Abstract
To study air space fluid clearance (AFC) under conditions that resemble the clinical setting of pulmonary edema in patients, we developed a new perfused human lung preparation. We measured AFC in 20 human lungs rejected for transplantation and determined the contribution of AFC to lung fluid balance. AFC was then compared with air space and perfusate levels of a biological marker of epithelial injury. The majority of human lungs rejected for transplant had intact basal (75%) and β2-adrenergic agonist-stimulated (70%) AFC. For lungs with both basal and stimulated AFC, the basal AFC rate was 19 ± 10%/h, and the β2-adrenergic-stimulated AFC rate was 43 ± 13%/h. Higher rates of AFC were associated with less lung weight gain (Pearson coefficient −0.90, P < 0.0001). Air space and perfusate levels of the type I pneumocyte marker receptor for advanced glycation end products (RAGE) were threefold and sixfold higher, respectively, in lungs without basal AFC compared with lungs with AFC (P < 0.05). These data show that preserved AFC is a critical determinant of favorable lung fluid balance in the perfused human lung, raising the possibility that β2-agonist therapy to increase edema fluid clearance may be of value for patients with acute lung injury and pulmonary edema. Also, although additional studies are needed, a biological marker of alveolar epithelial injury may be useful clinically in predicting preserved AFC.
Keywords: alveolar epithelial fluid transport, acute lung injury, acute respiratory distress syndrome, lung transplantation, biological markers, pulmonary edema, primary graft failure, air space fluid clearance
Postlung transplant reperfusion pulmonary edema is a form of acute lung injury that contributes to early mortality in lung transplant patients (6-8, 37). Acute lung injury is characterized by the loss of alveolar epithelial barrier function, including increased permeability to protein and decreased fluid transport from the air spaces, resulting in pulmonary edema (6-8, 37). Removal of edema fluid from the air spaces, or distal air space fluid clearance (AFC), is dependent on a transepithelial sodium concentration gradient established by basolateral sodium-potassium ATPase (19, 20). The basal rate of alveolar epithelial ion and fluid transport can be increased by β2-adrenergic agonists (15, 17, 20, 22). Edema fluid cleared from the distal air spaces into the interstitial space is transported from the lungs primarily by direct absorption into the pulmonary venous circulation, but also by pulmonary lymphatic drainage and clearance into the pleural space (20, 30). Lung fluid balance is the sum of edema formation and lung fluid clearance.
Data from nonperfused human lungs obtained from donated organs rejected for transplant (39) and from patients with lung cancer (24, 26) have indicated that AFC rates in human lungs are low in the absence of perfusion compared with rates in other species (rats and mice) commonly used to study epithelial barrier function. In these previous animal studies, AFC was comparable when measured during perfusion or in situ, in the absence of perfusion; however, these measurements were made immediately upon cessation of perfusion with temperature maintained at 37°C and without allowing the lungs to cool. The majority of these studies was done in the absence of interstitial pulmonary edema. Before transplantation, human lungs are preserved at 4°C for several hours. The effect of this period of cold preservation and rewarming on AFC is uncertain. Our previously reported human lung studies of AFC have used nonperfused lungs only (24, 26, 39). Variables such as interstitial pulmonary edema, the rate and effectiveness of passive rewarming in a warm bath, and the surface area for fluid clearance could potentially be affected by perfusion in ex vivo human lungs. Therefore, although preserved epithelial fluid transport has been associated with increased survival among patients with acute lung injury (38) and more rapid resolution of posttransplant reperfusion pulmonary edema, the rate of basal and stimulated AFC in ex vivo human lungs has not been carefully evaluated in the presence of perfusion. Furthermore, the utility of biological markers of alveolar epithelial cell injury to predict the presence or absence of intact AFC in lungs being evaluated for transplant is uncertain.
Therefore, we developed a new perfused human lung preparation to answer three questions. First, does pulmonary vascular perfusion alter the rate of distal air space fluid transport in human lungs? Second, what are the relative contributions of epithelial permeability to protein and epithelial fluid transport to lung fluid balance in the perfused human lung? Third, is a biological marker of alveolar epithelial injury associated with the rate of alveolar epithelial fluid transport?
METHODS
Lung selection
Lungs from brain-dead organ donors that were rejected for transplantation by the Northern California Transplant Donor Network were used for this study. After obtaining informed consent from next-of-kin, lungs from 24 donors were removed en bloc, inflated, and transported on ice to the research laboratory. Lungs were not perfused with preservation fluid before being placed on ice. Inclusion of lungs in the study was random and was dependent on the availability of the surgical team to procure the lungs and the availability of the research team. Lungs were not included in this study if on arrival to the research laboratory there was gross rupture of the visceral pleura or if the surgical preparation at procurement excluded the possibility of either ventilation or pulmonary vascular perfusion. Demographic data were extracted from the donors' charts.
Preparation
The experimental preparation was done in a cold room (4°C) with the lungs on ice. The left and right lungs were separated. The left pulmonary artery (PA) was cannulated by passing a Foley catheter 2–3 cm into the surgical stump and securing it place with a purse-string suture. The Foley catheter was connected to sterile peristaltic tubing filled with DME-H21 medium containing 5% BSA. To measure PA pressure, a PA catheter (Cook) was passed through a side port in the tubing and advanced to the end of the Foley catheter. The left main stem bronchus was cannulated with a sterile 12-mm-diameter rigid plastic catheter. The lung preparation was then weighed and suspended within a sealed acrylic container from a mass transducer (Harvard Apparatus) (Fig. 1). The container was surrounded by a heated (38°C) water jacket, and the bottom of the inner container served as a reservoir for the perfusate solution (900 ml). The perfusion tubing was submerged in the perfusate reservoir, and perfusion with the warmed perfusate was begun at a constant pressure of 15 cmH2O. The temperature of the perfusate at the PA was held constant at 36°C. The pulmonary veins were not cannulated, and venous drainage was passive. The temperature of the perfusate at the pulmonary vein was continuously monitored with a digital thermometer. When the temperature of the venous drainage reached 36°C, the lungs were inflated with a continuous positive airway pressure of 10 cmH2O with 95% O2 and 5% CO2. PA pressure, airway pressure, and lung mass were continuously monitored using a computer-integrated data acquisition system (Biopac, Santa Barbara, CA). Perfusate flow rate was continuously monitored on the previously calibrated perfusion pump. Pulmonary capillary pressure was determined every 30 min by extrapolation of the second exponential phase of the postocclusion PA pressure toward the instant of occlusion as previously described (13). Perfusate pH, PO2 and PCO2 tensions were measured with a blood gas machine (Bayer RapidLab 248) every 30–60 min. After a stabilization period of 10–20 min, lung mass was recorded, and the experimental protocol was begun.
Fig. 1.
Isolated perfused lung preparation. The pulmonary artery and main bronchus of the left lung were cannulated, and the lung was placed in a heated humidified chamber. Perfusion was initiated and maintained at a constant pressure. Venous drainage from the lung into the recirculated perfusate reservoir was passive. After rewarming, continuous positive airway pressure (CPAP; 10 cmH2O) was applied to the airway with 95% oxygen and 5% carbon dioxide. Pulmonary artery pressure, airway pressure, perfusate flow, and lung weight were continuously monitored.
To further test the effect of perfusion on AFC in human lungs rejected for transplantation, four lungs were studied with and without perfusion. The lung was passively rewarmed as previously reported (26, 36, 39). Briefly, the lung was placed in a plastic bag and submerged in a 37°C water bath for 2 h. During this period, the lung was gently inflated with 10 cmH2O. AFC was then measured in one lung lobe as described below. The lung was then perfused as per the protocol above, and the AFC measurement was repeated in a separate lung lobe.
Measurement of distal AFC
Distal AFC was measured as previously described (24) with slight modification (Fig. 2). Briefly, following the stabilization period, a catheter (PE 240 tubing, BD) was passed through a side port in the endobronchial tube into the lung and advanced until gentle resistance was encountered. One-hundred fifty milliliters of warmed (36°C) normal saline containing 5% BSA was instilled through the catheter into the air spaces to the lung. After 5 min (time 0) and 35 min (time 30 min), samples were removed through the catheter by gentle aspiration. At the time 30 min time point, all easily aspirated air space fluid was removed from the lung (5–25 ml), the volume was removed, and change in lung mass was recorded. The change in concentration of protein in the time 30 min compared with the time 0 min samples was used to determine the volume of fluid cleared from the air spaces by the following equation: [distal AFC (%/h) = 2(1 – Ci/Cf)], where Ci is the protein concentration of the time 0 sample and Cf is the protein concentration of the time 30 min sample. Following collection of the time 30 min sample, a second catheter was placed into a separate subsegment of the lung, and 150 ml of a solution of warmed saline with 5% BSA and 10−5 M terbutaline, a selective β2-adrenergic agonist, were instilled into the lung. Samples were aspirated at 5 and 35 min, and β-adrenergic agonist-stimulated AFC was determined. Basal AFC was the rate of fluid clearance measured in the absence of β-adrenergic agonist; stimulated AFC was the rate of AFC measured with β-agonists. The term intact AFC describes the presence of basal AFC (>0%/h). Stimulated AFC was defined as any increase in AFC with β-agonist.
Fig. 2.
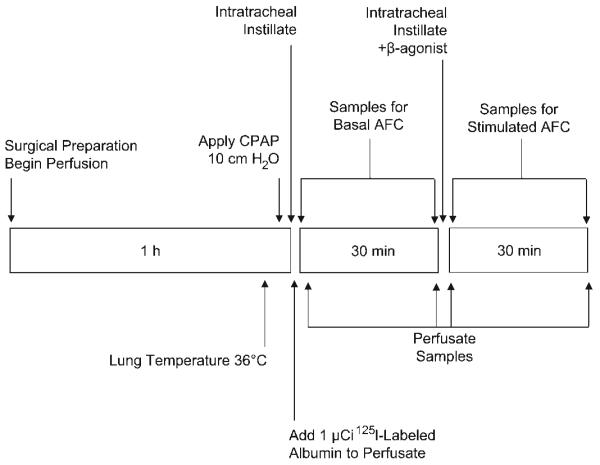
Experimental protocol. Following surgical preparation, rewarming to 36°C, and application of CPAP, 150 ml of isotonic instillate with 5% BSA was instilled into the air space, and 1 μCi of 125I-labeled albumin was added to the perfusate. Five minutes later, baseline samples of both air space fluid and perfusate were collected. Samples were taken again 30 min later. Immediately following the measurement of basal air space fluid clearance (AFC), a second volume of fluid containing terbutaline (10−5 M) was instilled into the lung. Air space and perfusate samples were subsequently collected at 5 min and 30 min for determination of AFC and epithelial albumin flux. Lung weight gain was measured from the start of the first measurement of AFC to the end of the protocol.
Measurement of epithelial barrier albumin flux and lung fluid balance
Lung alveolar epithelial albumin flux was measured as previously described (11) with minor modification. Briefly, 125I-labeled albumin (1 μCi) was added to the perfusate at the end of the stabilization period. A sample of perfusate (0.5 ml) was collected after 5 min and at the end of the entire protocol to coincide with collection of air space fluid from the final AFC measurement. Radioactivity in the perfusate and air space was then compared with estimate albumin flux using the following equation: [CtAS/(CtP/ml)]/VPI × 100, where CtAS is the radioactivity in the aspirated air space fluid at the end of the protocol, CtP/ml is the radioactivity per ml of perfusate averaged between the sample collected at 5 min and the sample collected at the end of the protocol, and VPI is the initial volume of the perfusate. Lung fluid balance was calculated as the net lung weight change over the course of the experimental protocol beginning at the time of rewarming to the end of the protocol (Fig. 2).
Measurement of RAGE
Air space and perfusate levels of the alveolar epithelial protein receptor for advanced glycation end products (RAGE) were measured with a specific ELISA as previously reported (14, 33) in a subset of nine lungs. Levels were measured in samples collected at baseline (time 0) and every 30 min thereafter.
Statistical analysis
Comparisons between groups were made using unpaired t-tests. Comparisons of repeated measures were made using paired t-tests. Nonparametric data were compared using the Mann-Whitney U-test. Univariate and multivariable logistic regression was determined using SPSS software. The multivariable model included AFC, albumin flux, and lung weight gain.
RESULTS
Donor demographics and procurement times
The lungs from 24 donors were used for these studies. Clinical demographic data are summarized in Table 1. The reasons for rejection included bilateral chest radiograph abnormalities (10), pneumonia (7), atelectasis (5), hypoxemia (3), pneumothorax (2), pulmonary contusion (2), aspiration (1), pulmonary edema (1), hemoptysis (1), pleural adhesions (1), emphysema (1), and hepatitis C virus infection (1). Lungs from three donors met criteria for acute lung injury, and lungs from four donors met criteria for acute respiratory distress syndrome (1). There was no significant association between the time of brain death to organ procurement or the time from procurement to the experiment and alveolar fluid clearance rates (r 2 = 0.03 and 0.02, respectively).
Table 1.
Donor clinical demographic data, procurement times, and time to warming
| Mean age (±SD) | 40±15 yr |
| Female | 55% |
| Ethnic/racial minority | 55% |
| Cause of death (number/total) | |
| Motor vehicle accident/head trauma | 7/20 |
| Intracranial hemorrhage | 10/20 |
| Gun shot wound to head | 2/20 |
| Anoxic brain injury | 1/20 |
| Clinical concern for lung injury* | 19/20 |
| Time from death to procurement (±SD) | 38±14h |
| Time from procurement to experiment | 18±11 h |
| Time from 4°C to 36°C with perfusion | 52±19 min |
Including hypoxemia, pneumonia by microbiological findings, radiographic infiltrates, history of aspiration, pulmonary contusion, and pulmonary edema.
Distal AFC in perfused human lungs
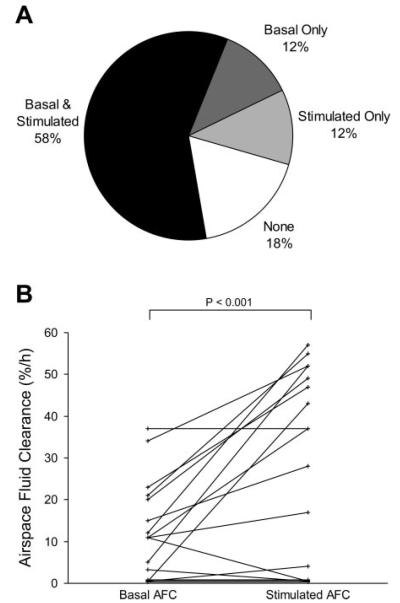
Basal AFC and β-adrenergic-stimulated AFC were measured in 17 lungs. In seven lungs, only basal AFC was studied. Of the lungs in which both basal and stimulated AFC were measured, 82% (14/17) had either basal or stimulated AFC, or both (Fig. 3). Of these, two had basal AFC with no increase in the fluid clearance rate after terbutaline was added. Five lungs had no basal AFC. Of these, two lungs demonstrated fluid clearance with terbutaline stimulation. Of the lungs with measurable basal and stimulated fluid clearance (n = 10), the mean basal AFC rate was 19 ± 10%/h, and the mean stimulated AFC was 43 ± 13%/h (P < 0.001) (Fig. 4). The mean basal AFC rate for all of the lungs studied (n = 24) was 16%/h (95% confidence interval 11–21%/h). The mean β-adrenergic-stimulated AFC for all of the lungs studied (n = 17) was 28%/h (95% confidence interval 17–39%/h, P < 0.05 compared with basal AFC).
Fig. 3.
Proportion of lungs rejected for transplantation with measurable basal and β-adrenergic-stimulated AFC. A: of the 17 lungs in which both basal and stimulated AFC were measured, the majority of lungs had a basal AFC rate that increased following the addition of terbutaline. A smaller proportion of lungs had no measurable AFC with or without the addition of terbutaline. B: basal and stimulated AFC in the 17 lungs from which both measurements were made (P < 0.001 by paired t-test).
Fig. 4.
Basal and β-adrenergic-stimulated AFC. The mean basal AFC rate was 19% in the lungs for which both basal and stimulated AFC were present. Terbutaline significantly increased AFC (*P < 0.001). Data shown are means ± SD for lungs with measured basal and stimulated AFC.
The effect of perfusion compared with passive rewarming on basal AFC
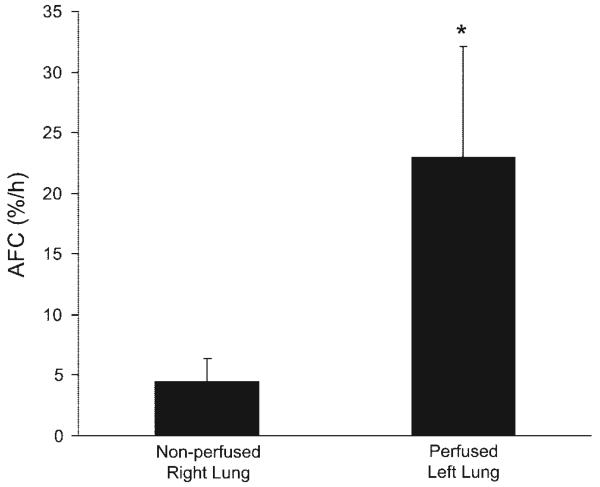
In four lungs, basal AFC was compared without and with perfusion. The lungs were passively rewarmed using our previously reported protocol (26, 36, 39), and then AFC was measured in one lobe. The lung was then perfused, and AFC was measured again in a separate lung lobe. All four lungs had measurable AFC with both measurements. In each experiment, the AFC rate was significantly greater with perfusion (Fig. 5).
Fig. 5.
Effect of perfusion compared with passive rewarming on basal AFC. Four lungs were used for this comparison. The lung was passively rewarmed with submersion in a 37°C water bath for 2 h (24, 39), and AFC was measured without perfusion. The lung was then perfused with 37°C perfusate for 1 h, and AFC was measured in a separate lung lobe. In all experiments, basal AFC was greater with perfusion (*P < 0.05).
Lung fluid balance
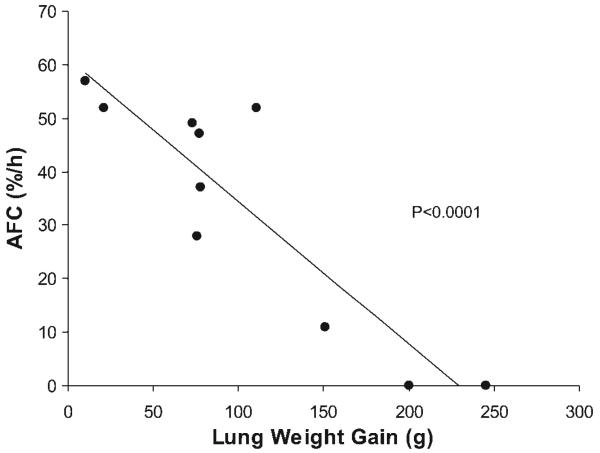
Alveolar epithelial albumin flux was measured in addition to basal and stimulated AFC in 10 lungs. There were no significant differences in pulmonary perfusion pressures, perfusate flow rates, capillary pressure, perfusate pH, PCO2, or PO2 during the protocol between lungs with and without basal AFC (Table 2). Maximal, β-agonist-stimulated AFC was inversely associated with lung weight gain [a measure of pulmonary edema; Pearson coefficient −0.90, P < 0.0001 (Fig. 6)]. Basal AFC was also associated with less lung weight gain (Pearson coefficient −0.070, P < 0.02). Results were not different if the change in lung weight were expressed as a percentage of the initial weight or as the absolute change in grams (not shown). Stimulated AFC was also inversely associated with albumin flux, but the correlation did not quite reach statistical significance (Pearson coefficient −0.65, P = 0.08). Multivariable logistic regression analysis including stimulated AFC, lung weight gain, and alveolar epithelial albumin flux revealed that AFC was inversely associated with lung weight gain and the association was independent of albumin flux in the model [correlation coefficient −0.33, (±−0.14), P < 0.005 for AFC and lung weight gain, and 0.24 (±0.53), P > 0.3 for AFC and albumin flux]. This analysis indicates that for each additional 10-g increase in lung weight, the AFC rate decreased by 3.3%.
Table 2.
Hemodynamic and gas exchange variables during ex vivo lung perfusion
| Basal AFC |
|||
|---|---|---|---|
| Present (n = 15) | Absent (n = 5) | P Value | |
| PaO2/FiO2 | |||
| Initial | 214±56 | 195±96 | 0.72 |
| Final | 205±127 | 163±44 | 0.61 |
| pH | |||
| Initial | 7.57±0.14 | 7.58±0.11 | 0.93 |
| Final | 7.35±0.22 | 7.41±0.14 | 0.67 |
| Perfusate flow rate (l/min) | |||
| Initial | 0.41±0.19 | 0.38±0.14 | 0.86 |
| Final | 0.50±0.29 | 0.50±0.11 | 0.99 |
| Capillary pressure (mmHg) | |||
| Initial | 6.5±1.2 | 7.6±1.6 | 0.29 |
| Final | 4.0±1.8 | 4.2±0.6 | 0.83 |
Values are means ± SE. AFC, air space fluid clearance.
Fig. 6.
AFC is inversely associated with pulmonary edema in the perfused lung model. For the 10 lungs in which albumin flux and AFC were measured, higher stimulated AFC rates were associated with less weight gain (pulmonary edema) (Pearson coefficient −0.90, r 2 = 0.8, P < 0.0001). Basal AFC was also associated with less lung weight gain (Pearson coefficient −0.70, r 2 = 0.5, P < 0.02) (not shown).
Air space and perfusate levels of biological markers of alveolar epithelial injury
RAGE levels were measured in perfusate and air space samples from nine lungs. Baseline air space levels of RAGE were nearly threefold higher in lungs without AFC (n = 3) than in lungs with measurable AFC (n = 6) (P < 0.05) (Fig. 7). Perfusate levels of RAGE were also more than sixfold higher at baseline in lungs without AFC (P < 0.0005) (Fig. 7). At the end of the perfusion period, perfusate RAGE levels were more than 10-fold higher in lungs without intact AFC (P < 0.05) (Fig. 7B). Air space levels of RAGE did not significantly change in either group during the experimental protocol (not shown). There was a significant inverse association between perfusate levels of RAGE and AFC rate (r 2 = 0.62, P = 0.01); however, there was not a significant association between albumin flux and perfusate levels of RAGE (r 2 = 0.28, P = 0.11). There was no significant association between the time from brain death to organ procurement or the time from procurement to the experiment and perfusate RAGE levels (r 2 = 0.15 and 0.05, respectively).
Fig. 7.
Baseline air space and perfusate receptor for advanced glycation end products (RAGE) levels. A: levels of RAGE were significantly higher at baseline in donor lungs without basal AFC (gray bars, n = 3) compared with lungs with intact AFC (open bars, n = 6) (*P < 0.01). B: perfusate levels of RAGE significantly increased during the experiment in lungs without AFC (*P < 0.05).
DISCUSSION
This perfused human lung model was developed to address three main objectives. The first objective was to determine whether AFC could be measured in the presence of pulmonary vascular perfusion in human lungs rejected for transplantation. Such a model would allow future studies incorporating the delivery of test substances through the vasculature. The second objective was to evaluate the contributions of epithelial fluid transport and epithelial protein permeability to lung fluid balance in the model. The final objective was to determine whether biological markers of alveolar epithelial injury were associated with alveolar epithelial fluid transport rates in the perfused human lung model.
Previous studies have found that most human lungs rejected for transplantation maintain alveolar epithelial fluid transport capacity (39). However, the measured basal AFC rates in these previous studies and in lung lobes resected from lung cancer patients were only 4%/h (24-26, 35, 39). These previous studies were done in ex vivo lungs and lung segments in the absence of pulmonary vascular perfusion that were preserved under hypothermic conditions for at least 12–24 h. The present study confirmed that, in passively rewarmed lungs, AFC was 5 ± 2%/h in the absence of perfusion. Compared with nonperfused conditions, perfusion resulted in markedly higher basal AFC rates (Fig. 5). Because vascular perfusion may affect both AFC and pulmonary edema formation, we simultaneously measured alveolar epithelial fluid transport, pulmonary vascular permeability to albumin, and lung fluid balance in these experiments. Measurable basal AFC was present in 79% (19/24) of the lungs studied. The mean basal AFC rate for lungs in which AFC was present was 19 ± 10%/h (Fig. 4). This AFC rate is comparable to that in other species commonly used in experimental studies such as rats and mice (20). The rate of AFC determined in this model is also comparable to estimated AFC rates in our previous clinical studies of patients with pulmonary edema (34, 38). It is also notable that because the intratracheal instillate and the perfusate have identical protein concentrations at the start of the experiment, increased albumin flux could potentially result in an underestimation of AFC in this model.
Why does pulmonary vascular perfusion significantly increase AFC in the ex vivo human lung model? In previous animal studies, AFC measured in situ immediately after the cessation of perfusion was comparable to AFC measured during perfusion. There are important differences between the present study and those prior animal studies. In the animal studies, lung temperature is held constant at 37°C, and AFC is immediately measured. However, donor human lungs are removed and then undergo cold preservation for several hours. Because temperature is inversely associated with AFC (29), it is reasonable to assume that temperature must be restored to normal for AFC in the ex vivo lung to be comparable to the in situ conditions used in previous experimental studies. The addition of perfusion to the human lung model may have resulted in more uniform and complete rewarming of the lung. Therefore, it is possible that in the perfused lung, the temperature in the interior of the lung is higher than in the ex vivo, nonperfused human lungs in which the passive rewarming protocol involves submerging the lungs in a water bath for 2 h. Without perfusion, it is difficult to determine the internal temperature of the passively rewarmed lungs. In addition, the surface area for fluid transport may be greater in the perfused lung preparation compared with the ex vivo protocol without perfusion. The surface area for fluid transport could be increased by the addition of perfusion to the pulmonary vasculature or the suspension of the lungs from the bronchus in an air-filled chamber, which may result in more uniform inflation of the lung. Finally, in the absence of functioning lymphatics and vascular perfusion, the capacity for accumulation of pulmonary interstitial space for fluid is fixed. Therefore, the measured AFC rate could be affected by preexisting air space and interstitial pulmonary edema, the volume of fluid instilled for the measurement, and the duration of the measurement period (12). The addition of perfusion could have increased the clearance of fluid from the interstitial space, potentially allowing for greater removal of fluid from the air spaces. The later explanation is least likely, but remains a possibility.
We also found that a β2-selective adrenergic agonist (terbutaline, 10−5 M) significantly increased basal AFC rates more than twofold in this model. The mean stimulated AFC rate was 43 ± 13%/h in the presence of perfusion (Fig. 4). Previous studies of nonperfused ex vivo human lungs have reported stimulated fluid clearance rates of ~7%/h (24). Of the lungs studied, 70% (12/17) demonstrated a significant increase in AFC rate with terbutaline (Fig. 3). These data confirm that the alveolar epithelium remains responsive to β2-adrenergic stimulation hours after organ procurement and that stimulated fluid transport rates in human lungs are comparable to those in commonly used experimental animal models. Experimental data in animal models indicate that increased alveolar epithelial fluid transport rates result in less pulmonary edema and more rapid improvement in oxygenation during hydrostatic pulmonary edema and acute lung injury (2, 3, 11, 21, 27, 28). Previous clinical data indicate that intact AFC is associated with improved survival in patients with acute lung injury and acute respiratory distress syndrome and with more rapid recovery from reperfusion pulmonary edema after lung transplantation (21, 36, 38) or hydrostatic pulmonary edema (34). Most of the lungs used in this study were rejected in part due to concern for acute lung injury, but a minority (7/24) of donors met criteria for acute lung injury or acute respiratory distress syndrome. However, these data support the plausibility of β2-adrenergic agonist therapy targeted at increasing AFC rates and effecting a more favorable lung fluid balance in patients with acute lung injury and in patients with posttransplant reperfusion pulmonary edema and primary graft failure. Because previous clinical studies have shown that AFC may be impaired in acute lung injury and acute respiratory distress syndrome patients (38), more studies are needed to determine if β2-adrenergic agonists increase AFC in this patient population.
To determine the contribution of AFC to lung fluid balance in the perfused human lung, we simultaneously measured epithelial albumin flux, AFC, and lung weight gain (as a measure of lung fluid balance) in 10 lungs. There was a strong inverse association between AFC rate and lung weight gain (Fig. 6). There was a weaker association between AFC and lung albumin flux. Multivariable regression analysis showed that the association between AFC and lung weight gain was largely independent of epithelial albumin flux in the model. Therefore, consistent with a previous study in rats (10), AFC can be preserved or increased in the perfused human lung in the presence of increased epithelial permeability. These results emphasize the critical contribution of AFC to favorable lung fluid balance and further highlight alveolar epithelial fluid transport as a potential target of pharmacological therapy in patients with pulmonary edema and acute lung injury.
Assessment of lung function before transplantation remains a challenge. A previous study found that many lungs rejected for transplantation based on conventional clinical criteria were without significant histological or functional abnormalities (39). Those data, along with patient outcome data from recipients of lungs not meeting all of the usual pretransplant clinical criteria (4, 9, 18, 31, 32), call into question the validity of certain clinical criteria used for screening donor organs. To investigate whether air space or circulating levels of biological markers of alveolar epithelial cell injury were associated with measures of epithelial barrier function, we also measured levels of RAGE in the perfusate and air spaces in these donor lungs. RAGE is made by alveolar epithelial cells and other epithelial cells. A recent report found that air space and plasma levels of RAGE were associated with lung injury severity in rats and in patients with acute lung injury (33). Although RAGE is also produced by epithelial cells in other organs, it has been used recently as a type I pneumocyte marker (33). In this isolated lung model, type I pneumocytes are probably the only source of RAGE. In the present study, alveolar epithelial injury as measured by baseline perfusate and air space levels of RAGE were significantly greater in lungs without AFC (Fig. 7). There was a significant inverse association between perfusate levels of RAGE and AFC rate. The average RAGE level in lungs without basal AFC was 544 ± 70 ng/ml compared with 76 ± 44 ng/ml in lungs with measurable AFC (P < 0.00001, Fig. 7B). There was a weaker association between perfusate RAGE levels and albumin flux in this model, suggesting that epithelial permeability may be influenced by factors other than epithelial cell injury alone. However, because of the new evidence that alveolar epithelial type I cells transport sodium and contribute to AFC (5, 16, 23), a marker of type I cell injury could be an especially useful marker of intact AFC. These results suggest that RAGE may be a useful biological marker of epithelial injury and barrier dysfunction clinically, although additional prospective validation is necessary. These data also raise the possibility that physiological and biochemical markers of alveolar epithelial injury could have predictive value in the assessment of lungs before transplantation.
Data from this new experimental model have potentially important clinical implications. First, these data demonstrate that β2-adrenergic agonists significantly increase AFC rates in perfused human lungs, raising the possibility that pharmacological therapy directed at augmenting AFC is plausible. Second, these data confirm previous reports that many donor lungs rejected for transplantation due to concern for lung injury maintain adequate epithelial barrier function. We also show for the first time that preserved AFC is associated with more favorable lung fluid balance in the perfused human lung. Finally, measurement of biological markers of alveolar epithelial injury may be useful in predicting the presence or absence of intact alveolar fluid clearance in donor lungs and perhaps in patients with acute lung injury. Therefore, functional and biochemical assessment of alveolar epithelial injury in donor lungs before lung transplantation may be useful clinically.
Acknowledgments
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-69900 and HL-51856 and the Northern California Transplant Donor Network.
REFERENCES
- 1.Artigas A, Bernard GR, Carlet J, Dreyfuss D, Gattinoni L, Hudson L, Lamy M, Marini JJ, Matthay MA, Pinsky MR, Spragg R, Suter PM. The American-European Consensus Conference on ARDS, part 2: ventilatory, pharmacologic, supportive therapy, study design strategies, and issues related to recovery and remodeling. Am J Respir Crit Care Med. 1998;157:1332–1347. doi: 10.1164/ajrccm.157.4.ats2-98. [DOI] [PubMed] [Google Scholar]
- 2.Berthiaume Y, Staub NC, Matthay MA. Beta-adrenergic agonists increase lung liquid clearance in anesthetized sheep. J Clin Invest. 1987;79:335–343. doi: 10.1172/JCI112817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertorello AM, Ridge KM, Chibalin AV, Katz AI, Sznajder JI. Isoproterenol increases Na+-K+-ATPase activity by membrane insertion of α-subunits in lung alveolar cells. Am J Physiol Lung Cell Mol Physiol. 1999;276:L20–L27. doi: 10.1152/ajplung.1999.276.1.L20. [DOI] [PubMed] [Google Scholar]
- 4.Bhorade SM, Vigneswaran W, McCabe MA, Garrity ER. Liberalization of donor criteria may expand the donor pool without adverse consequence in lung transplantation. J Heart Lung Transplant. 2000;19:1199–1204. doi: 10.1016/s1053-2498(00)00215-1. [DOI] [PubMed] [Google Scholar]
- 5.Borok Z, Liebler JM, Lubman RL, Foster MJ, Zhou B, Li X, Zabski SM, Kim KJ, Crandall ED. Na transport proteins are expressed by rat alveolar epithelial type I cells. Am J Physiol Lung Cell Mol Physiol. 2822002:L599–L608. doi: 10.1152/ajplung.00130.2000. [DOI] [PubMed] [Google Scholar]
- 6.Christie JD, Bavaria JE, Palevsky HI, Litzky L, Blumenthal NP, Kaiser LR, Kotloff RM. Primary graft failure following lung transplantation. Chest. 1998;114:51–60. doi: 10.1378/chest.114.1.51. [DOI] [PubMed] [Google Scholar]
- 7.Christie JD, Kotloff RM, Pochettino A, Arcasoy SM, Rosengard BR, Landis JR, Kimmel SE. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003;124:1232–1241. doi: 10.1378/chest.124.4.1232. [DOI] [PubMed] [Google Scholar]
- 8.Estenne M, Kotloff RM. Update in transplantation 2005. Am J Respir Crit Care Med. 2006;173:593–598. doi: 10.1164/rccm.2601012. [DOI] [PubMed] [Google Scholar]
- 9.Fischer S, Gohrbandt B, Struckmeier P, Niedermeyer J, Simon A, Hagl C, Kallenbach K, Haverich A, Struber M. Lung transplantation with lungs from donors fifty years of age and older. J Thorac Cardiovasc Surg. 2005;129:919–925. doi: 10.1016/j.jtcvs.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 10.Folkesson HG, Nitenberg G, Oliver BL, Jayr C, Albertine KH, Matthay MA. Upregulation of alveolar epithelial fluid transport after subacute lung injury in rats from bleomycin. Am J Physiol Lung Cell Mol Physiol. 1998;275:L478–L490. doi: 10.1152/ajplung.1998.275.3.L478. [DOI] [PubMed] [Google Scholar]
- 11.Frank JA, McAuley DF, Gutierrez JA, Daniel BM, Dobbs L, Matthay MA. Differential effects of sustained inflation recruitment maneuvers on alveolar epithelial and lung endothelial injury. Crit Care Med. 2005;33:181–188. doi: 10.1097/01.ccm.0000150663.45778.c4. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda N, Folkesson HG, Matthay MA. Relationship of interstitial fluid volume to alveolar fluid clearance in mice: ventilated vs. in situ studies. J Appl Physiol. 2000;89:672–679. doi: 10.1152/jappl.2000.89.2.672. [DOI] [PubMed] [Google Scholar]
- 13.Hakim TS, Maarek JM, Chang HK. Estimation of pulmonary capillary pressure in intact dog lungs using the arterial occlusion technique. Am Rev Respir Dis. 1989;140:217–224. doi: 10.1164/ajrccm/140.1.217. [DOI] [PubMed] [Google Scholar]
- 14.Ishizaka A, Matsuda T, Albertine KH, Koh H, Tasaka S, Hasegawa N, Kohno N, Kotani T, Morisaki H, Takeda J, Nakamura M, Fang X, Martin TR, Matthay MA, Hashimoto S. Elevation of KL-6, a lung epithelial cell marker, in plasma and epithelial lining fluid in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2862004:L1088–L1094. doi: 10.1152/ajplung.00420.2002. [DOI] [PubMed] [Google Scholar]
- 15.Jayr C, Garat C, Meignan M, Pittet JF, Zelter M, Matthay MA. Alveolar liquid and protein clearance in anesthetized ventilated rats. J Appl Physiol. 1994;76:2636–2642. doi: 10.1152/jappl.1994.76.6.2636. [DOI] [PubMed] [Google Scholar]
- 16.Johnson MD, Bao HF, Helms MN, Chen XJ, Tigue Z, Jain L, Dobbs LG, Eaton DC. Functional ion channels in pulmonary alveolar type I cells support a role for type I cells in lung ion transport. Proc Natl Acad Sci USA. 2006;103:4964–4969. doi: 10.1073/pnas.0600855103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lasnier JM, Wangensteen OD, Schmitz LS, Gross CR, Ingbar DH. Terbutaline stimulates alveolar fluid resorption in hyperoxic lung injury. J Appl Physiol. 1996;81:1723–1729. doi: 10.1152/jappl.1996.81.4.1723. [DOI] [PubMed] [Google Scholar]
- 18.Luckraz H, White P, Sharples LD, Hopkins P, Wallwork J. Short- and long-term outcomes of using pulmonary allograft donors with low PO2. J Heart Lung Transplant. 2005;24:470–473. doi: 10.1016/j.healun.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Matalon S, Lazrak A, Jain L, Eaton DC. Invited review: biophysical properties of sodium channels in lung alveolar epithelial cells. J Appl Physiol. 2002;93:1852–1859. doi: 10.1152/japplphysiol.01241.2001. [DOI] [PubMed] [Google Scholar]
- 20.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 21.McAuley DF, Frank JA, Fang X, Matthay MA. Clinically relevant concentrations of beta2-adrenergic agonists stimulate maximal cyclic adenosine monophosphate-dependent air space fluid clearance and decrease pulmonary edema in experimental acid-induced lung injury. Crit Care Med. 2004;32:1470–1476. doi: 10.1097/01.ccm.0000129489.34416.0e. [DOI] [PubMed] [Google Scholar]
- 22.Mutlu GM, Dumasius V, Burhop J, McShane PJ, Meng FJ, Welch L, Dumasius A, Mohebahmadi N, Thakuria G, Hardiman K, Matalon S, Hollenberg S, Factor P. Upregulation of alveolar epithelial active Na+ transport is dependent on β2-adrenergic receptor signaling. Circ Res. 2004;94:1091–1100. doi: 10.1161/01.RES.0000125623.56442.20. [DOI] [PubMed] [Google Scholar]
- 23.Ridge KM, Olivera WG, Saldias F, Azzam Z, Horowitz S, Rutschman DH, Dumasius V, Factor P, Sznajder JI. Alveolar type 1 cells express the alpha2 Na,K-ATPase, which contributes to lung liquid clearance. Circ Res. 2003;92:453–460. doi: 10.1161/01.RES.0000059414.10360.F2. [DOI] [PubMed] [Google Scholar]
- 24.Sakuma T, Folkesson HG, Suzuki S, Okaniwa G, Fujimura S, Matthay MA. Beta-adrenergic agonist stimulated alveolar fluid clearance in ex vivo human and rat lungs. Am J Respir Crit Care Med. 1997;155:506–512. doi: 10.1164/ajrccm.155.2.9032186. [DOI] [PubMed] [Google Scholar]
- 25.Sakuma T, Gu X, Wang Z, Maeda S, Sugita M, Sagawa M, Osanai K, Toga H, Ware LB, Folkesson G, Matthay MA. Stimulation of alveolar epithelial fluid clearance in human lungs by exogenous epinephrine. Crit Care Med. 2006;34:676–681. doi: 10.1097/01.CCM.0000201403.70636.0F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakuma T, Okaniwa G, Nakada T, Nishimura T, Fujimura S, Matthay MA. Alveolar fluid clearance in the resected human lung. Am J Respir Crit Care Med. 1994;150:305–310. doi: 10.1164/ajrccm.150.2.8049807. [DOI] [PubMed] [Google Scholar]
- 27.Saldias F, Lecuona E, Friedman E, Barnard ML, Ridge KM, Sznajder JI. Modulation of lung liquid clearance by isoproterenol in rat lungs. Am J Physiol Lung Cell Mol Physiol. 1998;274:L694–L701. doi: 10.1152/ajplung.1998.274.5.L694. [DOI] [PubMed] [Google Scholar]
- 28.Saldias FJ, Lecuona E, Comellas AP, Ridge KM, Rutschman DH, Sznajder JI. Beta-adrenergic stimulation restores rat lung ability to clear edema in ventilator-associated lung injury. Am J Respir Crit Care Med. 2000;162:282–287. doi: 10.1164/ajrccm.162.1.9809058. [DOI] [PubMed] [Google Scholar]
- 29.Serikov VB, Grady M, Matthay MA. Effect of temperature on alveolar liquid and protein clearance in an in situ perfused goat lung. J Appl Physiol. 1993;75:940–947. doi: 10.1152/jappl.1993.75.2.940. [DOI] [PubMed] [Google Scholar]
- 30.Staub NC. Pulmonary edema. Physiol Rev. 1974;54:678–811. doi: 10.1152/physrev.1974.54.3.678. [DOI] [PubMed] [Google Scholar]
- 31.Sundaresan S, Semenkovich J, Ochoa L, Richardson G, Trulock EP, Cooper JD, Patterson GA. Successful outcome of lung transplantation is not compromised by the use of marginal donor lungs. J Thorac Cardiovasc Surg. 1995;109:1075–1079. doi: 10.1016/S0022-5223(95)70190-7. discussion 1079–1080. [DOI] [PubMed] [Google Scholar]
- 32.Thabut G, Mal H, Cerrina J, Dartevelle P, Dromer C, Velly JF, Stern M, Loirat P, Bertocchi M, Mornex JF, Haloun A, Despins P, Pison C, Blin D, Simonneau G, Reynaud-Gaubert M. Influence of donor characteristics on outcome after lung transplantation: a multicenter study. J Heart Lung Transplant. 2005;24:1347–1353. doi: 10.1016/j.healun.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 33.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006;173:1008–1015. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verghese GM, Ware LB, Matthay BA, Matthay MA. Alveolar epithelial fluid transport and the resolution of clinically severe hydrostatic pulmonary edema. J Appl Physiol. 1999;87:1301–1312. doi: 10.1152/jappl.1999.87.4.1301. [DOI] [PubMed] [Google Scholar]
- 35.Ware LB, Fang X, Wang Y, Sakuma T, Hall TS, Matthay MA. Selected contribution: mechanisms that may stimulate the resolution of alveolar edema in the transplanted human lung. J Appl Physiol. 2002;93:1869–1874. doi: 10.1152/japplphysiol.00252.2002. [DOI] [PubMed] [Google Scholar]
- 36.Ware LB, Golden JA, Finkbeiner WE, Matthay MA. Alveolar epithelial fluid transport capacity in reperfusion lung injury after lung transplantation. Am J Respir Crit Care Med. 1999;159:980–988. doi: 10.1164/ajrccm.159.3.9802105. [DOI] [PubMed] [Google Scholar]
- 37.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 38.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 39.Ware LB, Wang Y, Fang X, Warnock M, Sakuma T, Hall TS, Matthay M. Assessment of lungs rejected for transplantation and implications for donor selection. Lancet. 2002;360:619–620. doi: 10.1016/s0140-6736(02)09774-x. [DOI] [PubMed] [Google Scholar]