1 abundance observed in this and other studies[11]. In this context, Zhang et al.[23] have proposed that the redox-sensitive transcriptional corepressor, CtBP, may regulate SIRT1 transcription.
The notion that changes in SIRT1 may regulate AMPK activity is also supported by other recent studies. Overexpression of SIRT1 has been found to increase the phosphorylation of AMPK and ACC both in vivo and in vitro [24], by a reaction dependent on the AMPK kinase LKB1 [24–26]. More specifically, we found that SIRT1 deacetylates lysine residue 48 of LKB1, and that this is associated with LKB1 movement from the nucleus to the cytoplasm, where it binds to STRAD (STE-related adapter) and MO25 (mouse protein 25) and is activated [26]. Likewise, Hou et al. [24] have found that pharmacologic and genetic inhibition of SIRT1 in HepG2 cells attenuates the increase in AMPK phosphorylation caused by polyphenol exposure, and it has been demonstrated that refeeding after a fast decreases SIRT1 [11], LKB1 and AMPK activity, and increases LKB1 acetylation in rodent liver [26].
The data presented in this report, together with several other recent studies [11, 24, 26], are consistent with a model in which alterations in the ambient glucose concentration influence SIRT1 and secondarily AMPK activity. In contrast, an alternative model to explain the effects of glucose concentration on SIRT1 has recently been proposed by Fulco et al. [27]. In brief they reported that glucose deprivation (5 vs. 25 mM) inhibited the differentiation of C2C12 myoblasts by leading to a decrease in cellular ATP and activation of AMPK, which in turn caused the activation of SIRT1. They also demonstrated that SIRT1 activation was enhanced by the AMPK activator AICAR, and that both glucose deprivation and AICAR induced transcription of the NAD+ biosynthetic enzyme Nampt (nicotinamide phosphoribosyltransferase), resulting in a decrease in the concentration of the SIRT1 inhibitor nicotinamide, an increase in the NAD+/NADH ratio, and activation of SIRT1. These findings suggest that the upstream event in glucose regulation of AMPK is a change in energy state, a finding also observed in pancreatic β-cells [28]. In contrast, in the present study as well as in earlier studies in incubated rat EDL muscle (30 min - 2 h) [7] and human umbilical vein endothelial cells (2 h) [29], glucose deprivation led to AMPK activation in the apparent absence of a decrease in cellular energy state, although the possibility that a transient alteration in energy state occurred at earlier time points was not excluded. Thus, whether glucose regulates SIRT1 by effects on cellular energy or redox state or by both mechanisms remains to be determined. If both mechanisms operate, it would suggest the existence of a SIRT1/AMPK cycle that links cellular redox and energy states.
In summary, the results presented here support a strong association between SIRT1 and AMPK in mammalian cells, as demonstrated by concurrent regulation of both molecules in response to varying concentrations of glucose and pyruvate. Additionally, exposure to SIRT1 activators and inhibitors resulted in AMPK activation and inhibition, respectively. The absence of significant changes in energy state (where measured) suggest that modulation of SIRT1 by redox changes could mediate the effects of glucose and pyruvate on its activity and abundance; however, additional studies are needed to establish this with greater certainty.
Figure 3. Nicotinamide increases PGC-1α acetylation and decreases the phosphorylation of AMPK and ACC.
(A) PGC-1α acetylation in HepG2 cells incubated ± 10 mM nicotinamide (NAM) for 6 h. (B) Quantification of Acetyl-Lys/HA-PGC-1α. Results are means ± SE, (n = 4); *, p<0.05. (C) Western blot analysis of p-AMPK and p-ACC. (D) Quantification of representative blot shown in C. Results are means ± SE, (n = 4); *, p<0.05.
Acknowledgments
This work was supported by NIH grants R01 DK19514, P01 HL08758, and DK67509 (N.R.). G.S. was the recipient of a mentor based grant from the ADA (N.R.). L.N. was supported by NIH training grant T32DK07201-31 and a fellowship award F30DK082136 from NIDDK. The authors would like to thank Jose Cacicedo and Fan Lan for helpful comments and technical advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–120. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- 2.Kahn B, Alquier T, Carling D, Hardie G. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metabolism. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Zang M, Zuccollo A, Hou X, Nagata D, Walsh K, Herscovitz H, Brecher P, Ruderman NB, Cohen RA. AMP-activated protein kinase is required for the lipid-lowering effect of metformin in insulin-resistant human HepG2 cells. J Biol Chem. 2004;279:47898–47905. doi: 10.1074/jbc.M408149200. [DOI] [PubMed] [Google Scholar]
- 4.Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, van Denderen B, Jennings IG, Iseli T, Michell BJ, Witters LA. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans. 2003;31:162–168. doi: 10.1042/bst0310162. [DOI] [PubMed] [Google Scholar]
- 5.Beauloye C, Marsin AS, Bertrand L, Krause U, Hardie DG, Vanoverschelde JL, Hue L. Insulin antagonizes AMP-activated protein kinase activation by ischemia or anoxia in rat hearts, without affecting total adenine nucleotides. FEBS Lett. 2001;505:348–352. doi: 10.1016/s0014-5793(01)02788-0. [DOI] [PubMed] [Google Scholar]
- 6.Frederich M, Balschi JA. The relationship between AMP-activated protein kinase activity and AMP concentration in the isolated perfused rat heart. J Biol Chem. 2002;277:1928–1932. doi: 10.1074/jbc.M107128200. [DOI] [PubMed] [Google Scholar]
- 7.Itani SI, Saha AK, Kurowski TG, Coffin HR, Tornheim K, Ruderman NB. Glucose autoregulates its uptake in skeletal muscle: involvement of AMP-activated protein kinase. Diabetes. 2003;52:1635–1640. doi: 10.2337/diabetes.52.7.1635. [DOI] [PubMed] [Google Scholar]
- 8.Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 10.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 11.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 12.Assifi MM, Suchankova G, Constant S, Prentki M, Saha AK, Ruderman NB. AMP-activated protein kinase and coordination of hepatic fatty acid metabolism of starved/carbohydrate-refed rats. Am J Physiol Endocrinol Metab. 2005;289:E794–E800. doi: 10.1152/ajpendo.00144.2005. [DOI] [PubMed] [Google Scholar]
- 13.Lagouge M, Argmann C, Gerhart-Hinesc Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Kurowski TG, Lin Y, Luo Z, Tsichlis PN, Buse MG, Heydrick SJ, Ruderman NB. Hyperglycemia inhibits insulin activation of Akt/protein kinase B but not phosphatidylinositol 3-kinase in rat skeletal muscle. Diabetes. 1999;48:658–663. doi: 10.2337/diabetes.48.3.658. [DOI] [PubMed] [Google Scholar]
- 15.Gutmann WA. L(+) lactate: determination with lactate dehydrogenase and NAD. 1974 ed. [Google Scholar]
- 16.Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- 17.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 18.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu JM, Wang ZR, Hsieh TC, Bruder JL, Zou JG, Huang YZ. Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine (Review) Int J Mol Med. 2001;8:3–17. doi: 10.3892/ijmm.8.1.3. [DOI] [PubMed] [Google Scholar]
- 20.Borra MT, Smith BC, Denu JM. J Biol Chem. Vol. 280. 2005. Mechanism of human SIRT1 activation by resveratrol; pp. 17187–17195. [DOI] [PubMed] [Google Scholar]
- 21.Williamson DH, Lund P, Krebs HA. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967;103:514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buss GD, Constantin J, de Lima LC, Teodoro GR, Comar JF, Ishii-Iwamoto EL, Bracht A. The action of quercetin on the mitochondrial NADH to NAD(+) ratio in the isolated perfused rat liver. Planta Med. 2005;71:1118–1122. doi: 10.1055/s-2005-873174. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Q, Wang SY, Fleuriel C, Leprince D, Rocheleau JV, Piston DW, Goodman RH. Metabolic regulation of SIRT1 transcription via a HIC1:CtBP corepressor complex. Proc Natl Acad Sci U S A. 2007;104:829–833. doi: 10.1073/pnas.0610590104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008 doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lan F, Cacicedo JM, Ido Y. Activation of AMPKK-AMPK cascade by Silence Information Regulator 2 (Sir2) Diabetes. 2005;54:A383. [Google Scholar]
- 26.Lan F, Cacicedo JM, Ruderman N, Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1: possible role in AMP-actived protein kinase activation. J Biol Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salt IP, Johnson G, Ashcroft SJ, Hardie DG. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic beta cells, and may regulate insulin release. Biochem J. 1998;335(Pt 3):533–539. doi: 10.1042/bj3350533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ido Y, Carling D, Ruderman N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: inhibition by the AMP-activated protein kinase activation. Diabetes. 2002;51:159–167. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]