Abstract
Prolonged anoxia has deleterious effects on islets. Gas-permeable cell culture devices can be used to minimize anoxia during islet culture and especially during shipment when elimination of gas-liquid interfaces is required to prevent the formation of damaging gas bubbles. Gas-permeable bags may have several drawbacks, such as propensity for puncture and contamination, difficult islet retrieval, and significantly lower oxygen permeability than silicone rubber membranes (SRM). We hypothesized that oxygen permeability of bags may be insufficient for islet oxygenation. We measured oxygen transmission rates through the membrane walls of three different types of commercially available bags and through SRM currently used for islet shipment. We found that the bag membranes have oxygen transmission rates per unit area about 100-fold lower than SRM. We solved the oxygen diffusion-reaction equation for 150-μm diameter islets seeded at 3000 islet equivalents per cm2, a density adequate to culture and ship an entire human or porcine islet preparation in a single gas-permeable device, predicting that about 40% of the islet volume would be anoxic at 22°C and about 70% would be anoxic at 37°C. Islets of larger size or islets accumulated during shipment would be even more anoxic. The model predicted no anoxia in islets similarly seeded in devices with SRM bottoms. We concluded that commercially available bags may not prevent anoxia during islet culture or shipment; devices with SRM bottoms are more suitable alternatives.
Isolated islets are especially susceptible to damage from anoxia due to their large size relative to single cells, high oxygen consumption rate (OCR), and low levels of enzymes necessary for energy production under anaerobic conditions.1 Effects of short periods of anoxia may be reversible,2 but as few as 6 hours of anoxia can result in extensive and irreversible damage to islets.2–4 Islets cultured at high surface densities in T-flasks also exhibit low viable tissue recovery, viability, and potency.5–8 These effects can be prevented by culturing islets in gas-permeable (GP) devices, which increase oxygen availability to islets.5,7,8
Oxygen solubility in culture media is low. The oxygen content per unit volume in media is only about 2% to 4% of that in air, depending on the temperature. It is estimated that when fully viable islets are cultured at the standard density of 1000 islet equivalents (IE)/mL, the oxygen dissolved in media equilibrated with air is depleted in less than 1 hour at 37°C, if oxygen is not replenished from the ambient atmosphere. This is particularly important during shipment when a gas headspace in the devices is avoided, because it may cause gas bubble formation that potentially damages the islets by shear stress, as observed with cells in other systems.9 Islet shipping devices (tubes, T-flasks, bags, bottles) are filled with media and caps tightened to eliminate the gas phase, leaving the islets to rely only on the oxygen dissolved in the media for oxygenation. Cooling decreases islet OCR and increases the medium oxygen solubility, and using lower islet volume densities increases the amount of dissolved oxygen per IE. However, not all dissolved oxygen is necessarily readily available to the islets as the media may not be well mixed and thus oxygen partial pressure (pO2) gradients may develop in the media. These gradients depend on the level of convection in the media inside the shipping device. Ideally, shipping conditions should simulate optimal, accepted culture conditions, including temperature. Based on current standard culture practice, islets should be shipped at 22°C or 37°C, depending on the donor species, rather than at colder temperatures, especially because islet viability, function, or recovery upon return to standard culture or in vivo temperatures have not been well characterized for lower shipping temperatures.
To alleviate the problem of low and decreasing oxygen availability during even express shipment, commercially available GP cell culture bags are being used by some isolation centers for shipping islets. However, cell culture bags are not tailored specifically to islet culture or shipping. As a result, besides their general drawbacks, such as propensity for puncture and contamination during tissue transfer, bags have drawbacks that are specific to islets, including difficulty in islet retrieval and insufficient tissue oxygenation.
Significant pO2 gradients develop inside isolated islets owing to their large size. In fully viable, 150-μm diameter islets, a 30 to 40 mm Hg pO2 gradient may develop between the islet surface and its center. For larger islets, the magnitude of this gradient increases with the square of the islet diameter.10 In addition to the internal pO2 gradients, when islets are seeded at high surface densities their high oxygen demand can result in fast depletion of the oxygen dissolved in the media and in development of significant steady-state pO2 gradients outside the islets. The size of the pO2 gradient across the bag membrane on which the islets have settled is inversely proportional to the membrane oxygen permeability, Pmem. Although Pmem for GP membranes of commercially available bags is sufficiently high for adequately oxygenating individual cells, it may be suboptimal for large cell clusters, like islets. The cumulative effect of the internal and external gradients is that islet cores can become anoxic under culture or shipping conditions in which single cells would be fully oxygenated. The potential shortage of oxygen for islets in bags can be exacerbated by a number of factors: potential obstruction of the access of ambient gas to the GP membrane on which islets reside; formation of wrinkles and “valleys” in the pliable membrane material in which islets can accumulate; and displacement from the horizontal position of the bag during shipment, leading to islets getting trapped in ports or accumulating on the narrow side of the bag.
We investigated the possibility that other device configurations and GP membranes with higher Pmem, such as silicone rubber membranes (SRM), might be more suitable for islet culture and shipment by means of increasing the oxygen transmission rate per unit area of membrane (OTR).
MATERIALS AND METHODS
Commercially available GP cell culture bags currently used by investigators for islet shipments were used for the OTR tests reported. The three types of tested bags were: VueLife 2PF-0290 (American Fluoroseal Corporation, Gaithersburg, Md) and PermaLife (various sizes, OriGen Biomedical, Austin, Tex), both made of fluorinated ethylene propylene (FEP) copolymers; and LIFECELL (3-L bag, Baxter, Deerfield, Ill) made of proprietary polyolefin blends. We also investigated SRM used as GP bottoms for the commercially available 500-mL capacity devices (Wilson Wolf, New Brighton, Minn) that are currently used at our site for islet culture and shipment. OTR were measured according to ASTM F-1927 standards by MOCON Testing Service (Minneapolis, Minn) using two different samples from each material. Measurements were conducted at 22°C and 37°C, using the same two samples at both temperatures for consistency. Pure oxygen at 1 atm and 85% relative humidity diffused through the membranes and was removed by and measured in a nitrogen/hydrogen mixture with virtually zero pO2. The pO2 on the oxygen side was estimated from psychrometric charts to account for the water vapor partial pressure, pH2O. The uncompressed thickness L of all membranes was measured with a dial caliper, a digital optical comparator, and a drop gauge with a dial indicator. All thickness measurements were comparable, but those deemed most accurate and reported were those taken with the optical comparator. The effective Pmem for each membrane was calculated from the OTR, L, and the ΔpO2 across the membrane. Pmem values at different temperatures were fitted to Arrhenius-type functions to derive the effective activation energy for Pmem for each material that can be then used to estimate Pmem at other temperatures.
We used the finite elements software package COMSOL (COMSOL, Inc., Burlington, Mass) to solve the oxygen diffusion-reaction equation. This method has been previously applied for the modeling of islet oxygenation in vitro and in vivo.11,12 We derived the steady-state pO2 profiles at 22°C and 37°C and fully humidified 95% air/5% CO2 ambient atmosphere for pure islets seeded at 3000 IE/cm2. OCR was assumed to follow Michaelis-Menten kinetics with Km = 0.44 mm Hg and to cease at a local pO2 = 0.1 mm Hg at all temperatures.13 Islet tissue exposed to 0.1 mm Hg local pO2 was characterized as anoxic. A value for OCR at high pO2 levels of Vmax = 50 nmol/cm3 · s was assumed at 37°C, which is representative of human and porcine islet preparations with high viability measured at the UMN. Vmax was adjusted for temperature using an Arrhenius-type function with E/R = 7500 K, derived from OCR measurements at different temperatures. An accurate 2D, axisymmetric approximation of the 3D geometry, maintaining the same islet surface density, was defined to reduce the high computational load, partly related to the assumed discontinuity in OCR at pO2 = 0.1 mm Hg that makes convergence difficult.11 In bags, oxygen transfer was assumed to occur from both the lower and upper membrane by diffusion only (medium assumed to be stagnant). Medium depth in bags was assumed to be 1 cm. In SRM devices, oxygen transfer was assumed to occur only through the SRM bottom, making steady-state oxygen profiles independent of medium depth. Islets were assumed to be spherical with 150-μm diameter and homogeneously dispersed throughout the membrane in a square array conformation. We used oxygen permeability values of 35.3 fmol/(cm · mm Hg · s) in media and 12.4 fmol/(cm · mm Hg · s) in islet tissue at 37°C,14 adjusted for temperature using an Arrhenius-type function with E/R = 954 K, derived from the combined effect of temperature on oxygen diffusivity and solubility.
RESULTS
Table 1 summarizes the OTR and membrane thickness measurements for the three commercially available bags and the devices with SRM bottoms. OTR was found to be similar for all bags, both at 22°C (92 to 129 cc/100 in2 · day) and 37°C (171 to 218 cc/100 in2 · day), with the OriGen bag having the highest OTR at both temperatures. Values at 22°C were in reasonably good agreement with the values provided by manufacturers at 23 to 25°C (American Fluoroseal website, DuPont literature referenced in OriGen website15). In sharp contrast, the SRM had an OTR 100 to 140 times higher at 22°C (12,610 cc/100 in2 · day) and 65 to 85 times higher at 37°C (14,322 cc/100 in2 · day). The coefficient of variation (CV) for OTR measured for the two samples was less than 6% in all eight cases (four materials at two temperatures). The replicate CV for the same sample was determined only for one of the two SRM samples at both temperatures and was found to be 6% to 8% for two to three replicate measurements.
Table 1.
Measured Values of Oxygen Transmission Rate Per Unit Area (OTR) and Membrane Thickness for Three Commercially Available Bags (from American Fluoroseal, Baxter, and OriGen) and Silicone Rubber Membranes (SRM) Used as Bottoms in Commercially Available Devices (from Wilson Wolf)
| OTR [cc/(100 in2 · day)] |
|||||
|---|---|---|---|---|---|
| Company | Product | Material | Thickness (10−3 in) | 22°C | 37°C |
| American Fluoroseal | VueLife 2PF-0290 | FEP | 5.4 ± 0.4 | 103.6 ± 2.6 | 171.1 ± 7.0 |
| Baxter | LIFECELL | Polyolefin Blend | 12.5 ± 0.3 | 92.4 ± 2.5 | 184.3 ± 2.2 |
| OriGen | PermaLife Series | FEP | 4.9 ± 0.1 | 129.3 ± 0.3 | 217.8 ± 5.3 |
| Wilson Wolf | PR-5-0003-2 | Silicone Rubber | 7.4 ± 0.1 | 12610 ± 473 | 14322 ± 811 |
Although the polyolefin Baxter bag had a calculated Pmem more than double that of the two FEP bags, it had the lowest OTR at 22°C and the second lowest OTR at 37°C among the three commercially available bags, due to its much larger thickness. The SRM’s thickness (185 to 190 μm) was larger than that of the two FEP bags (125 to 145 μm measured, 127 μm advertised) and about 60% of the Baxter bag thickness (311 to 323 μm measured). Therefore, the ~100-fold difference in OTR was due to the SRM’s much higher Pmem.
The SRM’s calculated Pmem was higher than that of the three commercially available bags by a factor of 70 to 170 at 22°C and 45 to 115 at 37°C. Earlier OTR measurements for SRM of different thicknesses (4 to 10 thousandths of an inch or 101.6 to 254 μm) verified that the oxygen diffusive resistance of the SRM varies linearly with its thickness (UMN, unpublished data).
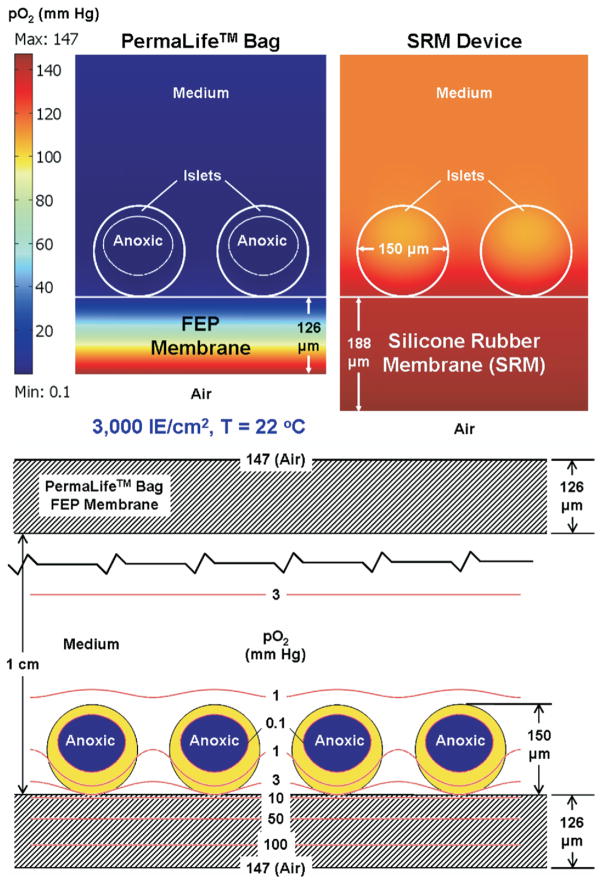
Using the measured and estimated parameters, we determined the steady-state pO2 profiles across the membranes, in the medium, and inside the islets for the chosen islet surface density of 3000 IE/cm2. For the three commercially available bags, the islet volume fraction predicted to be anoxic was 37% to 43% at 22°C and 67% to 70% at 37°C. Minimum pO2 in the islets was 0.1 mm Hg, as imposed by the model assumptions. The predicted maximum pO2 in the islets ranged for the three bags from 1.7 to 3.0 mm Hg at 22°C and from 1.5 to 2.2 mm Hg at 37°C. In contrast, no anoxia was predicted with the SRM, despite oxygen being supplied just from the bottom. The predicted pO2 in the islets ranged from 107 to 141 mm Hg at 22°C and from 26 to 123 mm Hg at 37°C. The upper panel of Figure 1 compares the predicted oxygen pO2 profiles at 22°C for the OriGen bag that had the highest OTR of all three bags to those for the device with the SRM bottom. The lower panel of Figure 1 shows contour plots for the case of the OriGen bag.
Fig 1.
Upper panel. Predicted oxygen partial pressure (pO2) surface plots in mm Hg at 22°C in OriGen’s PermaLife bags and in Wilson Wolf devices with silicone rubber membrane (SRM) bottoms, seeded with islets at 3000 IE/cm2. Predicted pO2 levels to which islets are exposed in American Fluoroseal and Baxter bags or at higher temperatures are even lower (not shown). Lower panel. pO2 contour plots in the vicinity of the islets for the OriGen bag case shown in the upper panel.
DISCUSSION
Recent efforts by the Islet Cell Resources (ICR) consortium focused on controlling and standardizing islet shipping temperature conditions. Controlling pressure and pO2 of the ambient gas has also been proposed by the UMN ICR center as necessary for good process control during shipment. Another issue to be considered is the practicality of shipping islets at extremely low islet densities, even when GP bags are utilized. For example, the University of Wisconsin has been shipping no more than 5000 to 20,000 IE per OriGen bag, depending on the bag size, at surface densities below 35 to 55 IE/cm2 (Matthew Hanson, University of Wisconsin, personal communication). The University of Miami has shipped 50,000 to 200,000 IE per Baxter bag, depending on the bag size, at a target surface density of around 270 IE/cm2, with both total IE number and IE surface density adjusted downward for impure preparations.16 Shipping islets at such low surface densities necessitates the use of multiple bags for some research and for all potential clinical shipments.
Islet shipment in GP bags is an improvement over shipment in gas-impermeable tubes or T-flasks. However, islet accumulation has been observed in the center of bags during culture (Matthew Hanson, University of Wisconsin, personal communication) and is even more likely to occur during shipment. Racks used for transporting multiple bags to allow oxygen access16 could cause valleys in the pliable material, resulting in localized islet accumulation. Such accumulation can be simulated to some extent by assuming an increase in local islet surface density close to that of a confluent islet monolayer in a square array conformation (4444 IE/cm2 for 150-μm diameter islets). Accumulated islets could even surpass that surface density by packing even tighter in a monolayer (e.g., hexagonal array) or by forming multilayers. Furthermore, with a 270 IE/cm2 surface density, a high-purity preparation of 500,000 IE would require a total footprint of 1850 cm2. For impure preparations, the required footprint would be even higher. Cooling during shipment can reduce the islet oxygen requirements. However, cooling the islets constitutes a significant deviation from standard islet culture conditions, which is generally undesirable. Improving oxygen supply is another approach that could reduce variability and problems related to islet accumulation during shipment.
Our model predictions, using the Pmem values we measured, suggest that devices with SRM bottoms can adequately oxygenate islets seeded at high surface densities. Independent culture studies that validated the model entailed comparison of standard culture of human5 and porcine7,8 islets at 200 IE/cm2 and 1000 IE/mL in T-flasks (control) to culture in devices with SRM bottoms at 1000 to 4000 IE/cm2 and 130 to 1000 IE/mL. In these studies, islet viability and viable tissue recovery were assessed based on OCR/DNA and total OCR measurements, respectively. Potency was assessed based on diabetes reversal rates in streptozotocin-induced diabetic nude mice after transplantation of 2000 IE under the kidney capsule. Islet viability and nude mouse diabetes reversal rates were similar in the high-density SRM and the control groups. Viable tissue recovery was statistically higher for high-density culture on SRM relative to control for both 2-day8 and 7-day culture17 (P = .04 in both cases), despite the high variability observed.
Based on the present data and analysis, we conclude that using a commercially available 500 mL, 100 cm2 footprint device with a SRM bottom (Wilson Wolf, New Brighton, Minn), in combination with a gyroscopic container, could make shipping an entire high-purity human or porcine islet preparation of up to 400,000 IE in a single device possible. The SRM bottom in this device is supported and flat and the gyroscopic container would keep the device horizontal during shipment, thereby ensuring that islets reside directly upon the SRM at high pO2. The device would be filled with media to minimize liquid movement. Oxygenation only through the bottom membrane is adequate in such devices, allowing higher media heights that can be exploited to extend culture and shipping time without media changes. Larger footprint SRM devices are under development for larger and/or impure islet preparations, whereas smaller footprint devices are available for research islet shipments. Surrounding the SRM device or the gyroscopic shipping container with phase-change materials could provide temperature control.18 The gyroscopic container could also be designed to create an air-tight seal that locks in the ambient conditions present during the placement of the SRM device inside the gyroscopic container, such as atmospheric pO2, 5% CO2, and high relative humidity (or other conditions determined to be optimal during the culture stage). In this configuration, elevating the ambient pO2 above atmospheric can be also accomplished. With sufficient oxygen and nutrient supply to the islets and control of ambient conditions, islet shipment could become a mere extension of islet culture.
The ability to place islets in a highly oxygenated environment, without the problems of random islet accumulation and limited Pmem associated with GP bags, creates a superior overall shipping process and justifies shipping both research and clinical islets in single SRM devices rather than multiple GP bags. This is important for shipment of fractions of research preparations and even more so for shipment of entire islet clinical preparations.
Acknowledgments
We are thankful for the financial support from NIH (NCRR and NIDDK), JDRF, the Iacocca Foundation, the Schott Foundation, and the Carol Olson Memorial Diabetes Research Fund. We also thank Matthew Hanson (University of Wisconsin) for helpful discussions and for providing extra OriGen bags for assessment of thickness variability; American Fluoroseal and OriGen for providing free samples of their commercially available bags; Michael Kragness of MOCON Testing Service for OTR and thickness measurements; and Denice Dudero, Laurie Macleod, Dr. Kristen Maynard, Heather Nelson, Christine Vincent, and Gina Wildey (UMN, DIIT) for administrative help.
Supported by grants from the NIH (NCRR U42 RR016598 and NIDDK R43 DK069865), the JDRF (7-2005-1167 and 21-2006-881), the Iacocca Foundation, the Schott Foundation, and the Carol Olson Memorial Diabetes Research Fund.
References
- 1.Jijakli H, Rasschaert J, Nadi AB, et al. Relevance of lactate dehydrogenase activity to the control of oxidative glycolysis in pancreatic islet β-cells. Arch Biochem Biophys. 1996;327:260. doi: 10.1006/abbi.1996.0119. [DOI] [PubMed] [Google Scholar]
- 2.Papas KK, Colton CK, Gounarides JS, et al. NMR spectroscopy in β cell engineering and islet transplantation. Ann NY Acad Sci. 2001;944:96. doi: 10.1111/j.1749-6632.2001.tb03826.x. [DOI] [PubMed] [Google Scholar]
- 3.Ichii H, Inverardi L, Pileggi A, et al. A novel method for the assessment of cellular composition and beta-cell viability in human islet preparations. Am J Transplant. 2005;5:1635. doi: 10.1111/j.1600-6143.2005.00913.x. [DOI] [PubMed] [Google Scholar]
- 4.Papas KK, Bauer AC, Avgoustiniatos ES, et al. Vitamin E homologs protect cultured islets from anoxia-induced death: implications in islet processing and engraftment. Diabetologia. 2005;48(Suppl 1):A185. [Google Scholar]
- 5.Papas KK, Avgoustiniatos ES, Tempelman LA, et al. High-density culture of human islets on top of silicone rubber membranes. Transplant Proc. 2005;37:3412. doi: 10.1016/j.transproceed.2005.09.086. [DOI] [PubMed] [Google Scholar]
- 6.Olsson R, Carlsson P-O. Oxygenation of cultured pancreatic islets. Adv Exp Med Biol. 2006;578:263. doi: 10.1007/0-387-29540-2_42. [DOI] [PubMed] [Google Scholar]
- 7.Papas KK, Hering BJ, Rozak PR, et al. Improved islet culture on top of gas-permeable membranes. Am J Transplant. 2007;7(Suppl 2):568. [Google Scholar]
- 8.Avgoustiniatos ES, Hering BJ, Rozak PR, et al. Islet culture at high surface densities on top of silicone rubber membranes compares favorably to standard culture. Xenotransplantation. 2007;14:489. [Google Scholar]
- 9.Ma N, Chalmers JJ, Aunins JG, et al. Quantitative studies of cell-bubble interactions and cell damage at different pluronic F-68 and cell concentrations. Biotechnol Prog. 2004;20:1183. doi: 10.1021/bp0342405. [DOI] [PubMed] [Google Scholar]
- 10.Avgoustiniatos ES, Colton CK. Design considerations in immunoisolation. In: Lanza RP, Langer R, Chick WL, editors. Principles of tissue engineering. Austin, Tex: RG Landes; 1997. p. 333. [Google Scholar]
- 11.Avgoustiniatos ES. PhD Thesis, Massachusetts Institute of Technology. Cambridge, Mass: 2002. Oxygen diffusion limitations in pancreatic islet culture and immunoisolation. [Google Scholar]
- 12.Fraker CA, Álvarez S, Papadopoulos P, et al. Enhanced oxygenation promotes beta cell differentiation in vitro. Stem Cells. 2007 doi: 10.1634/stemcells.2007-0445. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Avgoustiniatos ES, Colton CK. Effect of external oxygen mass transfer resistances on viability of immunoisolated tissue. Ann N Y Acad Sci. 1997;831:145. doi: 10.1111/j.1749-6632.1997.tb52192.x. [DOI] [PubMed] [Google Scholar]
- 14.Avgoustiniatos ES, Dionne KE, Wilson DF, et al. Measurements of the effective diffusion coefficient of oxygen in pancreatic islets. Ind Eng Chem Res. 2007;46:6157. [Google Scholar]
- 15.Teflon FEP Film. DuPont Bulletin E-80412 [Google Scholar]
- 16.Ichii H, Sakuma Y, Pileggi A, et al. Shipment of human islets for transplantation. Am J Transplant. 2007;7:1010. doi: 10.1111/j.1600-6143.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- 17.Papas KK, Avgoustiniatos ES, Rozak PR, et al. Seven-day culture enhances potency and reduces immunogenicity of porcine islets. Xenotransplantation. 2007;14:420. [Google Scholar]
- 18.Rozak PR, Avgoustiniatos ES, Weegman BP, et al. Phase change material maintains temperature control in an islet shipping container. Xenotransplantation. 2007;14:489. [Google Scholar]