Abstract
Objective
The coagulation and inflammatory cascades may be linked in the pathogenesis of acute lung injury and acute respiratory distress syndrome. However, direct evidence for the contribution of abnormalities in coagulation and fibrinolysis proteins to outcomes in patients with acute lung injury/acute respiratory distress syndrome is lacking.
Design
Retrospective measurement of plasma levels of protein C and plasminogen activator inhibitor-1 in plasma samples that were collected prospectively as part of a large multicenter clinical trial. The primary outcome was hospital mortality. To evaluate the potential additive value of abnormalities of these biomarkers, the excess relative risk of death was calculated for each combination of quartiles of protein-C and plasminogen activator inhibitor-1 levels.
Setting
Ten university medical centers.
Patients
The study included 779 patients from a multicenter clinical trial of a protective ventilatory strategy in acute lung injury/acute respiratory distress syndrome and 99 patients with acute cardiogenic pulmonary edema, as well as ten normal controls.
Measurements and Main Results
Compared with plasma from controls and patients with acute cardiogenic pulmonary edema, baseline protein-C levels were low and baseline plasminogen activator inhibitor-1 levels were elevated in acute lung injury/acute respiratory distress syndrome. By multivariate analysis, lower protein C and higher plasminogen activator inhibitor-1 were strong independent predictors of mortality, and ventilator-free and organ-failure-free days. Plasminogen activator inhibitor-1 and protein C had a synergistic interaction for the risk of death.
Conclusions
Early acute lung injury/acute respiratory distress syndrome is characterized by decreased plasma levels of protein C and increased plasma levels of plasminogen activator inhibitor-1 that are independent risk factors for mortality and adverse clinical outcomes. Measurement of plasminogen activator inhibitor-1 and protein-C levels may be useful to identify those at highest risk of adverse clinical outcomes for the development of new therapies.
Keywords: acute respiratory distress syndrome, protein C, plasminogen activator inhibitor-1, coagulation, fibrinolysis
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are life-threatening disorders characterized by severe inflammation in the lungs and frequent occurrence of multiple organ failure (1). Despite numerous randomized controlled trials of therapies aimed at modulating the inflammatory response in ALI/ARDS (2), the only therapy that has been proven to reduce mortality is a protective ventilatory strategy (3). Although mortality from ALI/ARDS has declined in clinical trials, epidemiologic studies that include all patients with ALI/ARDS still report mortality rates >50% (4). Insight into the pathophysiological derangements that characterize early clinical ALI/ARDS may help to guide the development of new therapies for this devastating disorder.
The degree of alterations in coagulation and fibrinolysis may be an important pathogenetic and prognostic determinant of mortality in ALI/ARDS. Although altered coagulation and fibrinolysis, both systemically and in the alveolar compartment, were first described in patients with ARDS some years ago (5-7), the relationship of procoagulant to proinflammatory events in causing organ injury has been better appreciated recently (8, 9). Alterations in coagulation and fibrinolysis proteins have not been systematically studied in large numbers of well-characterized patients with ALI/ARDS using the standard definitions (10) that are now used in most clinical trials. We previously measured circulating levels of the endogenous anticoagulant protein C (11) and the fibrinolysis inhibitor plasminogen activator inhibitor-1 (PAI-1) (12) in patients with ALI/ARDS in two small single-center pilot studies. Compared with control patients with acute cardiogenic pulmonary edema, levels of plasma protein C were low and levels of plasma PAI-1 were high in ALI/ARDS.
Based on these preliminary findings, we hypothesized that in a large multicenter study, low circulating levels of protein C and high levels of PAI-1 would be present and would have a major impact on the risk of death and other major outcomes in ALI/ARDS. In addition, because prognostic indices in lung injury have limited value when based on a single biomarker alone (13), we determined whether the combination of protein C and PAI-1 would have a higher prognostic value for important clinical outcomes. Understanding the relationship of alterations in coagulation and fibrinolysis proteins on the pathogenesis and prognosis of ALI could be a valuable step toward the development of new therapeutic strategies to target these pathways in this important cause of acute respiratory failure.
MATERIALS AND METHODS
ALI/ARDS Patients
Patients enrolled between 1996 and 1999 in the ARDS Network multicentered trial (3) of tidal volume of 6 mL/kg vs. 12 mL/kg were included if baseline plasma was available (779 of 861 patients). Plasma was not available from 82 patients because it was not collected or was utilized for prior studies. The study was approved by each hospital’s institutional review board. Informed consent was obtained from patients or surrogates; this requirement was waived at one hospital.
Clinical Trial Procedures
Trial procedures were previously reported (3). Ethylenediamine-tetraacetic-acid plasma was collected on days 0 and 3 and stored at -80°C at each study site until batched shipment for long-term storage at the National Institutes of Health Sample Repository. On day 0, plasma was collected at the time of enrollment (typically between 8 am and 5 pm). On day 3, plasma was collected at 8 am. Plasma from 10 healthy volunteers was collected for comparison.
Clinical Data
Clinical data were collected prospectively. Acute Physiology and Chronic Health Evaluation (APACHE) III scores were calculated at baseline (14). The primary clinical risk factor for ALI/ARDS was determined at randomization and classified as direct (pneumonia or aspiration) or indirect (sepsis, trauma, other) (15). The presence of sepsis as the clinical risk factor for ALI/ARDS was ascertained at baseline by the clinical coordinator and physician investigator at each study site based on clinical judgment, rather than on strictly specified criteria, a method that is likely to match that used in clinical practice. The primary outcome was mortality before discharge home with unassisted breathing. Secondary outcomes included ventilator-free days and nonpulmonary-organ-failure-free days over the 28 days after enrollment (3).
Cardiogenic Pulmonary Edema Patients
Ethylenediamine-tetraacetic-acid plasma was obtained from 99 subjects with acute cardiogenic pulmonary edema drawn from patients enrolled in 2003 in a study of N-terminal pro-brain-natriuretic-peptide for the diagnosis of acute destabilized heart failure in patients with acute dyspnea (16). In order to be included in the current study, patients had to have acute destabilized heart failure as the cause of dyspnea, and have radiographic findings consistent with pulmonary edema. Plasma was collected at enrollment and stored at -80°C. None of these patients were mechanically ventilated.
Measurement of Protein C and PAI-1
Protein-C antigen (Helena Laboratories, Beaumont, TX) (11) and PAI-1 antigen (American Diagnostica, Stamford, CT) (12) were measured in duplicate by enzyme-linked immunosorbent assay. Protein C is expressed as a percentage of a pooled plasma standard provided by the manufacturer. The intra-assay coefficient of variation was 4.1%. PAI-1 is expressed in ng/mL compared with a standard curve of known PAI-1 concentrations. The intra-assay coefficient of variation was 4.3%. The samples from the ARDS Network study were analyzed in 2001. The samples from the N-terminal pro-brain-natriuretic-peptide study were analyzed in 2005. The samples from healthy volunteers were drawn in 2000 and analyzed in 2001.
Statistical Analysis
Statistical analysis was done using SAS 9.1 (SAS Institute, Cary, NC). Normally and non-normally distributed data are expressed as mean ± sd and median (interquartile range), respectively. For bivariate analyses, the Student’s t-test, Wilcoxon’s rank-sum test, or chi-square test were used. For the repeated-measurements comparisons (day 3 vs. day 0), the paired Student’s t-test was used on rank-transformed values. Based on the distribution, protein-C and PAI-1 levels were divided into quartiles for analysis. Logistic regression analysis was used to examine the impact of protein C and PAI-1 on the risk of death in both individual and mutually adjusted models. Multivariate logistic regression analysis was used to control for potential confounding effects of age, illness severity (APACHE III), sepsis, and ventilator group based on reported risk factors for mortality (15). To evaluate the relationship of changes in PAI-1 and protein C over the first 3 days to study outcomes, we repeated the multivariate logistic regression analysis with indicator variables to code for an increase or decrease in PAI-1 or protein-C level over time (referent group = no change).
Interactions between each biomarker and sepsis were tested in logistic-regression models. To examine the impact of protein C and PAI-1 on ventilator-free days and organ-failure-free days, we used multivariate linear regression. Protein C and PAI-1 were evaluated for evidence of interaction, either synergism or antagonism. Interaction was studied on an additive scale, which is more appropriate than the multiplicative scale implicit in logistic regression (17). Using the odds ratio as an estimate of relative risk (RR), the formula RR-1 was used to calculate the relative excess risk (18, 19).
RESULTS
Patient Characteristics
Baseline demographic and clinical characteristics for the 779 ALI/ARDS patients are summarized in Table 1. The most common underlying cause of ALI/ARDS was pneumonia (36%), followed by sepsis (27%), aspiration of gastric contents (15%), trauma (10%), and other causes (13%). The patients were equally divided between the two tidal-volume groups. Patients who were included in the study (n = 779) had demographic characteristics and severity of illness similar to patients who were not included in the study because of lack of available plasma (n = 82) with the exception of a modestly higher (149 ± 63 vs. 131 ± 62) Pao2/Fio2 ratio in the group that was included. For example, the APACHE III score was similar in the two groups (76 ± 32 vs. 76 ± 27). Baseline demographics for the comparison group of 99 patients with acute cardiogenic pulmonary edema are also provided in Table 1.
Table 1.
Baseline demographic and clinical characteristics of 779 patients with acute lung injury/acute respiratory distress syndrome (ALI/ARDS) and 99 patients with acute cardiogenic pulmonary edema
| Variable | ALI/ARDS (n = 779) |
Acute Cardiogenic Pulmonary Edema (n = 99) |
|---|---|---|
| Age (years), mean ± sd | 52 ± 17 | 74 ± 13 |
| Male gender (%) | 461 (59) | 53 (53) |
| White race (%) | 570 (73) | 93 (94) |
| APACHE III score, mean ± sd | 76 ± 27 | NA |
| Platelet count (×109/L), mean ± sd | 160 ± 112 | NA |
| Creatinine (mg/dL), mean ± sd | 1.7 ± 1.6 | 1.3 ± 0.4 |
| Pao2/Fio2 ratio, mean ± sd | 131 ± 62 | NA |
| 6 mL/kg tidal-volume protocol (%) | 391 (50) | NA |
| Vasopressor administration (%) | 321 (41) | 0 (0) |
| Sepsis as clinical risk factor for ALI (%) | 207 (27) | 0 (0) |
APACHE III, Acute Physiology and Chronic Health Evaluation III; NA, not available.
Protein-C and PAI-1 Levels
Plasma protein-C levels were significantly decreased in ALI/ARDS compared with normal controls (p < .0001) (Table 2) and compared with patients with acute cardiogenic pulmonary edema (p < .0001) (Table 3). Plasma protein-C levels rose between day 0 and day 3 in patients with ALI/ARDS (Table 2). Plasma PAI-1 levels were significantly elevated compared with normal controls (p < .0001) (Table 2) or compared with patients with acute cardiogenic pulmonary edema (p = .0073) (Table 3). Plasma PAI-1 levels declined between day 0 and day 3 (Table 2). After controlling for age and the presence of sepsis, protein-C levels were lower and PAI-1 levels were higher in ALI/ARDS than acute cardiogenic pulmonary edema (Table 3). There was no meaningful correlation between PAI-1 levels and protein-C levels at baseline in the ALI/ARDS patients (r = .17).
Table 2.
Summary of protein C and plasminogen activator inhibitor-1 (PAI-1) plasma levels at baseline and day 3 in acute lung injury (ALI) and acute respiratory distress syndrome patients with and without sepsis
| Protein C, % Control |
PAI-1, ng/mL |
|||||||
|---|---|---|---|---|---|---|---|---|
| n | Baseline | n | Day 3 | n | Baseline | n | Day 3 | |
| All patients | 779 | 47 (32-64) | 690 | 53 (37-76)a | 779 | 73 (41-162) | 690 | 51 (34-109)a |
| Sepsis | 207 | 38 (27-53) | 181 | 48 (32-68) | 207 | 84 (44-206) | 181 | 53 (34-53) |
| Other ALI risk factor | 572 | 50 (35-67)b | 509 | 55 (39-76)c | 572 | 69 (40-149)d | 509 | 50 (34-106)e |
p < .0001 for protein C and PAI-1 (comparison of day 3 with day 0 for all patients using paired-sample t-test with rank-transformed data)
p < .0001
p < .0008
p = .03
p = .64 (comparison of non-sepsis with sepsis group by Wilcoxon’s rank-sum test).
The median protein C level in 10 normal volunteers was 127% (100-142%) of control. The median PAI-1 level in 10 normal controls was 8.0 (6.7-9.3) ng/mL. Data are median (interquartile range).
Table 3.
Comparison of protein C and plasminogen activator inhibitor-1 (PAI-1) levels in 779 patients with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) to 99 patients with acute cardiogenic pulmonary edema
| ALI/ARDS (n = 779) |
Acute Cardiogenic Pulmonary Edema (n = 99) |
p Value |
p Value, Controlling for Age and Sepsis |
|
|---|---|---|---|---|
| Protein C, % control | 47 (32-64) | 76 (52-119) | <.0001 | <.0001 |
| PAI-1, ng/mL | 73 (41-162) | 60 (33-126) | .0073 | .028 |
Data are median (25th to 75th interquartile range).
Although abnormal levels of both protein C and PAI-1 were present in all ALI/ARDS patients, the abnormalities were more severe at baseline (but not day 3) in the group of 207 patients with sepsis as the underlying cause of lung injury (Table 2). The treatment strategy (6 vs. 12 mL/kg tidal volume) had no effect on PAI-1 levels over the first 3 days (p = .94). Protein-C levels were slightly lower in the 12-mL/kg group compared with the 6-mL/kg group at day 3 with a mean proportional increase of 7.5% (0.5-15%; p = .036) in the 6-mL/kg group compared with the 12-mL/kg group.
Association With Clinical Outcomes in ALI/ARDS
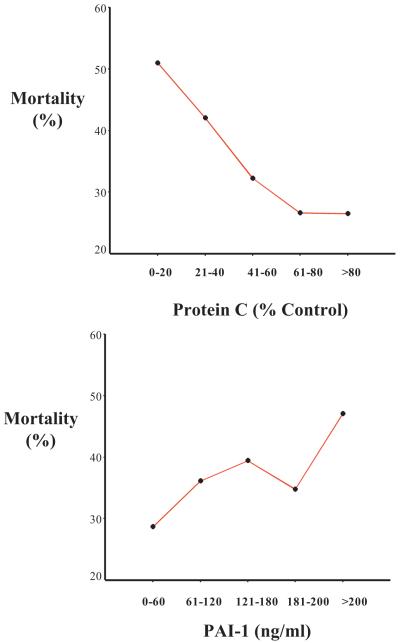
Lower levels of protein C and higher levels of PAI-1 were associated with hospital mortality (Fig. 1) (p < .05 for linear-dose-response trend for both protein C and PAI-1). In a bivariate analysis, lower levels of protein C by quartile and higher levels of PAI-1 by quartile were associated with higher mortality in ALI/ARDS (Table 4). In a multivariate analysis controlling for protein C, PAI-1, age, APACHE III, sepsis, and the tidal volume group, lower levels of protein C and higher levels of PAI-1 were strong independent predictors of mortality (Table 4). As an alternative, the multivariate analysis for mortality was repeated using protein C and PAI-1 as continuous variables. Controlling for age, APACHE III, sepsis, and ventilator group, the odds ratio per log10 increment of protein C was 0.42 (95% confidence interval [CI], 0.20-0.87) and the odds ratio per log10 increment of PAI-1 was 1.56 (95% CI, 1.08-2.25). The multivariate analysis also was repeated excluding the 207 patients with sepsis as the clinical risk factor for lung injury, and lower levels of protein C and higher levels of PAI-1 remained strong independent predictors of mortality (Table 5). Lower levels of protein C and higher levels of PAI-1 also were associated with both fewer ventilator-free days and fewer organ-failure-free days in multivariate analysis (Table 6).
Figure 1.
Association between plasma levels of protein C (top) and plasminogen activator inhibitor-1 (PAI-1) (bottom) and hospital mortality in 779 patients with acute lung injury/acute respiratory distress syndrome. p < .05 for linear-dose-response trend for both protein C and PAI-1.
Table 4.
Baseline protein C and plasminogen activator inhibitor-1 (PAI-1) levels and clinical outcomes among 779 patients with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS)
| Baseline Measurement | Risk of Death Unadjusted Odds Ratio (95% CI) |
Risk of Death Protein C and PAI-1 Mutually Adjusted Odds Ratio (95% CI) |
Risk of Death Multivariate Odds Ratio (95% CI) |
|---|---|---|---|
| Protein C level | |||
| Lowest quartile (referent) | 1.0 | 1.0 | 1.0 |
| Second quartile | 0.54 (0.35-0.81) | 0.55 (0.36-0.83) | 0.57 (0.36-0.90) |
| Third quartile | 0.52 (0.34-0.78) | 0.54 (0.36-0.82) | 0.60 (0.38-0.96) |
| Fourth quartile | 0.40 (0.26-0.61) | 0.43 (0.28-0.67) | 0.52 (0.32-0.85) |
| p value for linear trend across quartiles | <.0001 | <.0001 | <.0001 |
| PAI-1 level | |||
| Lowest quartile (referent) | 1.0 | 1.0 | 1.0 |
| Second quartile | 1.62 (1.04-2.53) | 1.20 (0.79-1.82) | 1.32 (0.80-2.20) |
| Third quartile | 2.04 (1.32-3.17) | 1.26 (0.83-1.92) | 2.16 (1.33-3.49) |
| Fourth quartile | 2.47 (1.60-3.82) | 1.92 (1.27-2.90) | 3.30 (2.06-5.29) |
| p value for linear trend across quartiles | <.0001 | .0023 | <.0001 |
CI, confidence interval.
The quartile values for protein C levels were: lowest quartile (5.2-32.3%), second quartile (32.4-46.3%), third quartile (46.4-63.5%), and fourth quartile (63.6-257%). For PAI-1, the quartile values were lowest quartile (9.0-41.3 ng/mL), second quartile (41.4-72.4 ng/mL), third quartile (72.5-161.0 ng/mL), and fourth quartile (161.1-4148 ng/mL). In the unadjusted analysis, protein C and PAI-1 were evaluated in separate logistic-regression models. In the mutually adjusted analysis, protein C and PAI-1 were included in the same logistic-regression model. The multivariate analysis includes protein C, PAI-1, age, Acute Physiology and Chronic Health Evaluation III, sepsis as the clinical risk factor for ALI/ARDS, and ventilator group.
Table 5.
Baseline protein C and plasminogen activator inhibitor-1 (PAI-1) levels and clinical outcomes among 572 nonseptic patients with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS)
| OR (95% CI) |
|||
|---|---|---|---|
| Baseline Measurement | Risk of Death Unadjusted |
Risk of Death Protein C and PAI-1 Mutually Adjusted |
Risk of Death Multivariate |
| Protein C level | |||
| Lowest quartile (referent) | 1.0 | 1.0 | 1.0 |
| Second quartile | 0.52 (0.31-0.87) | 0.52 (0.31-0.88) | 0.57 (0.32-1.003) |
| Third quartile | 0.46 (0.28-0.76) | 0.48 (0.29-0.80) | 0.53 (0.31-0.93) |
| Fourth quartile | 0.43 (0.26-0.70) | 0.44 (0.26-0.74) | 0.51 (0.29-0.91) |
| p value for linear trend across quartiles |
.0012 | .0043 | .033 |
| PAI-1 level | |||
| Lowest quartile (referent) | 1.0 | 1.0 | 1.0 |
| Second quartile | 1.34 (0.79-2.26) | 1.38 (0.81-2.35) | 1.22 (0.69-2.15) |
| Third quartile | 2.45 (1.47-4.06) | 2.43 (1.45-4.05) | 2.18 (1.25-3.78) |
| Fourth quartile | 2.00 (1.19-3.36) | 1.84 (1.08-3.12) | 1.54 (0.86-2.74) |
| p value for linear trend across quartiles |
.0012 | .0041 | .035 |
OR, odds ratio; CI, confidence interval.
In the unadjusted analysis, protein C and PAI-1 were evaluated in separate logistic-regression models. In the mutually adjusted analysis, protein C and PAI-1 were included in the same logistic-regression model. The multivariate analysis includes protein C, PAI-1, age, Acute Physiology and Chronic Health Evaluation III, sepsis as the clinical risk factor for ALI/ARDS, and ventilator group.
Table 6.
Baseline protein C and plasminogen activator inhibitor-1 (PAI-1) levels and ventilator-free and organ-failure-free days among 779 patients with acute lung injury and acute respiratory distress syndrome
| Mean Days (95% CI) |
||
|---|---|---|
| Baseline Measurement | Change in Ventilator-Free Days Compared with Lowest Quartile |
Change in Nonpulmonary- Organ-Failure-Free Days Compared With Lowest Quartile |
| Protein C level | ||
| Lowest quartile (referent) | 1.0 | 1.0 |
| Second quartile | 3.4 (1.4-5.4) | 3.0 (1.0-5.0) |
| Third quartile | 3.2 (1.2-5.3) | 3.8 (1.7-5.9) |
| Fourth quartile | 5.0 (2.9-7.2) | 4.7 (2.6-6.8) |
| p value for linear trend across quartiles |
<.0001 | <.0001 |
| PAI-1 level | ||
| Lowest quartile (referent) | 1.0 | 1.0 |
| Second quartile | -0.5 (-2.5-1.5) | 0.3 (-1.7-2.3) |
| Third quartile | -1.4 (-3.4-0.6) | -1.3 (-3.3-0.7) |
| Fourth quartile | -1.9 (-4.0-0.1) | -2.1 (-4.2 to -0.04) |
| p value for linear trend across quartiles |
.04 | .018 |
CI, confidence interval.
Mutually adjusted multivariate linear regression analysis including protein C, PAI-1, age, Acute Physiology and Chronic Health Evaluation III, sepsis, and ventilator group.
To evaluate the relationship to outcomes of changes in PAI-1 and protein C over the first 3 days, the effect of an increase in PAI-1 or protein C over time was considered in a multivariate model controlling for age, APACHE III score, and ventilator protocol. The adjusted odds ratio for death if protein C increased from day 0 to 3 was 0.71 (95% CI, 0.49-1.009; p = .056). The adjusted odds ratio if PAI-1 increased from day 0 to 3 was 1.66 (95% CI, 1.16-2.38; p = .006).
Combined Predictive Value of Protein-C and PAI-1 Levels in ALI/ARDS
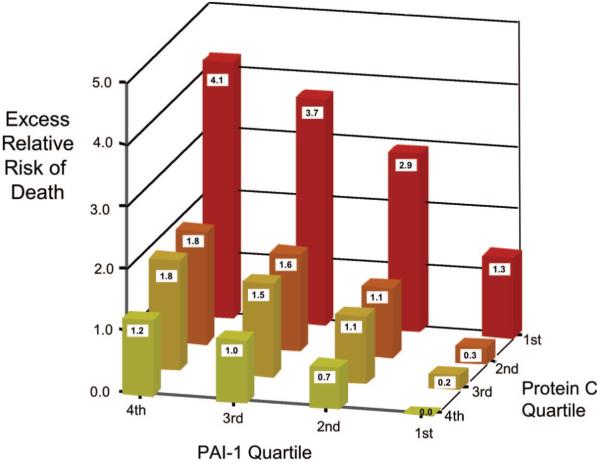
There was a statistically significant interaction between protein C and PAI-1 for mortality (p < .05). To evaluate whether levels of plasma protein C and PAI-1 were antagonistic or synergistic in terms of mortality, the excess relative risk of death was calculated for each combination of protein C and PAI-1 quartile (Fig. 2). On this additive scale, there was evidence of interaction of PAI-1 and protein C consistent with synergy. For example, as shown in Figure 2, the relative excess risk of death for the lowest quartile of protein C and highest quartile of PAI-1 was 4.14. This relative excess risk was greater than the simple sum of the relative excess risks for mortality in the most unfavorable quartiles of protein C (relative excess risk, 1.2) and PAI-1 (relative excess risk, 1.3) when examined independently (i.e., when coupled with the most favorable quartiles of PAI-1 and protein C).
Figure 2.
Combined effects of protein C and plasminogen activator inhibitor-1 (PAI-1) on mortality as measured by the excess relative risk of death. The probability of death was calculated from the logistic-regression model. The excess probability of death was calculated by subtracting the probability for the best combination of protein C (highest quartile) and PAI-1 (lowest quartile) (18, 19).
DISCUSSION
tk;4In a multicenter study of 779 patients with ALI/ARDS, both coagulation (as measured by plasma levels of protein C) and fibrinolysis (as measured by plasma levels of PAI-1) were markedly abnormal. This procoagulant, antifibrinolytic phenotype was present regardless of the underlying cause of lung injury, and similar alterations were not observed in severely ill patients with acute cardiogenic pulmonary edema. Furthermore, the alterations in plasma markers of coagulation and fibrinolysis in ALI/ARDS were strongly and independently associated with adverse clinical outcomes including death and fewer ventilator-free and organ-failure-free days. The combination of low levels of protein C and high levels of PAI-1 was particularly deleterious, with evidence of synergy between the two biomarkers as predictors of mortality. These findings indicate that alterations in systemic coagulation and fibrinolysis are not only prevalent in acute lung injury but may be pathogenetically important.
Findings in the current study can be compared with prior studies of patients with sepsis, a disorder that has many clinical similarities to ALI/ARDS, including the common occurrence of multiple organ failure (20). Both the coagulation and fibrinolytic pathways are abnormal in patients with severe sepsis and the severity of these abnormalities correlates with clinical outcomes (21-24). In the largest study to date (n = 70), Dr. Yan and colleagues (25) reported that protein-C levels in the plasma were reduced in severe sepsis with a mean level of 1.2 ± 0.5 μg/mL (normal range, 1.8-3.9 μg/mL). Our study used a different immunoassay for protein C with a median level (relative to normal pooled plasma) of 38% in the sepsis patients. Dr. Liaw and colleagues (26) have reported mean levels of protein C that were 48% ± 23% of control in 32 patients with severe sepsis, somewhat higher than the protein-C levels in our study. In the current study, abnormalities in protein-C and PAI-1 levels were more severe in the subgroup of 207 patients with sepsis as the cause of lung injury; however, there were still major abnormalities in both protein-C and PAI-1 levels when the sepsis patients were excluded. Furthermore, the prognostic value of protein C and PAI-1 was not substantively changed by exclusion of the sepsis patients from the analysis. These findings indicate that the abnormalities of coagulation and fibrinolysis were not simply a manifestation of sepsis, but are a feature of ALI/ARDS, regardless of the underlying cause.
Our findings suggest that there are alterations in coagulation and fibrinolysis in ALI/ARDS and that activated protein C, or other anticoagulant strategies, could potentially be of therapeutic benefit in this disease. This hypothesis is supported by recent evidence that thrombin formation can inhibit alveolar fluid clearance (27), a process that is critical to recovery from ALI/ARDS (28). It should be noted that no patient in the current study was treated with activated protein C. Information on the use of other anticoagulants was not available. Also of note is the fact that a protective ventilatory strategy did not have a major effect on levels of protein C and PAI-1 over the first 3 days, indicating that the mechanism of lung protection is probably mediated by other pathways. Activated protein C has not been tested prospectively in patients with ALI/ARDS from causes other than severe sepsis. In the Protein C Worldwide Evaluation in Severe Sepsis study of activated protein C (29), patients who had respiratory system dysfunction had a significantly shorter time to resolution of respiratory dysfunction in the treated group compared with the placebo group (30). A multicenter phase II clinical trial of activated protein C for the treatment of ALI/ARDS is currently enrolling patients (http://ClinicalTrials.gov, identifier NCT00112164), as is a multicenter clinical trial in Europe.
The synergistic effects of low protein C and high PAI-1 on prediction of mortality suggest that the combination of a procoagulant and antifibrinolytic is particularly deleterious. One potential explanation for this synergy is that a patient with ALI/ARDS may be able to compensate for low levels of protein C and the resultant generation of thrombin by increasing fibrinolysis. Conversely, if fibrinolysis is impaired by increased levels of PAI-1, activation of the protein-C system may compensate by reducing thrombin generation. However, if low levels of protein C and high levels of PAI-1 occur simultaneously, then this ability to compensate is lost. Activated protein C is also an inhibitor of PAI-1 activity (31, 32). Thus, the antifibrinolytic state associated with high levels of PAI-1 could be worsened by low levels of protein C, the inactive precursor of protein C. This explanation is attractive because recent evidence (33) indicates that PAI-1 impairs alveolar epithelial repair, a critical step in successful recovery from ALI/ARDS (28, 34). Identification of two plasma biomarkers that are synergistically associated with adverse clinical outcomes may ultimately be a useful tool for selection of patients with ALI/ARDS for enrollment in clinical trials of new therapies.
Although levels of PAI-1 were significantly higher and levels of protein C were significantly lower in ALI/ARDS compared with patients with cardiogenic pulmonary edema, levels in cardiogenic pulmonary edema were not normal. There are several possible explanations for this finding. PAI-1 release has been associated with ischemic cardiovascular disease and heart failure (35, 36); lower levels of protein C also are associated with ischemic cardiovascular disease (37-39). The presence of circulatory shock has been associated with lower levels of protein C in patients with ALI/ARDS (11). Shock also could contribute to abnormal levels of protein C in patients with cardiogenic pulmonary edema.
There are some limitations of the study. First, the plasma samples were stored for varying lengths of time before assaying for PAI-1 and protein C. However, samples were stored at -80°C until the assays were done and freeze-thaw cycles were minimized. Second, although the day-3 samples were all drawn at 8 am, the enrollment (day 0) samples were not drawn at the same time of day. Thus, we cannot rule out an effect of circadian rhythm, particularly on PAI-1 levels. Third, we measured only two proteins that are important in coagulation and fibrinolysis. More studies are needed to address the potential importance of other coagulation proteins, as well as to assay function of coagulation and fibrinolysis proteins in addition to antigen levels. Fourth, the comparison group of patients with cardiogenic pulmonary edema was not mechanically ventilated. Thus, we cannot rule out an effect of mechanical ventilation on the levels of protein C and PAI-1 before enrollment in the ARDS Network study. In our prior single-center studies of protein C and PAI-1 (11, 12), levels of both mediators were significantly different between mechanically ventilated patients with cardiogenic edema and those with ALI/ARDS, suggesting that mechanical ventilation in and of itself cannot explain the observed differences. Finally, the cardiogenic pulmonary edema samples were obtained and assayed several years after the ARDS Network trial samples, so caution must be used in directly comparing the results. However, the interval of storage at -80°C was similar for both sample sets and they were assayed by the same laboratory personnel using the same procedures and equipment.
CONCLUSIONS
In summary, ALI/ARDS is characterized by marked alterations in circulating levels of protein C and PAI-1, a pattern not seen in subjects with acute cardiogenic pulmonary edema. Both lower levels of protein C and higher levels of PAI-1 are independently associated with higher mortality and other clinical outcomes, including greater organ-system failure. In addition, the combination of low protein C and high PAI-1 carries an even higher risk of mortality. These important findings indicate that abnormalities in levels of coagulation and fibrinolysis proteins may be central to the pathogenesis and prognosis of ALI/ARDS and warrant further study.
Acknowledgments
Supported, in part, by NIH HL 70521 and NIH HL 081332 (LW), NIH HL 51856 and P50 HL74005 (MM), NIH HL 04201 (ME), and contracts NO-1 HR 46054, 46055, 46056, 46057, 46058, 46060, 46061, 46062, 46063, 46064 with the National Heart Lung and Blood Institute, Bethesda, MD.
APPENDIX
National Institutes of Health’s National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome Network
Network Participants: Cleveland Clinic Foundation: Herbert P. Wiedemann, MD*; Alejandro C. Arroliga, MD; Charles J. Fisher, Jr, MD; John J. Komara, Jr, BA, RRT; Patricia Periz-Trepichio, BS, RRT; Denver Health Medical Center: Polly E. Parsons, MD; Denver VA Medical Center: Carolyn Welsh, MD; Duke University Medical Center: William J. Fulkerson, Jr, MD*; Neil MacIntyre, MD; Lee Mallatratt, RN; Mark Sebastian, MD; John Davies, RRT; Elizabeth Van Dyne, RN; Joseph Govert, MD; Johns Hopkins Bayview Medical Center: Jonathan Sevransky, MD; Stacey Murray, RRT; Johns Hopkins Hospital: Roy G. Brower, MD; David Thompson, MS, RN; Henry E. Fessler, MD; LDS Hospital: Alan H. Morris, MD*; Terry Clemmer, MD; Robin Davis, RRT; James Orme, Jr, MD; Lindell Weaver, MD; Colin Grissom, MD; Frank Thomas, MD; Martin Gleich, MD†; McKay-Dee Hospital: Charles Lawton, MD; Janice D’Hulst, RRT; MetroHealth Medical Center of Cleveland: Joel R. Peerless, MD; Carolyn Smith, RN; San Francisco General Hospital Medical Center: Richard Kallet, MS, RRT; John M. Luce, MD; Thomas Jefferson University Hospital: Jonathan Gottlieb, MD; Pauline Park, MD; Aimee Girod, RN, BSN; Lisa Yannarell, RN, BSN; University of California, San Francisco: Michael A. Matthay, MD*; Mark D. Eisner, MD, MPH; John Luce, MD; Brian Daniel, RCP, RRT; Rich Kallet, MS, RRT; University of Colorado Health Sciences Center: Edward Abraham, MD*; Fran Piedalue, RRT; Rebecca Jagusch, RN; Paul Miller, MD; Robert McIntyre, MD; Kelley E. Greene, MD; University of Maryland: Henry J. Silverman, MD*; Carl Shanholtz, MD; Wanda Corral, BSN, RN; University of Michigan: Galen B. Toews, MD*; Deborah Arnoldi, MHSA; Robert H. Bartlett, MD; Ron Dechert, RRT; Charles Watts, MD; University of Pennsylvania: Paul N. Lanken, MD*; Harry Anderson, III, MD; Barbara Finkel, MSN, RN; C. William Hanson, III, MD; University of Utah Hospital: Richard Barton, MD; Mary Mone, RN; University of Washington/Harborview Medical Center: Leonard D. Hudson, MD*; Greg Carter, RRT; Claudette Lee Cooper, RN; Annemieke Hiemstra, RN; Ronald V. Maier, MD; Kenneth P. Steinberg, MD; Utah Valley Regional Medical Center: Tracy Hill, MD; Phil Thaut, RRT; Vanderbilt University: Arthur P. Wheeler, MD*; Gordon Bernard, MD*; Brian Christman, MD; Susan Bozeman, RN; Linda Collins; Teresa Swope, RN; Lorraine B. Ware, MD.
Clinical Coordinating Center: Massachusetts General Hospital, Harvard Medical School: David A. Schoenfeld, PhD*; B. Taylor Thompson, MD; Marek Ancukiewicz, PhD; Douglas Hayden, MA; Francine Molay, MSW; Nancy Ringwood, BSN, RN; Gail Wenzlow, MSW, MPH; Ali S. Kazeroonin, BS.
NHLBI Staff: Dorothy B. Gail, PhD; Andrea Harabin, PhD*; Pamela Lew; Myron Waclawiw, PhD.
Steering Committee: Gordon R. Bernard, MD, chairman, and the principal investigators from each center indicated by asterisk, above.
Data and Safety Monitoring Board: Roger G. Spragg, MD, chairman; James Boyett, PhD; Jason Kelley, MD; Kenneth Leeper, MD; Marion Gray Secundy, PhD; Arthur Slutsky, MD.
Protocol Review Committee: Joe G. N. Garcia, MD, chairman; Scott S. Emerson, MD, PhD; Susan K. Pingleton, MD; Michael D. Shasby, MD; William J. Sibbald, MD.
Footnotes
See also p. 1980.
The authors have not disclosed any potential conflicts of interest.
Principal investigator also belonging to steering committee.
Deceased.
REFERENCES
- 1.Ware LB, Matthay MA. Medical progress: The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Brower RG, Ware LB, Berthiaume Y, et al. Treatment of ARDS. Chest. 2001;120:1347–1367. doi: 10.1378/chest.120.4.1347. [DOI] [PubMed] [Google Scholar]
- 3.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 4.Brun-Buisson C, Minelli C, Bertolini G, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Intensive Care Med. 2004;30:51–61. doi: 10.1007/s00134-003-2022-6. [DOI] [PubMed] [Google Scholar]
- 5.Idell S, Gonzalez K, Bradford H, et al. Procoagulant activity in bronchoalveolar lavage in the adult respiratory distress syndrome. Am Rev Respir Dis. 1987;136:1466–1474. doi: 10.1164/ajrccm/136.6.1466. [DOI] [PubMed] [Google Scholar]
- 6.Idell S, Koenig K, Fair D, et al. Serial abnormalities of fibrin turnover in evolving adult respiratory distress syndrome. Am J Physiol. 1991;261:L240–L248. doi: 10.1152/ajplung.1991.261.4.L240. [DOI] [PubMed] [Google Scholar]
- 7.Bertozzi P, Astedt B, Zenzius L, et al. Depressed bronchoalveolar urokinase activity in patients with adult respiratory distress syndrome. N Engl J Med. 1990;322:890–897. doi: 10.1056/NEJM199003293221304. [DOI] [PubMed] [Google Scholar]
- 8.Abraham E. Coagulation abnormalities in acute lung injury and sepsis. Am J Respir Cell Mol Biol. 2000;22:401–404. doi: 10.1165/ajrcmb.22.4.f184. [DOI] [PubMed] [Google Scholar]
- 9.Coughlin SR. Thrombin signaling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 10.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 11.Ware LB, Fang X, Matthay MA. Protein C and thrombomodulin in human acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;285:L514–L521. doi: 10.1152/ajplung.00442.2002. [DOI] [PubMed] [Google Scholar]
- 12.Prabhakaran P, Ware L, White K, et al. Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;285:L20–L28. doi: 10.1152/ajplung.00312.2002. [DOI] [PubMed] [Google Scholar]
- 13.Pittet JF, MacKersie RC, Martin TR, et al. Biological markers of acute lung injury: Prognostic and pathogenetic significance. Am J Respir Crit Care Med. 1997;155:1187–1205. doi: 10.1164/ajrccm.155.4.9105054. [DOI] [PubMed] [Google Scholar]
- 14.Knaus W, Wagner D, Draper E, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 15.Eisner M, Thompson T, Hudson L, et al. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:231–236. doi: 10.1164/ajrccm.164.2.2011093. [DOI] [PubMed] [Google Scholar]
- 16.Januzzi JL, Jr, Camargo CA, Anwaruddin S, et al. The N-terminal pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95:948–954. doi: 10.1016/j.amjcard.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 17.Rothman KJ, Greenland S. Modern Epidemiology. Lippincott-Raven; Philadelphia, PA: 1998. [Google Scholar]
- 18.Rothman K, Keller A. The effect of joint exposure to alcohol and tobacco on risk of cancer of the mouth and pharynx. J Chronic Dis. 1972;25:711–716. doi: 10.1016/0021-9681(72)90006-9. [DOI] [PubMed] [Google Scholar]
- 19.Cole P, MacMahon B. Attributable risk percent in case control studies. Br J Prev Soc Med. 1971;25:242–244. doi: 10.1136/jech.25.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wheeler AP, Bernard GR. Treating patients with severe sepsis. N Engl J Med. 1999;340:207–214. doi: 10.1056/NEJM199901213400307. [DOI] [PubMed] [Google Scholar]
- 21.Kinasewitz GT, Yan SB, Basson B, et al. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative microorganism. Crit Care. 2004;8:R82–R90. doi: 10.1186/cc2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fourrier F, Chopin C, Goudemand J, et al. Septic shock, multiple organ failure, and disseminated intravascular coagulation: Compared patterns of antithrombin III, protein C, and protein S deficiencies. Chest. 1992;101:816–823. doi: 10.1378/chest.101.3.816. [DOI] [PubMed] [Google Scholar]
- 23.Lorente JA, Garcia-Frade LJ, Landin L, et al. Time course of hemostatic abnormalities in sepsis and its relation to outcome. Chest. 1993;103:1536–1542. doi: 10.1378/chest.103.5.1536. [DOI] [PubMed] [Google Scholar]
- 24.Bauer PR. Microvascular responses to sepsis: Clinical significance. Pathophysiology. 2002;8:141–148. doi: 10.1016/s0928-4680(02)00007-x. [DOI] [PubMed] [Google Scholar]
- 25.Yan SB, Helterbrand JD, Hartman DL, et al. Low levels of protein C are associated with poor outcome in severe sepsis. Chest. 2001;120:915–922. doi: 10.1378/chest.120.3.915. [DOI] [PubMed] [Google Scholar]
- 26.Liaw PC, Esmon CT, Kahnamoui K, et al. Patients with severe sepsis vary markedly in their ability to generate activated protein C. Blood. 2004;104:3958–3964. doi: 10.1182/blood-2004-03-1203. [DOI] [PubMed] [Google Scholar]
- 27.Vadasz I, Morty RE, Olschewski A, et al. Thrombin impairs alveolar fluid clearance by promoting endocytosis of Na+,K+-ATPase. Am J Respir Cell Mol Biol. 2005;33:343–354. doi: 10.1165/rcmb.2004-0407OC. [DOI] [PubMed] [Google Scholar]
- 28.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 29.Bernard G, Vincent J-L, Laterre P-F, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 30.Dhainaut J-F, Laterre P-F, Janes JM, et al. Drotrecogin alfa (activated) in the treatment of severe sepsis patients with multiple-organ dysfunction: Data from the PROWESS trial. Intensive Care Med. 2003;29:894–903. doi: 10.1007/s00134-003-1731-1. [DOI] [PubMed] [Google Scholar]
- 31.Sakata Y, Curriden S, Lawrence D, et al. Activated protein C stimulates the fibrinolytic activity of cultured endothelial cells and decreases antiactivator activity. Proc Natl Acad Sci U S A. 1985;82:1121–1125. doi: 10.1073/pnas.82.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Hinsbergh VW, Bertina RM, van Wijngaarden A, et al. Activated protein C decreases plasminogen activator-inhibitor activity in endothelial cell-conditioned medium. Blood. 1985;65:444–451. [PubMed] [Google Scholar]
- 33.Lazar MH, Christensen PJ, Du M, et al. Plasminogen activator inhibitor-1 impairs alveolar epithelial repair by binding to vitronectin. Am J Respir Cell Mol Biol. 2004;31:672–678. doi: 10.1165/rcmb.2004-0025OC. [DOI] [PubMed] [Google Scholar]
- 34.Bachofen M, Weibel ER. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am Rev Respir Dis. 1977;116:589–615. doi: 10.1164/arrd.1977.116.4.589. [DOI] [PubMed] [Google Scholar]
- 35.Juhan-Vague I, Pyke SD, Alessi MC, et al. ECAT Study Group Fibrinolytic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. Circulation. 1996;94:2057–2063. doi: 10.1161/01.cir.94.9.2057. European Concerted Action on Thrombosis and Disabilities. [DOI] [PubMed] [Google Scholar]
- 36.Collet JP, Montalescot G, Vicaut E, et al. Acute release of plasminogen activator inhibitor-1 in ST-segment elevation myocardial infarction predicts mortality. Circulation. 2003;108:391–394. doi: 10.1161/01.CIR.0000083471.33820.3C. [DOI] [PubMed] [Google Scholar]
- 37.Pelkonen KM, Wartiovaara-Kautto U, Nieminen MS, et al. Low normal level of protein C or of antithrombin increases risk for recurrent cardiovascular events. Blood Coagul Fibrinolysis. 2005;16:275–280. doi: 10.1097/01.mbc.0000169220.00679.13. [DOI] [PubMed] [Google Scholar]
- 38.ECAT Angina Pectoris Study Group ECAT angina pectoris study: Baseline associations of haemostatic factors with extent of coronary arteriosclerosis and other coronary risk factors in 3000 patients with angina pectoris undergoing coronary angiography. Eur Heart J. 1993;14:8–17. doi: 10.1093/eurheartj/14.1.8. [DOI] [PubMed] [Google Scholar]
- 39.Sargento L, Do Rosario HS, Perdigao C, et al. Long-term prognostic value of the hemorheological profile in transmural myocardial infarction survivors: 60-month clinical follow-up. Rev Port Cardiol. 2002;21:1263–1275. [PubMed] [Google Scholar]