SUMMARY
Effective innate immunity against many microbial pathogens requires macrophage programs that upregulate phagocytosis and direct antimicrobial pathways, two functions generally assumed to be coordinately regulated. Here the regulation of these key functions was investigated in human blood-derived macrophages. IL-10 induced the phagocytic pathway, including CD209 and scavenger receptors, resulting in phagocytosis of mycobacteria and oxLDL. IL-15 induced the vitamin D-dependent antimicrobial pathway and CD209, yet the cells were less phagocytic. The differential regulation of macrophage functional programs was confirmed by analysis of the spectrum of leprosy lesions: the macrophage phagocytosis pathway was prominent in the clinically progressive, multibacillary form, whereas the vitamin D-dependent antimicrobial pathway predominated in the self-limited form of the disease and in patients undergoing reversal reactions from the multibacillary to the self-limited form. These data indicate that macrophage programs for phagocytosis and antimicrobial responses are distinct and differentially regulated in innate immunity in bacterial infections.
INTRODUCTION
Since 1884, when Metchnikoff discovered phagocytes (Metschnikoff, 1884), it has generally been believed that phagocytic cells both engulf foreign bacteria, parasites and spores, and subsequently destroy them. Since this discovery, immunologists have often linked these two key functions of the innate immune response, phagocytosis and antimicrobial responses, as being co-regulated for optimal host defense. Following phagocytosis, there are a variety of antimicrobial mechanisms that macrophages (MΦ) utilize to kill pathogens, including the generation of nitric oxide and superoxide radicals, and in humans the vitamin D-dependent induction of antimicrobial peptides including cathelicidin (Liu et al., 2006). Although a key cytokine of the acquired immune system, IFN-γ, can both reduce phagocytosis (Backman and Guyre, 1994; Konopski et al., 1994) and is also known to upregulate antimicrobial activity, the mechanisms by which these pathways are regulated by the innate immune system are less clear.
In addition to phagocytosis of microbial pathogens, MΦ also have a scavenger function to remove extracellular material including apoptotic cells, cellular debris and toxic metabolic products (Mosser and Edwards, 2008). In particular, MΦ phagocytosis of oxidized lipoproteins, such as oxidized low-density lipoprotein (oxLDL), maintains proper lipid homeostasis within tissues (Mosser and Edwards, 2008; Greaves and Gordon, 2008), but can lead to foam cell formation in a variety of chronic infectious and noninfectious inflammatory disorders including atherosclerosis (Li and Glass, 2002), Whipple disease (Desnues et al., 2006), xanthomas (Caputo et al., 1986), and mycobacterial diseases such as tuberculosis (Lucas, 1988; Hunter et al., 2007; Pagel, 1925; Virchow, 1860; Ridley and Ridley, 1987) and leprosy (Lucas, 1988; Virchow, 1863; Sakurai and Skinsnes, 1970). The ability of MΦ to endocytose macromolecules and particles in their environment involves several distinct mechanisms including pinocytosis, receptor-mediated endocytosis and phagocytosis (Mosser and Edwards, 2008; Greaves and Gordon, 2008).
The mechanisms which regulate these MΦ antimicrobial and phagocytic functions are central to our understanding of innate immune responses against microbial pathogens. Leprosy provides an ideal model to study the human innate immune response to microbial infection, since the disease forms a clinical spectrum in which the pathogen, Mycobacterium leprae infects MΦ, and its fate correlates with the type of immune response (Yamamura et al., 1991). Although MΦ infiltration is prominent in all lesions, MΦ in the self-healing tuberculoid (T-lep) form are well-differentiated and rarely contain bacteria, whereas, MΦ in the disseminated lepromatous (L-lep) form are characterized by abundant intracellular bacilli and foam cell formation as the result of the accumulation of host-and pathogen-derived lipids (Cruz et al., 2008). Cytokine patterns are also distinct, T-lep lesions express IFN-γ, TNF-α, and IL-15, whereas, L-lep lesions are characterized by the expression of IL-4 and IL-10 (Jullien et al., 1997; Yamamura et al., 1991). Of these cytokines, IL-15 and IL-10 are both produced by activation of the innate immune system and are known to regulate MΦ function, but are differentially expressed in leprosy lesions. The ability of IL-15 to induce MΦ antimicrobial activity is consistent with the expression of this cytokine in self-limited T-lep lesions (Jullien et al., 1997); however the comparative effects of IL-10 on MΦ function are not known. We therefore hypothesized that key cytokines of the innate immune system, IL-15 and IL-10, trigger distinct MΦ functional programs with relevance to host defense in human infection.
RESULTS
IL-10 differentiates monocytes into CD209+CD163+ MΦ
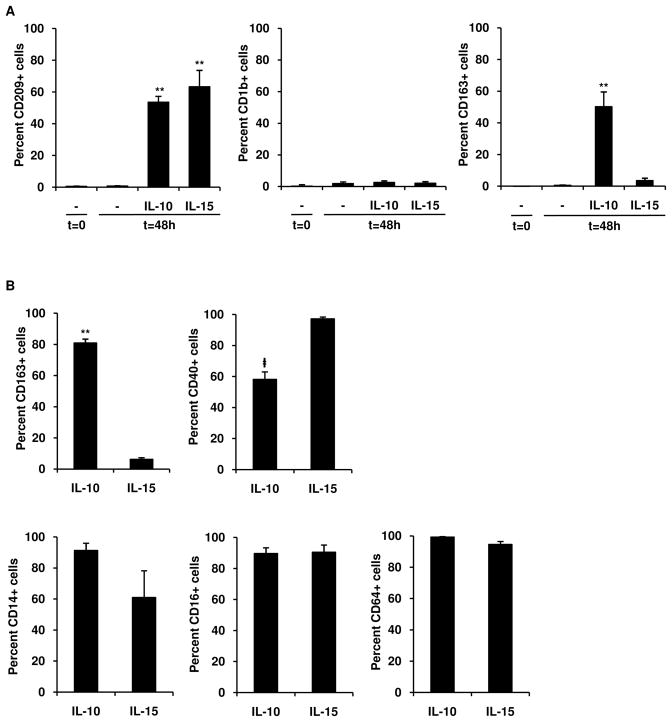
To compare the ability of two key innate immune cytokines, IL-10 and IL-15, to trigger MΦ functional programs (Sozzani et al., 1998; Krutzik et al., 2005), we cultured human peripheral blood monocytes with IL-10 or IL-15 for 2 days. Initially, we examined the expression of CD209, a C-type lectin receptor previously found to be expressed by tissue MΦ and induced by IL-15 (Krutzik et al., 2005), and known to be involved in mediating phagocytosis of M. leprae (Gringhuis et al., 2007). Both IL-10 and IL-15 induced monocytes to express CD209, but not the dendritic cell-specific marker, CD1b (Figure 1A). Maximal CD209 expression was achieved by 48 hours (data not shown). However, in addition to CD209, IL-10, but not IL-15 induced coexpression of CD163, the hemoglobin scavenger receptor (Kristiansen et al., 2001) (Figure 1A). In contrast, IL-15 induced greater expression of the co-stimulatory molecule CD40 (Figure 1B), while both CD209+ cell populations expressed MΦ specific markers CD14, CD16 (FcγRIII), and CD64 (FcγRI) (Figure 1B). Representative histograms of flow cytometry data can be seen in Figure S1. We also examined the mRNA expression of additional macrophage markers including NOS2, IL12B, MRC1, MGLL, ARG1 (Babu et al., 2009). Expression of MGLL mRNA was significantly higher in IL-10 vs. IL-15 stimulated monocytes (3.6E10 ± 4.6E9 vs. 8.1E9 ± 1.7E9 A.U., p < 0.05, n = 7). Although ARG1 was more strongly expressed in the IL-10 vs IL-15 stimulated monocytes, the difference was not significant (1.2E10 ± 5.8E9 vs. 6.2E9 ± 2.3E9 A.U., p = 0.26, n = 7). Taken together, these data provide evidence that IL-10 and IL-15 lead to the differential induction of MΦ cell surface markers.
Figure 1. IL-10 differentiates monocytes into CD209+CD163+ MΦ.
(A) Human peripheral monocytes were harvested at 0 h or stimulated for 48 h with IL-10, IL-15, or media and labeled with specific antibodies. Results shown as mean ± SEM (n ≥ 4). **p < 0.001 versus media (t = 48 h).
(B) IL-10 and IL-15 derived MΦ were double-labeled with antibodies against CD209 and specific markers. Results represent mean ± SEM (n ≥ 4) percent of CD209+ cells positive for indicated antibody. ‡p < 0.05 **p < 0.001 versus IL-15 derived MΦ.
IL-10, in comparison to IL-15, enhances endocytic and phagocytic activity
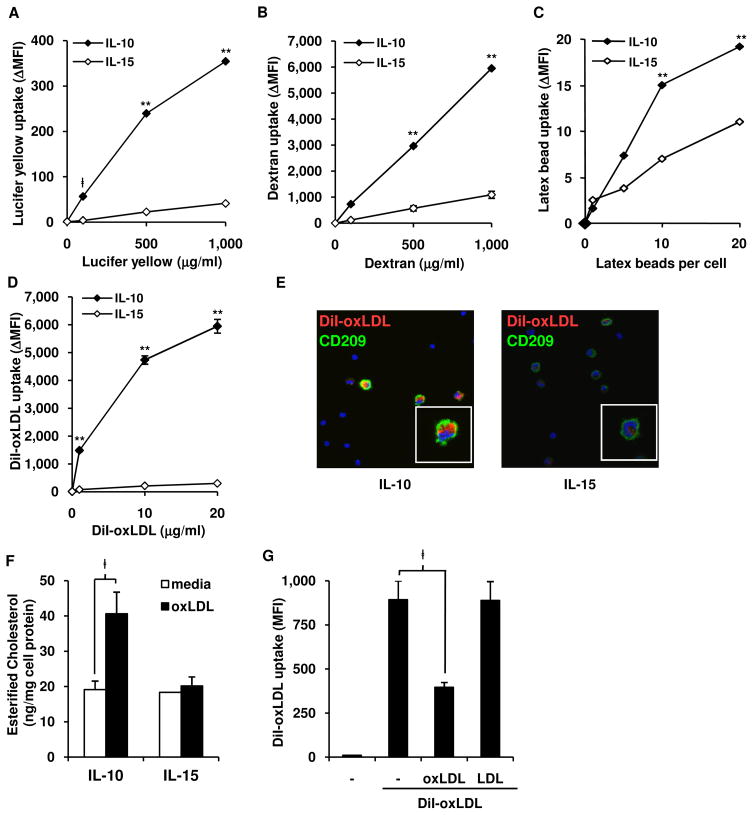
Given that IL-10 and IL-15 differentially induce MΦ cell-surface phenotypes, we next sought to determine if they induce distinct immune functions. A primary function for MΦ is the endocytosis and phagocytosis of particles to maintain tissue homeostasis and defend against microbial infection. We first investigated the ability of monocytes treated for 48 h with IL-10 vs. IL-15 to regulate pinocytosis according to uptake of, or binding with, lucifer yellow by flow cytometric analysis of CD209+ MΦ. Specific uptake was calculated as ΔMFI=MFIuptake-MFIbinding. Treatment of monocytes with IL-10 induced a 10-fold greater uptake of lucifer yellow (Figure 2A) at a range of concentrations, compared to treatment with IL-15. By the same method, we measured uptake of fluorescein-labeled dextran, which is taken up by pinocytosis and receptor-mediated endocytosis via the mannose receptor. Again, treatment of monocytes with IL-10 led to a 5-fold greater uptake of dextran at a range of concentrations, compared to treatment with IL-15 (Figure 2B). Binding alone with lucifer yellow and dextran had a similar trend as specific uptake but at a much lower level (Figure S2).
Figure 2. IL-10 derived MΦ have enhanced endocytic activity and develop into foam cell MΦ.
(A–D) Endocytosis assays comparing IL-10 and IL-15 programmed MΦ with (A) lucifer yellow (B) FITC-labeled dextran, (C) fluorescent latex beads, or (D) DiI-oxLDL, at indicated concentrations. Cells assayed for uptake at 37°C or binding at 4°C, labeled for CD209, data represented as net intracellular mean florescence intensity (ΔMFI=MFIuptake-MFIbinding) of indicated dye in CD209+ cells. Data represent the mean (n ≥ 3) ± SEM ‡p < 0.05, **p < 0.001 versus IL-15 derived MΦ.
(E) Confocal images of cells cultured and labeled as in (D). DiI-oxLDL, red; CD209, green; DAPI, blue.
(F) IL-10 or IL-15 derived MΦ were incubated with unlabeled oxLDL, lipids extracted and mean ± SEM (n = 3) of esterified cholesterol shown, normalized to amount of cell protein.
(G) Uptake of DiI-oxLDL was competed against excess amounts (30X of DiI-oxLDL) of unlabeled oxLDL, LDL or media and analyzed as in (D). Data represent the mean (n = 3) ± SEM ‡p < 0.05, *p < 0.005, **p < 0.001 versus IL-15 derived MΦ.
To measure phagocytosis, we evaluated the uptake of fluorescently-labeled latex beads. Treatment of monocytes with IL-10 as compared to IL-15 resulted in a two-fold greater level of phagocytosis (Figure 2C) and binding (Figure S2) of latex beads. In summary, IL-10, in comparison to IL-15, induces greater MΦ endocytic function.
IL-10 differentiates monocytes into foam cell MΦ
In order to maintain tissue homeostasis, a key function of MΦ is to scavenge accumulated metabolic products. For example, the accumulation of oxidized lipids in tissues is regulated by MΦ uptake. However, continued phagocytosis of oxidized lipids, such as oxLDL, can lead to the accumulation of intracellular lipid leading to foam cell formation (Li and Glass, 2002). Therefore, to investigate the capacity of IL-10 and IL-15 to induce foam cell formation, we measured binding and uptake of DiI (1, 1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate)-labeled CuSO4-oxidized low density lipoprotein (DiI-oxLDL). IL-10 derived MΦ were more efficient in uptake of oxLDL than IL-15, with over a 18-fold greater MFI (Figure 2D). Examination by confocal microscopy revealed that IL-10 treated cells contained a greater amount of intracellular DiI-oxLDL than IL-15 treated cells (Figure 2E). Similar results were observed with DiI-labeled acetylated LDL (data not shown), another modified lipoprotein used to evaluate foam cell formation.
A key measure of foam cell formation is the accumulation of esterified cholesterol (Li and Glass, 2002). Monocytes were treated with IL-10 vs. IL-15 for 48 h to derive MΦ, then incubated with unlabeled oxLDL (25 μg/ml) or media alone for 24 h, at which time cellular lipids were extracted assayed for cholesterol content. IL-10 treated monocytes incubated with oxLDL contained greater amounts of esterified cholesterol, a hallmark of foam cell formation, as compared to IL-10 treated cells with media alone or IL-15 treated monocytes incubated with oxLDL (Figure 2F). Free and total cholesterol were also elevated in IL-10 treated but not IL-15 treated cells (Figure S3A)
To confirm that uptake of DiI-oxLDL is specific for the oxidized form of LDL, we incubated cytokine-derived MΦ with DiI-LDL, and determined the DiI-LDL uptake was less than 7% of the uptake seen with DiI-oxLDL (Figure S3B). Furthermore, unlabeled oxLDL but not unlabeled LDL, was able to out-compete labeled DiI-oxLDL uptake by over 56% (Figure 2G). Collectively, these data demonstrate that IL-10, as compared to IL-15, programs MΦ for enhanced uptake of oxLDL resulting in foam cell formation.
SR-A and CD36 mediate oxLDL uptake in IL-10 programmed MΦ
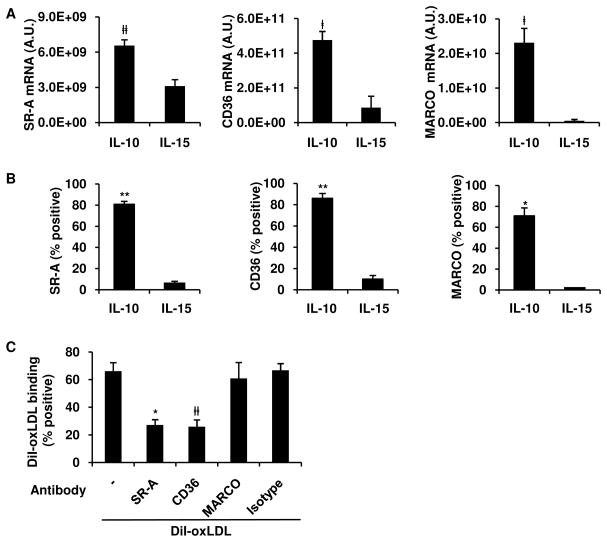
The uptake of oxLDL leading to foam cell formation is largely mediated via the scavenger receptors (SRs), a family of receptors defined through binding modified lipoproteins. The major scavenger receptors known to bind oxLDL and expressed on MΦ include the class A SR, scavenger receptor A (SR-A) and macrophage receptor with a collagenous structure (MARCO); and the class B SR, CD36. However, the relative role of each of these receptors in the pathogenesis of foam cell formation is unclear, particularly in human cells (Witztum, 2005). As an initial step to determine the mechanism for the differential oxLDL uptake by the IL-10 vs. IL-15 derived MΦ, we compared the gene expression program of SR by quantitative real-time PCR (qPCR). IL-10 derived MΦ had higher expression of SR-A, CD36, and MARCO than IL-15 derived MΦ (Figure 3A). Furthermore, flow cytometry showed that IL-10 derived MΦ were highly positive for SR-A (81%), CD36 (86%) and MARCO (71%), while surprisingly, IL-15 derived MΦ were largely negative for expression of any of the SRs (Figure 3B).
Figure 3. SR-A and CD36 mediate oxLDL uptake in IL-10 programmed MΦ.
(A–B) Monocytes were stimulated with IL-10 or IL-15 and scavenger receptor expression assessed by qPCR (A) after 24 r or surface protein expression (B) after 48 h on CD209+ cells by flow cytometry. Results show as mean ± SEM (n ≥ 3).
(C) Blocking antibodies against SR-A, CD36, MARCO, or isotype were pre-incubated with of IL-10 programmed MΦ and binding of DiI-oxLDL assayed. All results shown as mean ± SEM (n ≥ 3). ‡p < 0.05, #p < 0.01 *p < 0.005, **p < 0.001 versus IL-15 treated monocytes.
To evaluate if SRs are involved in oxLDL uptake, MΦ programmed by IL-10 were pretreated with the SR competitive inhibitors fucoidan and polyinosinic acid (poly-I), then incubated with DiI-oxLDL. Fucoidan (1–10 μg/mL) and poly-I (1–10 μg/mL), inhibited DiI-oxLDL uptake by 87% and 88% at their highest concentrations, respectively (Figure S4). Foam cell formation has also been reported to also be mediated by macropinocytosis (Kruth et al., 2005), however pretreatment of IL-10 programmed MΦ with pinocytosis inhibitor, dimethylamiloride (DMA), showed no reduction in uptake (Figure S4), indicating that uptake was not mediated by pinocytosis.
To determine the relative role of the each of the SRs to mediate binding of oxLDL to IL-10 programmed MΦ, we utilized available blocking monoclonal antibodies against SRs including SR-A (Fukuhara-Takaki et al., 2005), MARCO (Arredouani et al., 2005), and CD36 (Endemann et al., 1993). IL-10 programmed MΦ were pretreated with blocking antibodies or isotype control, and the percent of cells binding to DiI-oxLDL was measured. Blocking antibodies for SR-A and CD36, but not MARCO, were able to inhibit oxLDL uptake (Figure 3C). Taken together, our data demonstrate that the IL-10, as compared to IL-15, differentially induces a scavenger receptor program in CD209+ MΦ, with greater expression of SR-A and CD36, which mediate efficient binding to oxLDL.
Differential effect of IL-10 and IL-15 on MΦ phagocytosis of mycobacteria
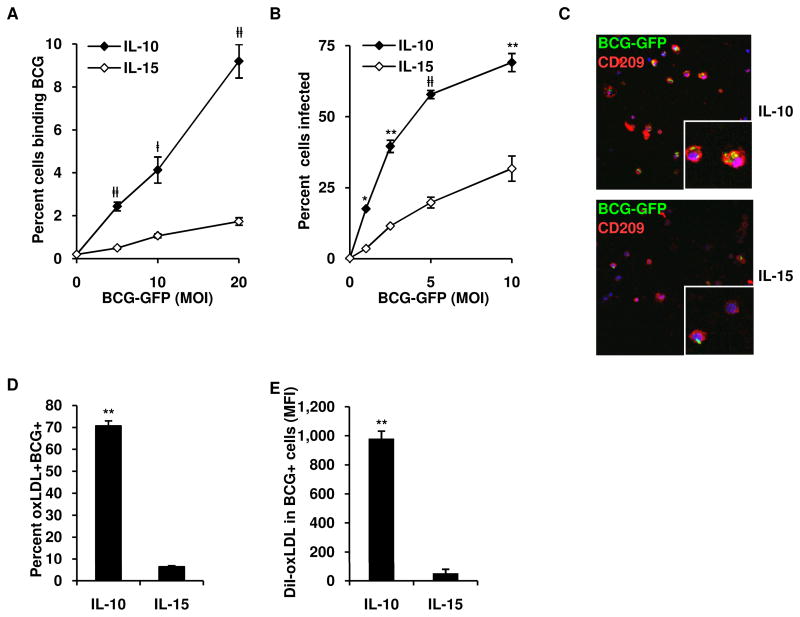
An important role of MΦ in host defense is the phagocytosis of microbial pathogens to facilitate their clearance. However, in mycobacterial disease, phagocytosis can contribute to disease progression if the bacteria persist. To first evaluate the ability of the cytokine-derived MΦ to bind mycobacteria, IL-10 and IL-15 derived adherent MΦ were incubated at 4°C for 2.5 h with Mycobacterium bovis, Bacille Calmette-Guérin (BCG) expressing green fluorescent protein (GFP). Across a range of multiplicities of infection (MOI) of BCG, 2–9% of IL-10 programmed MΦ bound BCG, while only 1–2% of IL-15 programmed MΦ exhibited BCG binding (Figure 4A). To evaluate the ability of MΦ to phagocytose and become infected with BCG, the MΦ were infected overnight at 37°C with BCG-GFP. Similar to the binding experiments, IL-10 programmed MΦ had a greater capacity to phagocytose BCG, with 18–69% bacteria containing cells, at a range of MOI from 1 to 10, compared to 4–32% for IL-15 programmed MΦ (Figure 4B). Confocal laser microscopy revealed that the overnight incubation with BCG resulted in complete internalization of mycobacteria as BCG were rarely observed bound to cell surface and at 2.5 MOI BCG, IL-10 derived macrophages contained twice as many bacilli per cell (2.4 ± 0.1 bacteria per cell) versus IL-15 derived macrophages (1.2 ± 0.1 bacteria per cell), p < 0.001, n = 4 (Figure 4C). Taken together, our data indicate that the IL-10, as compared to IL-15, is more effective at inducing a phagocytosis program for uptake of mycobacteria.
Figure 4. IL-10 derived MΦ coordinately phagocytose mycobacteria and oxLDL.
(A) Binding of BCG-GFP by IL-10 and IL-15 programmed MF
(B) Phagocytosis of BCG-GFP by IL-10 and IL-15 programmed MF. Results shown as mean ± SEM (n = 4) percent of CD209+ cells positive for BCG.
(C) Confocal images of MΦ cultured as in (B) (BCG-GFP, green; CD209, red; DAPI, blue).
(D–E) Coordinate uptake of oxLDL and BCG-GFP by IL-10 or IL-15 programmed MΦ, (D) percent of CD209+ cells positive for oxLDL and BCG. (E) Amount of DiI-oxLDL in CD209+ cells infected with BCG. Results show as mean ± SEM (n = 3). ‡p < 0.05, #p < 0.01, *p < 0.005, **p < 0.001 versus controls.
IL-10 programs MΦ to coordinately phagocytose mycobacteria and oxLDL
Although foam cell MΦ are present in several diseases, their presence is quite typical in disease lesions of leprosy and tuberculosis. The foam cell MΦ in mycobacterial disease, first identified by Virchow in 1863, contain mycobacteria, such that the accumulated lipid was thought to be derived from the intracellular bacteria (Virchow, 1863). Recently, we identified that the lipid within these foam cell MΦ is derived in part by the accumulation of host-derived oxidized lipids (Cruz et al., 2008). Therefore, we next compared the ability of the MΦ to coordinately take up both mycobacteria and oxLDL. IL-10 vs. IL-15 derived MΦ were incubated for 4 h at 37°C with BCG-GFP and DiI-oxLDL, labeled for CD209 and analyzed by flow cytometry as previously described. IL-10 programmed MΦ had a higher amount of mycobacteria+, oxLDL+ cells as compared to IL-15 programmed MΦ (71% vs 6%) (Figure 4D). Furthermore, of the cells that were mycobacteria+, oxLDL+ (double positive), IL-10 derived MΦ had a 10-fold higher oxLDL content than the IL-15 derived MΦ (Figure 4E).
IL-15 vs. IL-10 differentially programs the vitamin D antimicrobial pathway in MΦ
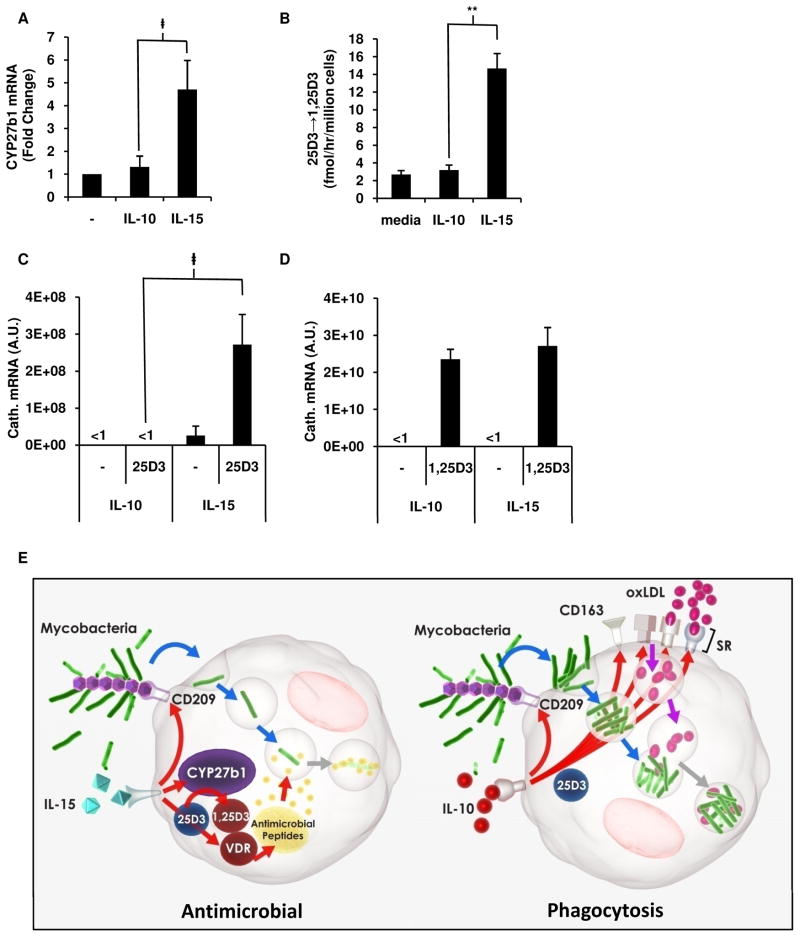
A key mechanism by which human MΦ kill intracellular mycobacteria is by an IL-15, vitamin D-dependent pathway involving the conversion of intracellular 25D3 to 1,25D3 by CYP27b1 resulting in the 1,25D3-dependent induction of the antimicrobial peptide cathelicidin (Liu et al., 2006; Liu et al., 2007). IL-15, but not IL-10, induces CYP27b1 after 24 h stimulation of monocytes (Figure 5A) To measure the ability of CYP27b1 to convert 25D3, monocytes were differentiated into MΦ with IL-10, IL-15, or media alone, then radiolabeled 25D3 was added for 6 h, and the amount of radiolabeled 1,25D3 assayed by HPLC. IL-15 programmed MΦ induced 7-fold greater rate of conversion to 1,25D3 as compared to IL-10 programmed MΦ or media treated cells (Figure 5b and Figure S5). Similarly, in IL-15 programmed MΦ, as compared to IL-10 programmed MΦ, 25D3 was sufficient by itself to induce cathelicidin mRNA (Figure 5C). The VDR was functional in all MΦ, since in both IL-15 and IL-10 derived MΦ 1,25D3 significantly induced cathelicidin mRNA (Figure 5D). These data suggest, that comparatively, IL-15 induces CYP27b1 and the vitamin D-dependent antimicrobial pathways in MΦ, while IL-10 induces a scavenger receptor program resulting in enhanced phagocytosis (Figure 5E).
Figure 5. IL-15 vs. IL-10 differentially programs the vitamin D antimicrobial pathway in MΦ.
(A) CYP27b1 expression measured by qPCR in monocytes stimulated with IL-10, IL-15 or media. Results shown as mean ± SEM (n = 3) fold change normalized to media stimulated cells.
(B) IL-10 or IL-15 derived MΦ cultured with radiolabeled 25D3 and rate of conversion to 1,25D3 measured by HPLC.
(C–D) Expression of cathelicidin mRNA after culturing IL-10 and IL-15 programmed MΦ with 25D3 (C) or 1,25D3 (D) vitamin D. All data represented as mean ± SEM (n = 3). ‡p < 0.05, **p < 0.001.
(E) Proposed model of divergence of phagocytosis and antimicrobial programs in MΦ. IL-10 induces a scavenger receptor program resulting in enhanced phagocytosis resulting in microbial persistence, while IL-15 induces the vitamin D mediated antimicrobial program resulting in microbial killing.
CD209+ MΦ in the different forms of leprosy lesions reflect the phenotype and function of the distinct IL-10 and IL-15 MΦ programs
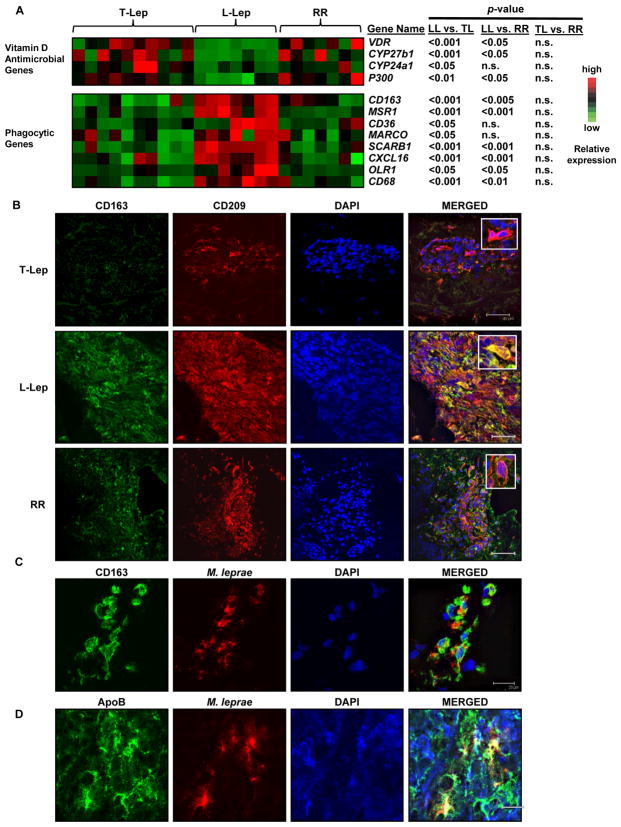
Given the dramatic dichotomy in the phenotype and function of the IL-10 vs. IL-15 programmed MΦ in vitro, we next evaluated the relevance of these phenotypic and functional subsets in leprosy, providing a model of human disease in which the innate and acquired immune response correlates with the clinical presentation of the disease. Previous data indicate that CD209+ MΦ are equally expressed in the lesions of the different clinical forms. However, gene expression profiles of lesions revealed that the phagocytic gene program, including gene expression of scavenger receptors was greater in L-lep vs. T-lep lesions. L-lep lesions were found to have higher expression of CD163, SR-A (MSR1), CD36, MARCO, and other scavenger receptors than T-lep lesions, correlating with the scavenger receptor gene expression program found in IL-10 derived MΦ (Figure 6A). In contrast, the vitamin D antimicrobial program was predominant in T-lep as compared to L-lep lesions, with greater expression of CYP27b1, the enzyme responsible for conversion of 25D3 to 1,25D3 and the VDR. Cathelicidin mRNA expression was below the limit of detection on the microarray with 100% of probes reporting absent. Finally, in patients with reversal reactions, in which there is a spontaneous conversion from the L-lep to T-lep form of the disease associated with enhanced immunity to M. leprae, there was upregulation of the vitamin D antimicrobial program and downregulation of the phagocytosis program, suggesting a plasticity of the responses in vivo depending on the cytokine environment.
Figure 6. Dynamics of the clinical response in leprosy patients reflects the phenotype and function of the IL-10 and IL-15 derived MΦ programs.
(A) Gene expression analysis of leprosy skin biopsies for vitamin D antimicrobial pathway and phagocytic program. Each column represents one donor. n.s. not significant.
(B) Phenotype of MΦ in human leprosy skin lesions: L-lep and T-lep skin lesions were labeled for CD163 (green), CD209 (red), and DAPI (blue). Scale bar 40μm.
(C–D) CD163+ MΦ in L-lep lesions labeled for accumulation of oxLDL and mycobacteria, by antibodies against (C) M. leprae or (D) ApoB. Representative data of at least three patients used for each groups. Scale bar 20μm.
To investigate the phenotype of MΦ in leprosy lesions, skin biopsy specimens from the L-lep and T-lep patients were labeled for the MΦ markers, CD209 and CD163. The majority of CD209+ cells present throughout the L-lep granulomas co-localized with CD163, with 64% of the area positive for CD209 also positive for CD163, correlating to the IL-10 derived MΦ phenotype. Conversely, in the T-lep lesions, CD209+ cells within the granulomas were largely negative for CD163, only 12% of CD209+ area also expressed CD163, correlating with the IL-15 derived MΦ phenotype (Figure 6B). CD209+CD163+ MΦ were less frequently present within the dermis of T-lep lesions, but outside the granulomas (data not shown), corresponding to the dermal tissue macrophage phenotype detected in normal skin (Ochoa et al., 2008). Again, reversal reactions were associated with a change in the CD209+ MΦ population, switching from CD163+ to CD163-, only 13% of area positive for CD209 colocalized with CD163.
A major histological feature of L-lep skin lesions is the presence of foamy MΦ containing large amounts of bacilli and lipid, called “Virchow” cells or “lepra” cells (Virchow, 1860; Virchow, 1863). Since the L-lep MΦ uniquely express CD209 and CD163, we investigated whether the CD163+ cells found in L-lep skin lesions corresponded to the Virchow cells. M. leprae was identified using a monoclonal antibody to its unique phenolic glycolipid, PGL-1. In L-lep lesions, approximately 50% of CD163+ MΦ were found to contain M. leprae PGL-1, often appearing in rod-shaped form (Figure 6C), consistent with the presence of bacilli in MΦ in L-lep lesions (although we cannot exclude that the PGL-1 antigen was taken up directly from the serum (Cho et al., 2001)). Since IL-10 derived MΦ coordinately take up both oxLDL and mycobacteria, we next evaluated if the M. leprae in L-lep MΦ co-localized with ApoB, the major lipoprotein in oxLDL, (Figure 6D). ApoB and M. leprae co-localized together in nearly all areas where M. leprae reactivity was seen, suggesting the CD163+ MΦ in L-lep lesions contain both mycobacteria and oxLDL. In summary, these data provide in vivo evidence that CD209+CD163+ MΦ take up both M. leprae and oxLDL, becoming the foam cells, and reflective of the IL-10 derived MΦ in vitro. Together these data indicate the differential expression of MΦ in leprosy lesions, as defined by phenotype and functional programs correlates with the outcome to the infection.
DISCUSSION
The modern view of the innate immune system, like that of Metchnikoff (Metschnikoff, 1884), has traditionally linked the phagocytic and antimicrobial function of MΦ. Our data however, indicate a divergence of the phagocytic and antimicrobial programs that corresponds to the outcome of the host response to the pathogen in human leprosy. Specifically, the phagocytic program was induced in MΦ by IL-10 and evident in the lesions of leprosy patients with the progressive multibacillary L-lep form of the disease. In contrast, the vitamin D antimicrobial program was induced in MΦ by IL-15 and evident in the lesions of leprosy patients with the self healing T-lep form of the disease. The distinct programs induced by IL-10 vs. IL-15 in MΦ in vitro are linked to the disease lesions in vivo in which the expression of IL-10 vs. IL-15 correlate with gene profiles for the phagocytic vs. antimicrobial programs. Importantly, in patients undergoing reversal reactions spontaneously converting from the L-lep to the T-lep form, there was a switch from the phagocytic program to the antimicrobial program, linking these pathways to disease outcome. These data establish that the innate immune response, by selectively inducing IL-10 vs. IL-15, differentially programs MΦ for phagocytosis vs. antimicrobial responses that largely determines the outcome of infection, either to host resistance or to pathogenesis in infectious disease.
What is the possible advantage of the host to having different programs or limiting the phagocytic activity of the macrophages with antimicrobial activity? One possibility is that there are limits on the capacity of macrophages to kill intracellular pathogen. It is possible that the phagocytic program in MΦ activated by IL-15 is limited to prevent the uptake of more microorganisms than can be killed by a given cell. In a MΦ model of Trypanosoma cruzi infection, it was determined that the amount of reactive oxygen intermediates generated was limiting, and under various conditions at most 1–6 parasites could be killed by a given activated MΦ (Tanaka et al., 1982); phagocytosis of greater than 6.7 parasites resulted in approximately 20% of MΦ that allowed the parasites to survive, replicate and spread. In this manner, limiting the phagocytic function of a given MΦ would optimize the antimicrobial response against the ingested organisms. Further limitations are imposed by the pathogen, which can secrete virulence factors that interfere with antimicrobial pathways. For example, mycobacteria secrete glycolipids that in mouse MΦ scavenge free oxygen radicals (Chan et al., 1991). Little is known about the amount of antimicrobial peptides generated in human MΦ, but it is likely the amount of antimicrobial peptides generated by a macrophage is limiting, and that only a defined number of mycobacteria can be efficiently killed in a single cell.
Previous studies indicate that IFN-γ can limit phagocytosis (Konopski et al., 1994; Backman and Guyre, 1994) but also induce an antimicrobial response, providing a mechanism by which the acquired T cell response regulates these pathways. The present study provides insight into mechanisms by which the innate immune response differentially regulates the phagocytic vs. antimicrobial pathways. The IL-15 derived MΦ program results in limited phagocytosis of mycobacteria along with upregulation of the vitamin D-dependent expression of the antimicrobial peptide cathelicidin, allowing the convergence of the phagocytic and antimicrobial programs for optimal host defense against microbial infection. In contrast, the IL-10 derived MΦ program involves significantly enhanced phagocytosis of both oxLDL and mycobacteria, without the ability to trigger the vitamin D-dependent antimicrobial pathway, indicating a divergence between the phagocytic and antimicrobial programs, providing a favorable intracellular environment for mycobacterial survival. The coordinate uptake of oxLDL and mycobacteria may contribute to the pathogenesis of chronic infection, providing a lipid substrate for energy via the glyoxylate shunt and synthesis of complex bacterial lipids (Ehrt and Schnappinger, 2007). Simultaneously, these lipids inhibit the innate immune response against the bacteria through diminishing TLR-induced antimicrobial activity and skewing the cytokine balance to induce more IL-10 and less IL-12 cytokine production (Cruz et al., 2008). The defining receptor of IL-10 derived MΦ, CD163, has been shown to mediate the uptake of hemoglobin-haptoglobin complex (Kristiansen et al., 2001), providing a source of iron required for mycobacterial survival (Ratledge and Dover, 2000) and also triggering further IL-10 production (Philippidis et al., 2004). MΦ similar to those derived by IL-10 were found in the progressive form of leprosy, with the colocalization of the CD209 and CD163 markers, and the presence of M. leprae, ApoB and host-derived oxidized phospholipids (Krutzik et al., 2005; Cruz et al., 2008).
A physiologic role for IL-10 programmed MΦ pertains to their ability to take up various biomolecules relevant for tissue repair, removal of excess metabolic products and debris clearance. IL-10 programmed MΦ were characterized by high expression of C-type lectin receptors (CD209, CD206) and scavenger receptors (CD163, SR-A, CD36, MARCO), implicating a role of IL-10 derived MΦ in the uptake of lipids (Terpstra et al., 1998), lipoproteins (Parthasarathy et al., 1987), apoptotic cells (Platt et al., 1996), and hemoglobin (Kristiansen et al., 2001), key functions of MΦ in maintaining tissue homeostasis. Furthermore, a variety of resident tissue MΦ, including skin (Ochoa et al., 2008; Zaba et al., 2007), lung (Soilleux et al., 2002a; Van den Heuvel et al., 1999), CNS (Fabriek et al., 2005), placenta (Bockle et al., 2008), and adipose tissue (Zeyda and Stulnig, 2007), share with IL-10 derived MΦ the expression of CD209 and CD163, as well as phagocytic function. The foam cells in atherosclerosis are similar to IL-10 programmed MΦ; foam cell MΦ express CD209 (Soilleux et al., 2002b), CD163 (Komohara et al., 2006) and the scavenger receptors SR-A and CD36 (Nakata et al., 1999). This type of MΦ is linked to other inflammatory and metabolic diseases in which IL-10 production and foam cell formation are prominent, including Whipple disease (Desnues et al., 2006) and xanthomas (Caputo et al., 1986). Although IL-10 may enhance foam cell formation by induction of a phagocytic program, other mechanisms may also contribute such as enhanced cell survival through decreased apoptosis (Halvorsen et al., 2005).
The relevance of the MΦ functional programs to the outcome in human infectious disease provides a challenge for investigation, in particular in leprosy and tuberculosis, in which there is no animal model which fully mimics the human condition. Fortunately, nature has provided us such an opportunity in leprosy, since it is not a static disease but an extremely dynamic condition, in which immune changes alter the clinical manifestations, in the form of “reactional states.” One of these reactional states, so-called “reversal reactions” provide a window onto the dynamic immune events associated with mechanisms of immunoregulation and immune-mediated tissue injury in human disease. Reversal reactions are clinically recognized by the upgrading from the L-lep towards the T-lep pole and clearance of bacilli from lesions (Waters et al., 1971). Evidence has suggested the pathogenesis of reversal reaction is a naturally occurring delayed-type hypersensitivity (DTH) or type IV immunologic response to M. leprae, involving influx of CD4+ T-cells (Modlin et al., 1983) and a change in the local cytokine pattern from type 2 to type 1, including a reduction in IL-10 expression (Godal et al., 1973; Barnetson et al., 1976; Bjune et al., 1976; Rea and Taylor, 1977; Yamamura et al., 1992). Here, we demonstrate the dynamic change in MΦ functional programs, with a switch from the phagocytic to the vitamin D antimicrobial profile. Furthermore, reversal reactions involved a change in MΦ phenotype, resulting in the loss of the scavenger receptor CD163 expression on CD209+ cells. These data provide evidence for MΦ plasticity in innate immunity, either in the programming of a given MΦ or by the influx of new pre-programmed MΦ and indicate that changes in MΦ functional programs are relevant to clinical outcome in human infectious disease.
A key question that remains is how the innate immune system regulates the differential production of IL-15 vs. IL-10. TLR activation induces both IL-15 (Krutzik et al., 2005) and IL-10 (Cruz et al., 2008) production. In addition, immune complexes, known to be abundant in the L-lep form, trigger the differentiation of MΦ which produce IL-10 (Mosser and Edwards, 2008; Tripp et al., 1995) with the potential to amplify the IL-10 induced MΦ program. Nevertheless, our investigation provides insight into the mechanisms by which the innate immune response, principally through the production of IL-15 vs. IL-10, regulates the divergent MΦ functional programs for phagocytosis vs. antimicrobial responses. The differential regulation of these pathways optimizes antimicrobial efficiency required for host defense against microbial pathogens, yet the divergence of these programs can also contribute to the pathogenesis of infectious disease. Therefore, understanding how to modulate these MΦ functional programs may be useful to developing new interventions in human infectious and metabolic diseases in which macrophage function is central.
EXPERIMENTAL PROCEDURES
Antibodies and cytokines
Antibodies used for cell surface markers were as follows: CD1b (OKT6, Bcd3.1, ATCC), CD14, CD40, and IgG controls (Invitrogen), MARCO (Hycult Biotechnology), SR-A (R&D Systems), CD16, CD36, CD64, CD80, CD86, CD163, CD209, HLA-DR (BD Pharmingen). Recombinant human IL-10 and IL-15 (R&D Systems) cytokines were used to differentiate monocytes into MΦ.
Macrophage differentiation
Human peripheral blood was obtained from healthy donors (UCLA Institutional Review Board # 92-10-591-31) and adherent monocytes were isolated as previously described (Liu et al., 2006). Monocytes were then stimulated with media, IL-10 (10 ng/ml), or IL-15 (200 ng/ml) in 10% FCS in RPMI (Invitrogen) for 48 h at 37°C.
Cell surface labeling
Surface expression was determined using specific antibodies. A phycoerythrin-conjugated secondary antibody (Invitrogen) was used for CD1b, SR-A, and MARCO. Cells were acquired and analyzed as described (Krutzik et al., 2005).
Endocytosis and binding assays
IL-10 or IL-15 derived MΦ were incubated with the following: lucifer yellow or fluorescein-labeled dextran (Invitrogen) for 1 h 37°C. BCG-GFP (gift from Dr. Barry Bloom, Harvard) or 1μm yellow-green labeled polystyrene latex beads (Sigma) was incubated with cytokine-derived MΦ for 16 h 37°C as previously described (Krutzik et al., 2005; Krutzik et al., 2008; Cruz et al., 2008). Binding was assayed by incubating macrophages with lucifer yellow, FITC-dextran or BCG-GFP for 2.5 h at 4°C. Cells were then harvested, labeled with CD209, and analyzed by flow cytometry. Intracellular uptake shown as ΔMFI=MFIuptake-MFIbinding, unless binding and uptake MFI are shown separately. BCG–GFP infected MΦ were also visualized and quantified via confocal microscopy as described (Ochoa et al., 2008). Intracellular BCG was quantified from confocal images of CD209+ cells.
Foam cell formation
IL-10 and IL-15 programmed MΦ were cultured with DiI(1, 1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate)-labeled CuSO4-oxidized low density lipoprotein (Intracel) for 2.5 h, 4°C for binding, or 4 h, 37°C, for uptake. Cells were harvested, labeled with CD209, and analyzed by flow cytometry and confocal microscopy. Blocking assays were performed by pre-incubating MΦ with media, poly-I, fucoidan (Sigma), isotype, SR-A specific antibody (Cosmo Bio USA), or CD36 specific antibody (Becton Dickinson) for 30 min at 4°C then incubated with DiI-oxLDL for 2.5 h at 4°C for binding and 37° for uptake. For cellular lipid analysis, monocytes, differentiated with IL-10 or IL-15 for two days, were incubated with CuSO4-oxidized low density lipoprotein (oxLDL was generously provided by the lipid core of the Atherosclerosis Research Unit at UCLA) for 24 h then washed with cold PBS and lipids extracted by 3:2 (hexane:isopropanol) as described (Bligh and Dyer, 1959). Cholesterol measured by Amplex Red (Invitrogen) and protein measured by BCA protein assay (Pierce) according to manufacturer protocols.
Real time quantitative PCR
Monocytes were stimulated with IL-10, IL-15, or media for 24 h or differentiated MΦ were stimulated with media, 25D3, or 1,25D3 (Biomol) for 24 h. RNA was isolated and synthesized cDNA as described (Liu et al., 2006). Quantitect primers (Qiagen) were used for determining mRNA expression of MSR1 (QT00064141), CD36 (QT01674008), MARCO (QT00011977) and CAMP (QT00010458), NOS2 (QT00068740), IL12B (QT00000364), ARG1 (QT00068446), MGLL (QT00039837) MRC1 (QT00012810). Reactions used Sybr Green PCR Master Mix (BioRad), normalized to h36B4, and relative arbitrary units calculated as described (Liu et al., 2006).
Measurement of Vitamin D bioconversion by MΦ
CYP27b1 activity was assessed in IL-10 and IL-15 derived MΦ as previously described(Krutzik et al., 2008). Briefly, [3H]-25D3 was added to 106 cells in 200 ul serum-free medium then incubated for 5 h at 37°C. Vitamin D metabolites then extracted and separated by HPLC and elution profiles determined by UV absorbance at 264nm. Lauralite 3 software (LabLogic) was used to quantify peaks of radioactivity corresponding to 25D3 or 1,25D3.
Patients and clinical specimens
We classified patients with leprosy according to the criteria of Ridley and Jopling, all T-lep patients classified as borderline tuberculoid (BT) and all L-lep patients had lepromatous leprosy (LL). All T-lep and L-lep specimens were taken at the time of diagnosis before treatment, and reversal reaction biopsies were upgrading from patients originally diagnosed with borderline lepromatous leprosy (Krutzik et al., 2005), therefore starting from a different part of the disease spectrum than the L-lep group. All leprosy patients were recruited with approval from the Institutional Review Board of University of Southern California School of Medicine and the Institutional Ethics Committee of Oswald Cruz Foundation.
Microarray data analysis
Microarrays using Affymetrix Human U133 Plus 2.0 array comparing 24 leprosy patients (T-lep, n = 10; L-lep, n = 7; RR n = 7) and analyzed as previously been described (Bleharski et al., 2003). A supervised analysis was performed for scavenger receptors/phagocytosis genes and vitamin D mediated antimicrobial pathway. Only genes that met differential expression criteria between groups (p < 0.05; fold change > 1.5) were depicted. The raw gene expression data analyzed in this study are available online through the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo/) in series entity GSE17763 (provisional).
Immunofluorescence
Double immunofluorescence on leprosy skin lesions was performed and examined as described (Krutzik et al., 2005). Briefly, antibodies against CD163 (AbD Serotec) or ApoB (MB47, gift from Dr. Joseph Witztum UCSD), and were incubated with tissue sections followed by an Alexa-Fluor 488 isotype-specific secondary (Invitrogen). Sections were washed, incubated with antibodies for CD209, Mycobacterium leprae PGL-1 (gift from Dr. Patrick Brennan) followed by an Alexa Fluor 568 isotype-specific secondary (Invitrogen). Cells were then preserved with Prolong Gold with DAPI (Invitrogen). Colocalization analysis performed by Andor IQ RGB analysis tool (Andor Technoology, Ireland).
Statistical analyses
Statistical significance was calculated by paired two-tailed Students t-test.
Supplementary Material
Acknowledgments
Funding provided by Louis Stokes Alliance for Minority Participation (LSAMP) National Science Foundation (NSF) Predoctoral Fellowship, and by NIH Minority Supplement (NIH R01 AR 40312). Much appreciation to Barry Bloom, Judith Berliner, Patrick Brennan, XinMin Li and Philip Liu for helpful scientific discussions. We also thank Yubal Ebenstein and Matthew Schibler at the Advanced Light Microscopy core facility, California NanoSystems Institute for help in confocal image analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arredouani MS, Palecanda A, Koziel H, Huang YC, Imrich A, Sulahian TH, Ning YY, Yang Z, Pikkarainen T, Sankala M, Vargas SO, Takeya M, Tryggvason K, Kobzik L. MARCO is the major binding receptor for unopsonized particles and bacteria on human alveolar macrophages. J Immunol. 2005;175:6058–6064. doi: 10.4049/jimmunol.175.9.6058. [DOI] [PubMed] [Google Scholar]
- Babu S, Kumaraswami V, Nutman TB. Alternatively activated and immunoregulatory monocytes in human filarial infections. J Infect Dis. 2009;199:1827–1837. doi: 10.1086/599090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman KA, Guyre PM. Gamma-interferon inhibits Fc receptor II-mediated phagocytosis of tumor cells by human macrophages. Cancer Res. 1994;54:2456–2461. [PubMed] [Google Scholar]
- Barnetson RS, Bjune G, Pearson JMH, Kronvall G. Cell mediated and humoral immunity in “reversal reactions”. Int J Lepr. 1976;44:267–273. [PubMed] [Google Scholar]
- Bjune G, Barnetson RS, Ridley DS, Kronvall G. Lymphocyte transformation test in leprosy: correlation of the response with inflammation of lesions. Clin Exp Immunol. 1976;25:85–94. [PMC free article] [PubMed] [Google Scholar]
- Bleharski JR, Li H, Meinken C, Graeber TG, Ochoa MT, Yamamura M, Burdick A, Sarno EN, Wagner M, Rollinghoff M, Rea TH, Colonna M, Stenger S, Bloom BR, Eisenberg D, Modlin RL. Use of genetic profiling in leprosy to discriminate clinical forms of the disease. Science. 2003;301:1527–1530. doi: 10.1126/science.1087785. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bockle BC, Solder E, Kind S, Romani N, Sepp NT. DC-sign+ CD163+ macrophages expressing hyaluronan receptor LYVE-1 are located within chorion villi of the placenta. Placenta. 2008;29:187–192. doi: 10.1016/j.placenta.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Caputo R, Monti M, Berti E, Gasparini G. Normolipemic eruptive cutaneous xanthomatosis. Arch Dermatol. 1986;122:1294–1297. [PubMed] [Google Scholar]
- Chan J, Fan XD, Hunter SW, Brennan PJ, Bloom BR. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun. 1991;59:1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SN, Cellona RV, Villahermosa LG, Fajardo TT, Jr, Balagon MV, Abalos RM, Tan EV, Walsh GP, Kim JD, Brennan PJ. Detection of phenolic glycolipid I of Mycobacterium leprae in sera from leprosy patients before and after start of multidrug therapy. Clin Diagn Lab Immunol. 2001;8:138–142. doi: 10.1128/CDLI.8.1.138-142.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz D, Watson AD, Miller CS, Montoya D, Ochoa MT, Sieling PA, Gutierrez MA, Navab M, Reddy ST, Witztum JL, Fogelman AM, Rea TH, Eisenberg D, Berliner J, Modlin RL. Host-derived oxidized phospholipids and HDL regulate innate immunity in human leprosy. J Clin Invest. 2008;118:2917–2928. doi: 10.1172/JCI34189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnues B, Ihrig M, Raoult D, Mege JL. Whipple’s disease: a macrophage disease. Clin Vaccine Immunol. 2006;13:170–178. doi: 10.1128/CVI.13.2.170-178.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrt S, Schnappinger D. Mycobacterium tuberculosis virulence: lipids inside and out. Nat Med. 2007;13:284–285. doi: 10.1038/nm0307-284. [DOI] [PubMed] [Google Scholar]
- Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- Fabriek BO, Van Haastert ES, Galea I, Polfliet MM, Dopp ED, Van den Heuvel MM, Van den Berg TK, De Groot CJ, Van DV, Dijkstra CD. CD163-positive perivascular macrophages in the human CNS express molecules for antigen recognition and presentation. Glia. 2005;51:297–305. doi: 10.1002/glia.20208. [DOI] [PubMed] [Google Scholar]
- Fukuhara-Takaki K, Sakai M, Sakamoto Y, Takeya M, Horiuchi S. Expression of class A scavenger receptor is enhanced by high glucose in vitro and under diabetic conditions in vivo: one mechanism for an increased rate of atherosclerosis in diabetes. J Biol Chem. 2005;280:3355–3364. doi: 10.1074/jbc.M408715200. [DOI] [PubMed] [Google Scholar]
- Godal T, Myrvang B, Samuel DR, Ross WF, Lofgren M. Mechanism of reactions in borderline tuberculoid (BT) leprosy. Acta Pathol Microbiol Scand. 1973;236(suppl):45–53. [PubMed] [Google Scholar]
- Greaves DR, Gordon S. The macrophage scavenger receptor at 30 years of age - current knowledge and future challenges. J Lipid Res. 2008 doi: 10.1194/jlr.R800066-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringhuis SI, den DJ, Litjens M, van Het HB, van KY, Geijtenbeek TB. C-type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity. 2007;26:605–616. doi: 10.1016/j.immuni.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Halvorsen B, Waehre T, Scholz H, Clausen OP, von der Thusen JH, Muller F, Heimli H, Tonstad S, Hall C, Froland SS, Biessen EA, Damas JK, Aukrust P. Interleukin-10 enhances the oxidized LDL-induced foam cell formation of macrophages by antiapoptotic mechanisms. J Lipid Res. 2005;46:211–219. doi: 10.1194/jlr.M400324-JLR200. [DOI] [PubMed] [Google Scholar]
- Hunter RL, Jagannath C, Actor JK. Pathology of postprimary tuberculosis in humans and mice: contradiction of long-held beliefs. Tuberculosis (Edinb) 2007;87:267–278. doi: 10.1016/j.tube.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Jullien D, Sieling PA, Uyemura K, Mar ND, Rea TH, Modlin RL. IL-15, an immunomodulator of T cell responses in intracellular infection. J Immunol. 1997;158:800–806. [PubMed] [Google Scholar]
- Komohara Y, Hirahara J, Horikawa T, Kawamura K, Kiyota E, Sakashita N, Araki N, Takeya M. AM-3K, an anti-macrophage antibody, recognizes CD163, a molecule associated with an anti-inflammatory macrophage phenotype. J Histochem Cytochem. 2006;54:763–771. doi: 10.1369/jhc.5A6871.2006. [DOI] [PubMed] [Google Scholar]
- Konopski Z, Seljelid R, Eskeland T. IFN-gamma inhibits internalization of soluble aminated beta-1,3-D-glucan by macrophages and thereby down-regulates the glucan induced release of TNF-alpha and IL-1 beta. Scand J Immunol. 1994;40:57–63. doi: 10.1111/j.1365-3083.1994.tb03433.x. [DOI] [PubMed] [Google Scholar]
- Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- Kruth HS, Jones NL, Huang W, Zhao B, Ishii I, Chang J, Combs CA, Malide D, Zhang WY. Macropinocytosis is the endocytic pathway that mediates macrophage foam cell formation with native low density lipoprotein. J Biol Chem. 2005;280:2352–2360. doi: 10.1074/jbc.M407167200. [DOI] [PubMed] [Google Scholar]
- Krutzik SR, Hewison M, Liu PT, Robles JA, Stenger S, Adams JS, Modlin RL. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol. 2008;181:7115–7120. doi: 10.4049/jimmunol.181.10.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutzik SR, Tan B, Li H, Ochoa MT, Liu PT, Sharfstein SE, Graeber TG, Sieling PA, Liu YJ, Rea TH, Bloom BR, Modlin RL. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med. 2005;11:653–660. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nat Med. 2002;8:1235–1242. doi: 10.1038/nm1102-1235. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- Lucas SB. Histopathology of leprosy and tuberculosis--an overview. Br Med Bull. 1988;44:584–599. doi: 10.1093/oxfordjournals.bmb.a072269. [DOI] [PubMed] [Google Scholar]
- Metschnikoff E. Ueber eine Sprosspilzkrankheit der Daphnien. Beitrag zur Lehre uber den Kampf der Phagocyten gegen Krankheitserrenger. Archiv f pathologische Anatomie und Physiologie und f klinische Medicin. 1884;96:177–195. [Google Scholar]
- Modlin RL, Gebhard JF, Taylor CR, Rea TH. In situ characterization of T lymphocyte subsets in the reactional states of leprosy. Clin Exp Immunol. 1983;53:17–24. [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata A, Nakagawa Y, Nishida M, Nozaki S, Miyagawa J, Nakagawa T, Tamura R, Matsumoto K, Kameda-Takemura K, Yamashita S, Matsuzawa Y. CD36, a novel receptor for oxidized low-density lipoproteins, is highly expressed on lipid-laden macrophages in human atherosclerotic aorta. Arterioscler Thromb Vasc Biol. 1999;19:1333–1339. doi: 10.1161/01.atv.19.5.1333. [DOI] [PubMed] [Google Scholar]
- Ochoa MT, Loncaric A, Krutzik SR, Becker TC, Modlin RL. “Dermal dendritic cells” comprise two distinct populations: CD1+ dendritic cells and CD209+ macrophages. J Invest Dermatol. 2008 doi: 10.1038/jid.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel W. Zur Histochemie der Lungentuberkulose, mit besonderer Berrucksichtung der Fettsubstanzen und Lipode. (Fat and Lipoid content to tuberculous tissue Histochemical investigation.) Virchows Archiv A Pathol Anat. 1925;256:629–640. [Google Scholar]
- Parthasarathy S, Fong LG, Otero D, Steinberg D. Recognition of solubilized apoproteins from delipidated, oxidized low density lipoprotein (LDL) by the acetyl-LDL receptor. Proc Natl Acad Sci U S A. 1987;84:537–540. doi: 10.1073/pnas.84.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippidis P, Mason JC, Evans BJ, Nadra I, Taylor KM, Haskard DO, Landis RC. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res. 2004;94:119–126. doi: 10.1161/01.RES.0000109414.78907.F9. [DOI] [PubMed] [Google Scholar]
- Platt N, Suzuki H, Kurihara Y, Kodama T, Gordon S. Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc Natl Acad Sci U S A. 1996;93:12456–12460. doi: 10.1073/pnas.93.22.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria. Annu Rev Microbiol. 2000;54:881–941. doi: 10.1146/annurev.micro.54.1.881. [DOI] [PubMed] [Google Scholar]
- Rea TH, Taylor CR. Serum and tissue lysozyme in leprosy. Infect Immun. 1977;18:847–856. doi: 10.1128/iai.18.3.847-856.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley DS, Ridley MJ. Rationale for the histological spectrum of tuberculosis. A basis for classification. Pathology. 1987;19:186–192. doi: 10.3109/00313028709077132. [DOI] [PubMed] [Google Scholar]
- Sakurai I, Skinsnes OK. Lipids in leprosy. 2 Histochemistry of lipids in human leprosy. Int J Lepr Other Mycobact Dis. 1970;38:389–403. [PubMed] [Google Scholar]
- Soilleux EJ, Morris LS, Leslie G, Chehimi J, Luo Q, Levroney E, Trowsdale J, Montaner LJ, Doms RW, Weissman D, Coleman N, Lee B. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol. 2002a;71:445–457. [PubMed] [Google Scholar]
- Soilleux EJ, Morris LS, Trowsdale J, Coleman N, Boyle JJ. Human atherosclerotic plaques express DC-SIGN, a novel protein found on dendritic cells and macrophages. J Pathol. 2002b;198:511–516. doi: 10.1002/path.1205. [DOI] [PubMed] [Google Scholar]
- Sozzani S, Ghezzi S, Iannolo G, Luini W, Borsatti A, Polentarutti N, Sica A, Locati M, Mackay C, Wells TN, Biswas P, Vicenzi E, Poli G, Mantovani A. Interleukin 10 increases CCR5 expression and HIV infection in human monocytes. J Exp Med. 1998;187:439–444. doi: 10.1084/jem.187.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Kiyotaki C, Tanowitz H, Bloom BR. Reconstitution of a variant macrophage cell line defective in oxygen metabolism with a H2O2-generating system. Proc Natl Acad Sci U S A. 1982;79:2584–2588. doi: 10.1073/pnas.79.8.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra V, Bird DA, Steinberg D. Evidence that the lipid moiety of oxidized low density lipoprotein plays a role in its interaction with macrophage receptors. Proc Natl Acad Sci U S A. 1998;95:1806–1811. doi: 10.1073/pnas.95.4.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp CS, Beckerman KP, Unanue ER. Immune complexes inhibit antimicrobial responses through interleukin-10 production. Effects in severe combined immunodeficient mice during Listeria infection. J Clin Invest. 1995;95:1628–1634. doi: 10.1172/JCI117837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel MM, Tensen CP, van As JH, Van den Berg TK, Fluitsma DM, Dijkstra CD, Dopp EA, Droste A, Van Gaalen FA, Sorg C, Hogger P, Beelen RH. Regulation of CD 163 on human macrophages: cross-linking of CD163 induces signaling and activation. J Leukoc Biol. 1999;66:858–866. doi: 10.1002/jlb.66.5.858. [DOI] [PubMed] [Google Scholar]
- Virchow R. Die krankhaften Geschwülste. Berlin: August Hirschwald; 1863. [Google Scholar]
- Virchow R. Cellular Pathology as based upon physiological and pathological histology. London: 1860. (reprinted by The Classics of Medicine Library, 1978, Birmingham, AL.)) [DOI] [PubMed] [Google Scholar]
- Waters MFR, Turk JL, Wemambu SNC. Mechanisms of reaction in leprosy. Int J Lepr. 1971;39:417–428. [PubMed] [Google Scholar]
- Witztum JL. You are right too! J Clin Invest. 2005;115:2072–2075. doi: 10.1172/JCI26130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura M, Uyemura K, Deans RJ, Weinberg K, Rea TH, Bloom BR, Modlin RL. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991;254:277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]
- Yamamura M, Wang XH, Ohmen JD, Uyemura K, Rea TH, Bloom BR, Modlin RL. Cytokine patterns of immunologically mediated tissue damage. J Immunol. 1992;149:1470–1475. [PubMed] [Google Scholar]
- Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11cBDCA-1 dendritic cells and CD163FXIIIA macrophages. J Clin Invest. 2007;117:2517–2525. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyda M, Stulnig TM. Adipose tissue macrophages. Immunol Lett. 2007;112:61–67. doi: 10.1016/j.imlet.2007.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.