Abstract
Background
Untreated HIV infection may increase risk for cardiovascular disease, and arterial elasticity is a marker of cardiovascular risk and early disease.
Methods
HIV-infected participants not taking antiretroviral therapy (n = 32) were compared with HIV-negative controls (n = 30). Large and small artery elasticity (LAE and SAE) were estimated via analysis of radial pulse waveforms. Differences in LAE and SAE by HIV status were compared using analysis of covariance, with and without adjustment for Framingham risk (model 1); covariates that differed between groups [smoking, injection drug use, hepatitis C, and high-density lipoprotein cholesterol (HDLc); model 2]; or age, sex, race/ethnicity, smoking, injection drug use, hepatitis C, HDLc, and non-HDLc (model 3).
Results
HIV infection was associated with impaired LAE (−2.55 mL/mm Hg × 10; P = 0.02) and SAE (−1.50 mL/mm Hg × 100; P = 0.02). Associations with traditional risk factors were often stronger for SAE than LAE, including with Framingham score (per 1% higher; SAE −0.18, P = 0.01; LAE −0.19, P = 0.13). Fasting lipid levels were not significantly associated with LAE and SAE. After adjustment, differences between HIV-infected and HIV-uninfected participants were similar in model 1 (−2.36 for LAE, P = 0.04; −1.31 for SAE, P = 0.04), model 2 (−2.67 for LAE, P = 0.02; −1.13 for SAE, P = 0.07) and model 3 (−2.91 for LAE, P = 0.02; −1.34 for SAE, P = 0.03). CD4 count and HIV RNA level were not associated with LAE and SAE among HIV-infected participants.
Conclusions
Untreated HIV infection is associated with impaired arterial elasticity, of both the large and small vasculature, after controlling for additional risk factors. Pulse waveform analysis is a noninvasive technique to assess cardiovascular disease risk that should be evaluated in larger studies of HIV-infected persons.
Keywords: arterial elasticity, arterial stiffness, cardiovascular disease, endothelial dysfunction, HIV, untreated HIV infection
INTRODUCTION
Premature atherosclerotic cardiovascular disease (CVD) is an important cause of morbidity and mortality in patients with HIV infection.1,2 CVD risk is influenced by traditional risk factors [ie, blood pressure (BP), blood lipids, and smoking] and antiretroviral therapy (ART)-related mechanisms including metabolic changes (lipid levels and insulin resistance).3,4 In addition, HIV infection itself may promote atherogenesis, in part, via a decrease in high-density lipoprotein cholesterol (HDLc) and increase in triglyceride (TG) levels, immune activation and inflammation, increase in thrombosis and fibrinolysis, and endothelial dysfunction.5–8 Characterizing CVD risk attributed specifically to untreated HIV infection is important and has implications when assessing the risks and benefits of ART use.
Noninvasive markers of early CVD are important tools in understanding the pathogenesis and progression of premature CVD and the impact of therapy. To date, research among HIV-infected patients using carotid intima-media thickness and brachial artery flow-mediated dilation (FMD) has been inconsistent and has primarily focused on the influences of ART use and traditional CVD risk factors.7,9–22 However, a recent report of 82 treatment-naive patients showed improved FMD measures within 4 weeks of initial ART use, and FMD improvement at 24 weeks was associated with declines in HIV RNA.19 Thus, studies are needed using reproducible markers of CVD that distinguish the influence of traditional risk factors, HIV infection, and ART on disease risk.23
In the general population, arterial stiffness is a CVD marker associated with atherosclerotic disease and traditional CVD risk factors in cross-sectional studies, as well as risk for CVD events in longitudinal studies.24–26 There are several techniques to estimate arterial stiffness or elasticity (conceptually the inverse of stiffness). Analysis of the diastolic radial pulse waveform provides an assessment of artery elasticity at the level of the aorta and conduit arteries [large artery elasticity (LAE)] and the peripheral branches and microvascular components [small artery elasticity (SAE)] that contains information about structural and functional vascular changes, including endothelial dysfunction.27–29 This is the first study to examine SAE specifically, in addition to a large artery assessment, within healthy HIV-negative and HIV-infected participants not receiving ART.
METHODS
Study Population
The protocol was preapproved by the Hennepin County Medical Center (HCMC) Human Subjects Research Committee, and informed consent was signed before participant enrollment. HIV-infected participants had not taken ART in the previous year. Exclusion criteria included pregnancy, current/active bacterial infection, recent hospitalization, systemic vasculitis, valvular disease (including heart valve replacement), atrial fibrillation, and presence of an upper extremity arterial-venous fistula. Participants with clinical atherosclerotic disease (previous myocardial infarction or chronic angina or claudication by history) were also excluded as the focus of this study was to examine early subclinical CVD risk. Participants were recruited through informational flyers and referrals from patients and providers, at an urban HIV clinic (HCMC, Minneapolis, MN). The HIV-negative group was recruited in the same way, and efforts were made to enroll participants so that the control group did not differ from the HIV-infected group with regard to age, sex, race/ethnicity, smoking status, and the presence of diabetes mellitus (DM).
Study Procedures
Study participants presented for a single visit at HCMC, where a medical history, peripheral blood draw, and arterial elasticity measurements were performed. A medical history was taken (including a review of the medical record) that included duration of HIV infection, prior AIDS-defining event, prior ART use, history of injection drug use (IDU), smoking status (defined as current), a diagnosis of DM, a list of current medications, and a blood CD4 count within the past 3 months (when available). Study participants were instructed to fast and avoid antihistamines and nonsteroidal anti-inflammatory drugs during the 8-hour period before the visit and avoid alcohol and illicit drugs for at least 24 hours prior, as these substances may affect arterial elasticity estimates. Blood samples were tested for the following: HIV antibody (for HIV-negative participants), HIV RNA level (for HIV-infected participants), serologies for hepatitis B (infection defined as HBsAb negative and hepatitis B surface antigen positive) and hepatitis C (infection defined as hepatitis C virus antibody positive), serum creatinine, total serum cholesterol (TC), low-density lipoprotein cholesterol (LDLc), HDLc, and TG. Non-HDLc levels were calculated as TC minus HDLc and provided a single estimate of all potentially proatherogenic lipid particles, including LDLc particles, TGs, and very low density lipoprotein remnants.30,31 Framingham 10-year CVD risk was estimated from a National Heart, Lung, and Blood Institute calculator (http://hp2010.nhlbihin.net/atpiii/calculator.asp), after entering age, sex, smoking status, TC, HDLc, systolic blood pressure (SBP), and use of BP-lowering medication.
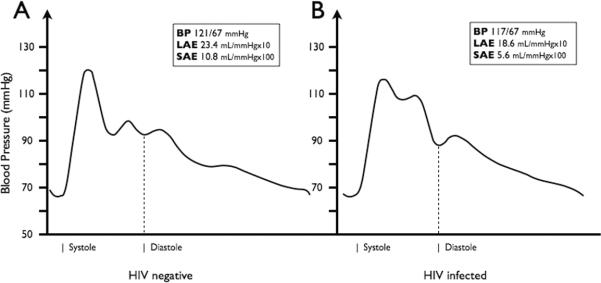
Arterial elasticity was assessed via pulse waveform analysis of the diastolic decay curve, and values of LAE and SAE were calculated using a modified Windkessel model of the circulation (model HDI/PulseWave CR-2000, Eagan, MN).27,29 A tonometer was placed at the dominant wrist overlying the radial artery of resting participants. A stable 30-second measure of the radial pulse waveform was achieved, excluding the dicrotic notch, and was digitized at 200 samples per second. Before and during the waveform assessment, an automated oscillatory BP measurement is taken on the contralateral arm. A typical waveform will have 2 maxima present within the diastolic decay curve. The first occurs at the beginning of diastole and represents the capacitance of the proximal aorta and major branches after cardiac ejection. The second maximum results from a reflective, or oscillatory, wave, corresponding to elasticity in the smaller arteries. Representative pulse waveforms are shown for an HIV-negative and HIV-infected participant in Figure 1. These methods have been previously described in detail and are consistent with established protocols using this technique in large cohort studies.27,29,32,33
FIGURE 1.
Representative radial artery pulse waveforms are shown for an HIV-negative control (A) and an HIV-infected participant (B). Waveforms are plotted with BP along the y axis over time along x axis. Differences in the pulse contour, including the diastolic decay, can be appreciated. Resting BP along with the corresponding large and small arterial elasticity (LAE and SAE) measurements is reported, and the beginning of systole and diastole is estimated.
A single investigator performed the pulse waveform assessments in triplicate, and the mean (average of 3 values for each participant) LAE and SAE indices were used in statistical analyses. The average SD among the 3 readings for each participant was 1.6 mL/mm Hg × 10 for LAE and 0.5 mL/mm Hg × 100 for SAE. A group of 12 participants (6 were not part of the cross-sectional comparison) were studied at 2 visits approximately 1–4 months apart to assess repeatability of measurements over time. The mean (±SD) difference between the 2 time points was 1.0 (±2.9) mL/mm Hg × 10 for LAE and −0.2 (±1.2) mL/mm Hg × 100 for SAE. The within-person variability between visits was 8.2 for LAE and 1.4 for SAE, and the values exhibited a very high correlation between visits (LAE R = 0.84; SAE R = 0.94).
Statistical Analyses
Data were analyzed by use of SAS software (Version 9.1; SAS Institute, Inc, Cary, NC). Descriptive statistics are reported as means ± SDs and medians with interquartile range (IQR). Student t test for independent groups and the χ2 test for categorical variables were used to compare HIV-infected and HIV-negative groups. Adjusted mean differences between HIV-infected and HIV-negative groups were obtained using analysis of covariance. To account for traditional CVD risk factors, Framingham risk score was considered (model 1). Given the small sample size, a parsimonious model was next considered including covariates that differed between groups in Table 1: smoking status, IDU, hepatitis C, and HDLc (model 2). Finally, a fully adjusted model included age, sex, race/ethnicity, smoking status, IDU, hepatitis C infection, HDLc, and non-HDLc (model 3). SBP, diastolic blood pressure (DBP), and heart rate are incorporated into LAE and SAE estimates, so these parameters were omitted from adjusted models. Framingham 10-year CVD risk score includes SBP, but smoking status and lipid levels largely explain differences in Framingham risk between groups in this study (Table 1). To explore potential explanations for impaired arterial elasticity among HIV-infected participants, regression models were performed between LAE and SAE indices and log10 transformed HIV RNA levels, CD4 count before and after square root transformation, hepatitis C infection, IDU, and Framingham risk score. The within-participant variability over time, and the correlation of repeat measures, was estimated using data from the 12 participants presenting for 2 study visits.
TABLE 1.
Baseline and Demographic Characteristics
| Characteristic | HIV Infected (n = 32) | HIV Negative (n = 30)* | P |
|---|---|---|---|
| Mean (SD) or Percent (n) Unless Stated | |||
| Age (yrs) | 40.0 (9.6) | 40.4 (10.6) | 0.83 |
| Sex (men) | 88% (28) | 87% (26) | 0.92 |
| Race/ethnicity |
|||
| White |
44% (14) | 47% (14) | 0.48 |
| African American |
31% (10) | 40% (12) | |
| Other | 25% (8) | 13% (4) | |
| Smoker | 59% (19) | 40% (12) | 0.13 |
| IDU | 38% (12) | 13% (4) | 0.03 |
| Hepatitis C | 34% (11) | 13% (4) | 0.05 |
| Diabetes | 6% (2) | 7% (2) | 0.95 |
| Body mass index (kg/m2) | 26.0 (5.1) | 27.6 (4.5) | 0.20 |
| Heart rate (bpm) | 71.0 (13.0) | 64.2 (8.7) | 0.02 |
| SBP (mm Hg) | 127.7 (13.9) | 126.7 (12.5) | 0.77 |
| DBP (mm Hg) | 77.0 (11.3) | 74.5 (7.8) | 0.31 |
| BP medication† | 19% (6) | 10% (3) | 0.33 |
| Serum creatinine (mg/dL) | 0.9 (0.2) | 1.0 (0.2) | 0.04 |
| Lipids |
|||
| TC (mg/dL) |
167.0 (31.7) | 184.5 (43.6) | 0.08 |
| HDLc (mg/dL) |
37.5 (13.1) | 49.6 (13.4) | <0.01 |
| LDLc (mg/dL) |
107.9 (53.4) | 111.8 (35.6) | 0.74 |
| TG (mg/dL) |
151.0 (91.0) | 118.8 (56.4) | 0.11 |
| Non-HDLc (mg/dL) |
129.5 (28.7) | 134.9 (41.5) | 0.57 |
| TC:HDLc | 4.9 (1.7) | 3.9 (1.3) | 0.01 |
| Framingham 10-year CVD risk | 4.8% (4.6) | 3.6% (4.6) | 0.66 |
| Duration of infection (yrs) | 6.5 (6.6) | — | — |
| Prior AIDS | 7% (2) | — | — |
| Prior ART use | 16% (5) | — | — |
| CD4 count (cells/mm3) |
|||
| Mean (SD) |
391.1 (181.5) | — | — |
| Median (IQR) | 382 (255–514) | — | — |
| HIV RNA (log10 copies/mL) |
|||
| Mean (SD) |
4.15 (0.73) | — | — |
| Median (IQR) | 4.18 (3.58–4.78) | — | — |
Blood lipid tests are defined in text.
For lipid levels and serum creatinine, 1 HIV-negative participant did not provide blood (n = 29).
Use of beta-blocker, hydrochlorothiazide, calcium channel blocker, or angiotensin-converting enzyme inhibitor.
RESULTS
Characteristics of Study Sample
From March 2007 to August 2008, we enrolled 32 HIV-infected and 30 HIV-negative participants. The demographic and clinical characteristics of the participants are presented in Table 1. HIV-infected and HIV-negative participants did not differ with respect to age, sex, race/ethnicity, DM, SBP, DBP, and the use of BP-lowering drugs. One HIV-infected and 2 HIV-negative participants were using statin medications. The HIV-infected group had a higher mean heart rate, lower HDLc, and serum creatinine levels; were more likely to be infected with hepatitis C; and reported prior IDU. Although not significant, there were a greater number of smokers in the HIV-infected vs. control group. Only 1 participant had evidence of chronic hepatitis B infection (positive hepatitis B surface antigen) and he also had hepatitis C. Most HIV-infected participants (59%) had CD4 counts above the recommended threshold for initiation of ART (350 cells/mm3).34 The majority of HIV-infected participants were naive to ART (84%), and mean and median CD4 counts and HIV RNA levels are shown. No HIV-infected participants were in the acute/early stage of infection (ie, within 6 months of known seroconversion), and only 4 participants had a CD4 count <200 cells per cubic millimeter. Among the 5 participants with prior ART exposure, all had been off therapy for >2 years, at least 3 had protease inhibitor exposure, and 1 had 10 years of ART exposure (unknown duration for the other 4), and the median and mean duration of HIV infection (by self-report) was 13 and 14 years, respectively.
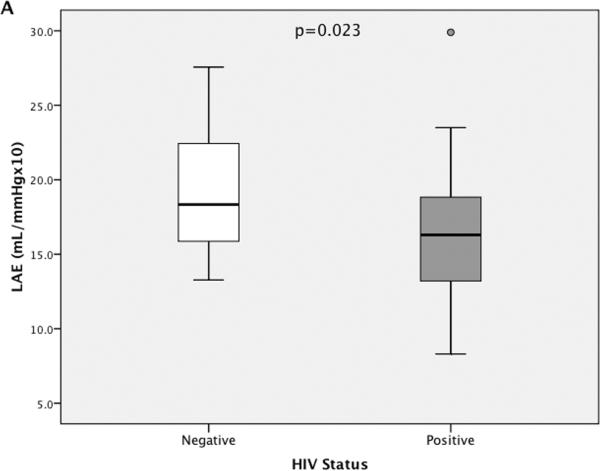
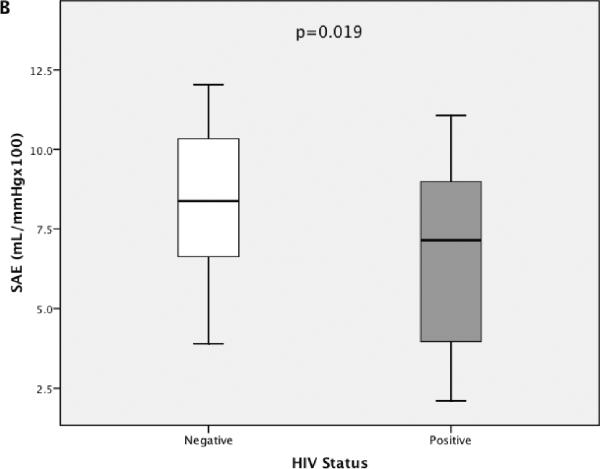
The distribution of LAE and SAE measurements for the full cohort, stratified by HIV status, is presented with box plots (including median and IQR) in Figure 2. The single outlier for LAE (value was >1.5 × IQR above the third quartile) was an HIV-infected 39-year-old African American male with an LAE estimate of 29.9mL/mm Hg × 10 and an SAE estimate of 7.6mL/mm Hg × 100. He was naive to ART, did not smoke; had no history of IDU; was hepatitis C antibody negative; and had a BP of 136/85, an HDLc of 25 mg/dL, a CD4 count of 184 cells per cubic millimeter, and an HIV RNA level of 21,570 copies per milliliter.
FIGURE 2.
The distribution of LAE (A) and SAE (B) estimates are presented, stratified by HIV status. Box plots represent the IQR with median values designated by a horizontal line. Error bars constitute minimum and maximum values. A, One high outlier (value >1.5 χ IQR higher than the third quartile) is designated for LAE within the HIV-infected group. P values represent a significantly lower mean LAE and SAE for HIV-infected participants compared with HIV-negative controls.
Univariate Associations With Arterial Elasticity
Mean (±SD) LAE and SAE were significantly lower for HIV-infected (LAE 16.4 ± 4.6 mL/mm Hg × 10; SAE 6.7 ± 2.5 mL/mm Hg × 100) compared with HIV-negative participants (LAE 18.9 ± 4.0 mL/mm Hg × 10; SAE 8.2 ± 2.4 mL/mm Hg × 100). Univariate associations between LAE and SAE and other baseline measurements are presented in Table 2. LAE was significantly lower with presence of smoking or hepatitis C infection and trended lower with IDU and a higher Framingham risk score. SAE was significantly lower with increased age, female sex, IDU, hepatitis C infection, or a higher Framingham risk score and trended lower with the presence of smoking. LAE and SAE exhibited inverse associations with SBP and DBP as expected because these are in the denominator of LAE and SAE indices. When comparisons were limited to ART-naive participants (n = 5 with prior ART exposure omitted), HIV infection remained associated with lower SAE (−1.50 mL/mm Hg × 100; P = 0.03) but did not reach significance for LAE (−2.28 mL/mm Hg × 10; P = 0.06).
TABLE 2.
Univariate Associations With LAE and SAE
| Variables | LAE | SAE | ||
|---|---|---|---|---|
| Regression Coefficient (95% CI) | P | Regression Coefficient (95% CI) | P | |
| HIV infected (vs. non infected) | −2.55 (−4.73 to −0.36) | 0.02 | −1.50 (−2.75 to −0.25) | 0.02 |
| Age (per 10 yrs older) | −0.55 (−1.68 to 0.59) | 0.34 | −1.08 (−1.67 to −0.49) | <0.01 |
| Women (vs. men) | −1.05 (−4.44 to 2.34) | 0.54 | −2.34 (−4.19 to −0.49) | 0.01 |
| Race/ethnicity | ||||
| African American (vs. white) | −0.65 (−3.23 to 1.92) | 0.61 | −0.24 (−1.71 to 1.23) | 0.75 |
| Other (vs. white) | −0.31 (−3.43 to 2.81) | 0.85 | −0.41 (−2.19 to 1.38) | 0.65 |
| Smoker (vs. nonsmoker) | −3.13 (−5.26 to −0.99) | <0.01 | −1.07 (−2.35 to 0.21) | 0.10 |
| IDU (vs. never) | −2.34 (−4.87 to 0.20) | 0.07 | −1.48 (−2.92 to −0.03) | 0.05 |
| Hepatitis C (vs. non infected) | −2.78 (−5.34 to −0.21) | 0.03 | −3.13 (−4.43 to −1.84) | <0.01 |
| SBP (per 10 units higher) | −1.08 (−1.91 to −0.25) | 0.01 | −0.44 (−0.93 to 0.05) | 0.08 |
| DBP (per 10 units higher) | −1.15 (−2.29 to −0.00) | 0.05 | −0.87 (−1.51 to −0.23) | 0.01 |
| TC (per 10 units higher) | −0.13 (−0.43 to 0.17) | 0.39 | 0.07 (−0.10 to 0.24) | 0.40 |
| HDLc (per 10 units higher) | −0.08 (−0.89 to 0.73) | 0.84 | 0.30 (−0.15 to 0.75) | 0.19 |
| LDLc (per 10 units higher) | −0.08 (−0.34 to 0.17) | 0.52 | 0.05 (−0.10 to 0.19) | 0.54 |
| TG (per 10 units higher) | 0.04 (−0.11 to 0.19) | 0.63 | 0.02 (−0.07 to 0.10) | 0.71 |
| Non-HDLc (per 10 units higher) | −0.14 (−0.47 to 0.19) | 0.39 | 0.04 (−0.15 to 0.22) | 0.71 |
| TC:HDLc (per 10 units higher) | 0.64 (−6.89 to 8.17) | 0.87 | −1.22 (−5.49 to 3.05) | 0.57 |
| Framingham 10-year CVD risk (per 1% higher) | −0.19 (−0.43 to 0.06) | 0.13 | −0.18 (−0.31 to −0.04) | 0.01 |
CI, confidence interval.
Multivariate Associations With Arterial Elasticity
Covariate adjustment did not have a large effect on the differences between HIV-infected and HIV-negative participants (Table 3). Parsimonious models were fit given the small sample size that considered Framingham score to adjust for traditional CVD risk factors (model 1) and the main covariates that differed in Table 1 (model 2). A fully adjusted model then provided similar estimates (model 3). When HIV-infected participants with prior ART exposure (n = 5) were excluded, HIV infection remained significantly associated with lower SAE (−1.37 mL/mm Hg × 100; P = 0.02) in model 3 but differences in LAE did not reach significance (−2.35 mL/mm Hg × 10; P = 0.07).
TABLE 3.
Multivariate Associations With LAE and SAE
| LAE | SAE | |||
|---|---|---|---|---|
| Regression Coefficient (95% CI) | P | Regression Coefficient (95% CI) | P | |
| Model 1 (Framingham score) | ||||
| HIV infected (vs. non infected) | −2.36 (−4.56 to −0.17) | 0.04 | −1.31 (−2.52 to −0.09) | 0.04 |
| Model 2 (smoking, IDU, hepatitis C, and HDLc) | ||||
| HIV infected (vs. non infected) | −2.67 (−4.97 to −0.38) | 0.02 | −1.13 (−2.35 to −0.09) | 0.07 |
| Model 3 (fully adjusted) | ||||
| HIV infected (vs. non infected) | −2.91 (−5.29 to −0.53) | 0.02 | −1.34 (−2.50 to −0.17) | 0.03 |
Regression coefficients represent the adjusted differences in LAE or SAE in HIV-infected participants vs. controls. Model 1 considers Framingham 10-year CVD risk score as a covariate; model 2 considers smoking status, history of IDU, hepatitis C infection, and HDLc level; and model 3 considers age, sex, race/ethnicity, smoking status, history of IDU, hepatitis C infection, HDLc, and non-HDLc levels.
CI, confidence interval.
Given differences in smoking status and IDU between groups in Table 1, the effect of HIV status on LAE and SAE was examined among nonsmokers and non-injection drug users. IDU was chosen over hepatitis C as most participants with hepatitis C also report IDU (73%). Power for these comparisons was limited, although differences between HIV-infected and HIV-negative groups did not differ significantly by smoking status (test for interaction: P = 0.06 for LAE and P = 0.55 for SAE) or IDU (test for interaction: P = 0.56 for LAE and P = 0.98 for SAE). Among nonsmokers, differences between HIV-infected and HIV-negative participants were −3.59 mL/mm Hg × 10 (P = 0.05) for LAE and −1.38 mL/mm Hg × 100 (P = 0.13) for SAE. The corresponding differences for smokers were −0.41 for LAE (P = 0.73) and −1.31 for SAE (P = 0.17). Among non-IDU participants, these differences were −2.05 mL/mm Hg × 10 (P = 0.15) for LAE and −1.60 mL/mm Hg × 100 (P = 0.03) for SAE. For participants reporting a history of IDU, differences were −2.51 for LAE (P = 0.15) and −2.80 for SAE (P = 0.92).
Univariate Associations With Arterial Elasticity for HIV-Infected Participants
Finally, factors that may account for lower LAE and SAE measures among HIV-infected participants were examined. No trends were apparent by CD4 count (P = 0.46 for LAE; P = 0.37 for SAE) and log10 transformed HIV RNA levels (P = 0.47 for LAE; P = 0.73 for SAE). After square root transformation of CD4 count, there remained no association (per 1 unit higher) with LAE (P = 0.39) or SAE (P = 0.35). Arterial elasticity did not differ between participants with CD4 counts <350 vs. ≥350 cells per cubic millimeter (P = 0.44 for LAE and P = 0.40 for SAE). Coinfection with hepatitis C was associated with lower SAE (−3.16 mL/mm Hg × 100; P < 0.01) but did not reach significance for LAE (−2.58 mL/mm Hg × 10; P = 0.13). IDU was not associated with LAE (P = 0.29) or SAE (P = 0.57) among HIV-infected participants. Finally, a higher Framingham risk score trended toward association with lower LAE (−0.32 mL/mm Hg × 10 per 1% higher; P = 0.07) and SAE (−0.14 mL/mm Hg × 100 per 1% higher; P = 0.15) among HIV-infected participants.
DISCUSSION
In this study, individuals with untreated HIV infection had lower levels of SAE and LAE than HIV-negative controls. The differences in LAE and SAE remained significant after adjusting for additional risk factors, including Framingham 10-year CVD risk score. The degree of HIV-related impairment reported in arterial elasticity is clinically relevant.26,33 This is the first study using pulse waveform analysis to assess both small and large artery elasticity among HIV-infected patients. The low within-participant variability in LAE and SAE measures, and associations with traditional CVD risk factors, suggests that this noninvasive technique will be a valuable marker to consider in larger studies assessing CVD risk among HIV-infected patients.
Artery stiffness (the inverse of elasticity) can also be assessed via ultrasound estimates of arterial distensibility and compliance, measures of aortic pulse wave velocity (PWV), and the augmentation index (analysis of systolic wave reflection). A recent study of 77 HIV-infected and 52 HIV-negative participants showed impaired carotid and femoral arterial stiffness (distensibility and compliance) with HIV infection.22 In this study, 53 participants were taking ART and ART use was associated with stiffness of the femoral artery among HIV-infected persons.22 In another cross-sectional comparison of 39 untreated HIV-infected persons and 78 HIV-negative controls demonstrated higher PWV or impaired aortic stiffness, among HIV-infected persons.35 Additional studies using PWV and augmentation index have also reported impaired arterial stiffness to be associated with HIV infection and ART use, with one study specifically demonstrating a correlation between PWV and duration of protease inhibitor use.15,35–37 Our study is consistent with these data and shows that LAE is reduced among persons with untreated HIV infection.
We also report impaired elasticity specifically at the distal part of the circulation (SAE) as a result of HIV infection and demonstrate a high degree of reproducibility in SAE measurements within participants over time. In the general population, SAE is associated with traditional CVD risk factors and is a marker of endothelial dysfunction and future CVD event risk.26,28,33,38–40 In a study of 419 individuals (41% with a CVD event), a 2-unit (mL/mm Hg × 100) decrease in SAE was associated with a 50% increased risk (95% confidence interval: 1.19 to 1.88) for CVD event after adjusting for age.26 Recent findings from 6047 participants in Multi-Ethnic Study of Atherosclerosis demonstrated that impaired SAE but not LAE was associated with future risk for CVD events after controlling for other surrogate risk markers including BP, carotid intima-media thickness, and coronary artery calcium score.33 Although the classic lesions of atherosclerotic disease (plaques) are described in large conduit arteries, the early events in this process begin with injury to endothelial surfaces throughout the vasculature, whether mediated through traditional risk factors, inflammation or oxidative stress, or other factors.41,42 Endothelial dysfunction promotes atherogenesis through alterations in vascular tone, inflammation, and thrombosis, and the microcirculation accounts for most of the vascular surface area.41,43 When endothelial dysfunction is induced in normal volunteers via an infusion of a nitric oxide (NO) inhibitor, L-NAME (NG-nitro-L-arginine-methyl ester), systemic vascular resistance increases and SAE measures become impaired, whereas LAE remains unchanged.28 Our findings suggest that HIV-mediated vessel damage and dysfunction are present throughout the arterial vasculature.
Inflammation and immune activation is both a hallmark of HIV infection and an important factor in the pathogenesis of atherosclerotic CVD.44,45 A recent randomized study of 100 healthy individuals reported acute worsening of large artery stiffness (PWV and augmentation index) after administration of Salmonella typhi vaccine, when compared with placebo.46 In addition, LAE and SAE measures were impaired, and C-reactive protein and soluble vascular cell adhesion molecule-1 levels were elevated in a study of 53 patients with rheumatoid arthritis, when compared with non-rheumatoid arthritis controls.47 Lower LAE measures have also been associated with higher C-reactive protein levels among asymptomatic individuals.48 Some of the most convincing evidence that untreated HIV infection increases risk for CVD comes from SMART (Strategies for Management of Antiretroviral Therapy), which reported that intermittent ART use, compared with continuous ART use, led to greater CVD and other end-organ disease events, and this risk then declined when ART was reinitiated.49,50 In SMART, baseline levels of inflammatory (IL-6) and thrombotic (D-dimer) markers were predictive of both CVD and non-CVD mortality, leading authors to suggest that upregulation of tissue factor coagulation pathways on endothelial surfaces was a potential mechanism.8 Additional studies are needed to further examine the relationship between HIV-mediated inflammation, endothelial dysfunction, thrombogenesis, and clinical events among persons with HIV infection.
We show an association between traditional CVD risk factors and lower LAE and SAE measures. This is important and further validates LAE and SAE as a noninvasive CVD risk marker among persons with HIV infection. In the general population, both LAE and SAE showed a greater association with Framingham risk score than with FMD in a study of 122 participants.24 In addition, FMD was not associated with Framingham risk score in a recent study of 100 HIV-infected and HIV-uninfected participants.18
There are several limitations to our study. The cross-sectional nature does not allow temporal relationships to be assessed, although artery elasticity will be assessed in a subset of participants within the Strategic Timing of Antiretroviral Therapy (START) trial. Given the size of this pilot study, there is also limited power to identify modest associations or to study the determinants of LAE and SAE among HIV-infected participants. By design, our study does not allow an assessment of ART use on differences in LAE and SAE. Finally, we were not able to specifically adjust for the presence of metabolic syndrome, but BP did not differ between groups, body mass index trended lower in HIV-infected vs. control group, only 2 participants from each group had DM, and HDLc was accounted for in adjusted models.
In summary, these data support the use of LAE and SAE measures, obtained via arterial pulse waveform analysis, as a marker of CVD risk in HIV-infected patients. Future research should focus on the HIV-related mechanisms of impaired artery elasticity and the influence of ART use and other treatments on these measures in HIV-infected patients.
ACKNOWLEDGMENTS
The authors would like to thank the participants who volunteered for this research and the staff of the HCMC Positive Care Center for their tireless support. We would specifically like to acknowledge the critical contributions of Edie Gunderson, Bette Borndenave, Miki Olson, Sarah Bruemmer, Jack Hall, and Rachel Prosser.
Supported by the National Institutes of Health (5 T32 GM12453-03) and the Minneapolis Medical Research Foundation.
REFERENCES
- 1.Lewden C, May T, Rosenthal E, et al. Changes in causes of death among adults infected by HIV between 2000 and 2005: the “Mortalite 2000 and 2005” surveys (ANRS EN19 and Mortavic) J Acquir Immune Defic Syndr. 2008;48:590–598. doi: 10.1097/QAI.0b013e31817efb54. [DOI] [PubMed] [Google Scholar]
- 2.Sackoff JE, Hanna DB, Pfeiffer MR, et al. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145:397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 3.Saves M, Chene G, Ducimetiere P, et al. Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clin Infect Dis. 2003;37:292–298. doi: 10.1086/375844. [DOI] [PubMed] [Google Scholar]
- 4.Grinspoon S, Carr A. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med. 2005;352:48–62. doi: 10.1056/NEJMra041811. [DOI] [PubMed] [Google Scholar]
- 5.Riddler SA, Smit E, Cole SR, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 6.de Gaetano Donati K, Rabagliati R, Iacoviello L, et al. HIV infection, HAART, and endothelial adhesion molecules: current perspectives. Lancet Infect Dis. 2004;4:213–222. doi: 10.1016/S1473-3099(04)00971-5. [DOI] [PubMed] [Google Scholar]
- 7.Solages A, Vita JA, Thornton DJ, et al. Endothelial function in HIV-infected persons. Clin Infect Dis. 2006;42:1325–1332. doi: 10.1086/503261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein JH, Klein MA, Bellehumeur JL, et al. Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation. 2001;104:257–262. doi: 10.1161/01.cir.104.3.257. [DOI] [PubMed] [Google Scholar]
- 10.Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603–1608. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 11.Blum A, Hadas V, Burke M, et al. Viral load of the human immunodeficiency virus could be an independent risk factor for endothelial dysfunction. Clin Cardiol. 2005;28(3):149–153. doi: 10.1002/clc.4960280311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charakida M, Donald AE, Green H, et al. Early structural and functional changes of the vasculature in HIV-infected children: impact of disease and antiretroviral therapy. Circulation. 2005;112:103–109. doi: 10.1161/CIRCULATIONAHA.104.517144. [DOI] [PubMed] [Google Scholar]
- 13.Currier JS, Kendall MA, Zackin R, et al. Carotid artery intima-media thickness and HIV infection: traditional risk factors overshadow impact of protease inhibitor exposure. AIDS. 2005;19:927–933. doi: 10.1097/01.aids.0000171406.53737.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnsen S, Dolan SE, Fitch KV, et al. Carotid intimal medial thickness in human immunodeficiency virus-infected women: effects of protease inhibitor use, cardiac risk factors, and the metabolic syndrome. J Clin Endocrinol Metab. 2006;91:4916–4924. doi: 10.1210/jc.2006-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Wijk JP, de Koning EJ, Cabezas MC, et al. Functional and structural markers of atherosclerosis in human immunodeficiency virus-infected patients. J Am Coll Cardiol. 2006;47:1117–1123. doi: 10.1016/j.jacc.2005.09.073. [DOI] [PubMed] [Google Scholar]
- 16.Currier JS, Kendall MA, Henry WK, et al. Progression of carotid artery intima-media thickening in HIV-infected and uninfected adults. AIDS. 2007;21:1137–1145. doi: 10.1097/QAD.0b013e32811ebf79. [DOI] [PubMed] [Google Scholar]
- 17.Bongiovanni M, Casana M, Cicconi P, et al. Predictive factors of vascular intima media thickness in HIV-positive subjects. J Antimicrob Chemother. 2008;61:195–199. doi: 10.1093/jac/dkm414. [DOI] [PubMed] [Google Scholar]
- 18.Mondy KE, de las Fuentes L, Waggoner A, et al. Insulin resistance predicts endothelial dysfunction and cardiovascular risk in HIV-infected persons on long-term highly active antiretroviral therapy. AIDS. 2008;22:849–856. doi: 10.1097/QAD.0b013e3282f70694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torriani FJ, Komarow L, Parker RA, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: the ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52:569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008;22:1615–1624. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorenz MW, Stephan C, Harmjanz A, et al. Both long-term HIV infection and highly active antiretroviral therapy are independent risk factors for early carotid atherosclerosis. Atherosclerosis. 2008;196:720–726. doi: 10.1016/j.atherosclerosis.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 22.van Vonderen MG, Smulders YM, Stehouwer CD, et al. Carotid intima-media thickness and arterial stiffness in HIV-infected patients: the role of HIV, antiretroviral therapy, and lipodystrophy. J Acquir Immune Defic Syndr. 2009;50:153–161. doi: 10.1097/QAI.0b013e31819367cd. [DOI] [PubMed] [Google Scholar]
- 23.Murphy R, Costagliola D. Increased cardiovascular risk in HIV infection: drugs, virus and immunity. AIDS. 2008;22:1625–1627. doi: 10.1097/QAD.0b013e328306a6db. [DOI] [PubMed] [Google Scholar]
- 24.Nair N, Oka RK, Waring LD, et al. Vascular compliance versus flow-mediated vasodilation: correlation with cardiovascular risk factors. Vasc Med. 2005;10:275–283. doi: 10.1191/1358863x05vm633oa. [DOI] [PubMed] [Google Scholar]
- 25.Duprez D, Cohen J. Arterial stiffness as a risk factor for coronary atherosclerosis. Curr Atheroscler Rep. 2007;9:139–144. doi: 10.1007/s11883-007-0010-y. [DOI] [PubMed] [Google Scholar]
- 26.Grey E, Bratteli C, Glasser SP, et al. Reduced small artery but not large artery elasticity is an independent risk marker for cardiovascular events. Am J Hypertens. 2003;16:265–269. doi: 10.1016/s0895-7061(02)03271-5. [DOI] [PubMed] [Google Scholar]
- 27.Finkelstein SM, Cohn JN. First- and third-order models for determining arterial compliance. J Hypertens Suppl. 1992;10(6):S11–S14. [PubMed] [Google Scholar]
- 28.Gilani M, Kaiser DR, Bratteli CW, et al. Role of nitric oxide deficiency and its detection as a risk factor in pre-hypertension. J Am Soc Hypertens. 2007;1(1):45–55. doi: 10.1016/j.jash.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Cohn JN, Finkelstein S, McVeigh G, et al. Noninvasive pulse wave analysis for the early detection of vascular disease. Hypertension. 1995;26:503–508. doi: 10.1161/01.hyp.26.3.503. [DOI] [PubMed] [Google Scholar]
- 30.Cui Y, Blumenthal RS, Flaws JA, et al. Non-high-density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch Intern Med. 2001;161:1413–1419. doi: 10.1001/archinte.161.11.1413. [DOI] [PubMed] [Google Scholar]
- 31.Miller M, Ginsberg HN, Schaefer EJ. Relative atherogenicity and predictive value of non-high-density lipoprotein cholesterol for coronary heart disease. Am J Cardiol. 2008;101:1003–1008. doi: 10.1016/j.amjcard.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 32.Valappil NI, Jacobs DR, Duprez DA, et al. Association between endothelial biomarkers and arterial elasticity in yound adults: the CARDIA Study. J Am Soc Hypertens. 2008;2:70–79. doi: 10.1016/j.jash.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duprez DA, Jacobs DR, Lutsey PL, et al. Small artery but not large artery elasticity predicts coronary heart disease events beyond coronary calcium score and carotid intima-media thickness in an asymptomatic population: results of the multiethnic study of atherosclerosis (MESA). Presented at: American College of Cardiology (ACC) 58th Annual Scientific Session; Orlando, FL. 2009. Abstract #1049–100. [Google Scholar]
- 34.Smit C, Geskus R, Walker S, et al. Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. AIDS. 2006;20:741–749. doi: 10.1097/01.aids.0000216375.99560.a2. [DOI] [PubMed] [Google Scholar]
- 35.Schillaci G, De Socio GV, Pucci G, et al. Aortic stiffness in untreated adult patients with human immunodeficiency virus infection. Hypertension. 2008;52:308–313. doi: 10.1161/HYPERTENSIONAHA.108.114660. [DOI] [PubMed] [Google Scholar]
- 36.Schillaci G, De Socio GV, Pirro M, et al. Impact of treatment with protease inhibitors on aortic stiffness in adult patients with human immunodeficiency virus infection. Arterioscler Thromb Vasc Biol. 2005;25:2381–2385. doi: 10.1161/01.ATV.0000183744.38509.de. [DOI] [PubMed] [Google Scholar]
- 37.Sevastianova K, Sutinen J, Westerbacka J, et al. Arterial stiffness in HIV-infected patients receiving highly active antiretroviral therapy. Antivir Ther. 2005;10:925–935. [PubMed] [Google Scholar]
- 38.McVeigh GE, Burns DE, Finkelstein SM, et al. Reduced vascular compliance as a marker for essential hypertension. Am J Hypertens. 1991;4(pt 1):245–251. doi: 10.1093/ajh/4.3.245. [DOI] [PubMed] [Google Scholar]
- 39.McVeigh GE, Brennan GM, Johnston GD, et al. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:771–776. doi: 10.1007/BF00429099. [DOI] [PubMed] [Google Scholar]
- 40.McVeigh GE, Morgan DJ, Finkelstein SM, et al. Vascular abnormalities associated with long-term cigarette smoking identified by arterial waveform analysis. Am J Med. 1997;102:227–231. doi: 10.1016/S0002-9343(96)00454-8. [DOI] [PubMed] [Google Scholar]
- 41.Faxon DP, Fuster V, Libby P, et al. Atherosclerotic vascular disease conference: writing group III: pathophysiology. Circulation. 2004;109:2617–2625. doi: 10.1161/01.CIR.0000128520.37674.EF. [DOI] [PubMed] [Google Scholar]
- 42.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 43.Duprez DA, Cohn JN. Identifying early cardiovascular disease to target candidates for treatment. J Clin Hypertens (Greenwich) 2008;10:226–231. doi: 10.1111/j.1751-7176.2008.07429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tracy RP. Epidemiological evidence for inflammation in cardiovascular disease. Thromb Haemost. 1999;82:826–831. [PubMed] [Google Scholar]
- 45.Sodora DL, Silvestri G. Immune activation and AIDS pathogenesis. AIDS. 2008;22:439–446. doi: 10.1097/QAD.0b013e3282f2dbe7. [DOI] [PubMed] [Google Scholar]
- 46.Vlachopoulos C, Dima I, Aznaouridis K, et al. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005;112:2193–2200. doi: 10.1161/CIRCULATIONAHA.105.535435. [DOI] [PubMed] [Google Scholar]
- 47.Wong M, Toh L, Wilson A, et al. Reduced arterial elasticity in rheumatoid arthritis and the relationship to vascular disease risk factors and inflammation. Arthritis Rheum. 2003;48(1):81–89. doi: 10.1002/art.10748. [DOI] [PubMed] [Google Scholar]
- 48.Duprez DA, Somasundaram PE, Sigurdsson G, et al. Relationship between C-reactive protein and arterial stiffness in an asymptomatic population. J Hum Hypertens. 2005;19:515–519. doi: 10.1038/sj.jhh.1001860. [DOI] [PubMed] [Google Scholar]
- 49.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 50.El-Sadr WM, Grund B, Neuhaus J, et al. Risk for opportunistic disease and death after reinitiating continuous antiretroviral therapy in patients with HIV previously receiving episodic therapy: a randomized trial. Ann Intern Med. 2008;149:289–299. doi: 10.7326/0003-4819-149-5-200809020-00003. [DOI] [PubMed] [Google Scholar]