Abstract
Background:
Clinical trials involving patients with glioblastoma (GBM) distinguish cohorts who are treated with enzyme-inducing anticonvulsants (EIAC). Such anticonvulsants induce hepatic P450 microsomal enzymes, which accelerate the metabolism of certain chemotherapy and molecular targeted agents. However, the resultant effect of such induction on patient outcome has received limited study.
Methods:
We performed a correlative analysis of baseline EIAC use with outcome, using a cross-sectional database of 620 patients with newly diagnosed GBM treated prospectively on North Central Cancer Treatment Group trials.
Results:
At registration, 72% were receiving treatment with EIAC; 2% were receiving non-EIACs, and the 26% were not receiving anticonvulsants (26%). Surprisingly, in the multivariable Cox model, overall survival (OS) and progression-free survival (PFS) showed a positive correlation with EIAC use (hazard ratio [HR] = 0.75, p = 0.0028 and HR = 0.80, p = 0.022), even after adjustment for the known prognostic factors of age, performance status, extent of resection, steroid use, and baseline neurocognitive function. Specifically, the median OS was longer in EIAC compared with non-EIAC patients (12.3 vs 10.7 months, p = 0.0002). Similarly, PFS was longer in EIAC patients (5.6 vs 4.8 months, p = 0.003). No differences in median OS or PFS were observed when comparing patients with or without a history of seizures at baseline.
Conclusions:
Paradoxically, enzyme-inducing anticonvulsant (EIAC) use correlated with superior outcome of patients with glioblastoma. These results suggest that in comparative clinical trials testing agents metabolized by P450 microsomal enzymes, treatment arms may need stratification for the proportion of patients receiving EIAC.
GLOSSARY
- CI
= confidence interval;
- CYP
= cytochrome P450;
- EIAC
= enzyme-inducing anticonvulsant;
- GBM
= glioblastoma;
- HR
= hazard ratio;
- MTD
= maximum tolerated dose;
- NCCTG
= North Central Cancer Treatment Group;
- OR
= odds ratio;
- OS
= overall survival;
- PFS
= progression-free survival.
Enzyme-inducing anticonvulsants (EIAC) induce P450 microsomal enzymes, resulting in enhanced metabolism of chemotherapeutic agents degraded by these enzymes. EIAC use has correlated with reduction in chemotherapy plasma levels in children with acute B-cell lymphocytic leukemia.1 Anticonvulsants are frequently prescribed to patients with glioblastoma (GBM), but the potential effect on outcome has not been systematically analyzed in prospective trials, with adjustment for known prognostic variables. We performed a correlative analysis of EIAC use with outcome, using a cross-sectional database of 620 patients with GBM treated on prospective North Central Cancer Treatment Group (NCCTG) studies to determine the relationship between EIAC use and overall survival (OS) and progression-free survival (PFS). Our hypothesis was that individuals taking EIACs would have poorer outcomes because of reduced chemotherapy plasma levels.
METHODS
The database was compiled from patients with newly diagnosed GBM enrolled prospectively on 3 NCCTG trials. These patients met the eligibility criteria of each trial on which they were enrolled, as reported previously.2–4 Patient, disease, treatment, and protocol specific variables were obtained from all patients. Baseline seizure history information was available only in the subgroup of patients treated at the Mayo Clinic.
EIAC use was coded as a dichotomous variable at baseline (registration on study). OS was measured from the date of initial surgical diagnosis until date of death, or last follow-up for those not deceased. PFS was measured from the start of chemotherapy to the date of documented progression. Follow-up of OS and PFS is ongoing for these patients. Patients who died without documented progression were assumed to have progressed at the time of death. Immediate progression was defined as a dichotomous variable in which the best objective tumor response was progression; the lack of immediate progression was defined as observation of complete response, partial response, regression, or stable disease. Other variables considered in the analysis included age at diagnosis (as a continuous variable); gender; baseline ECOG performance status; family history of cancer; baseline neurocognitive function (Folstein Mini-Mental State Examination score, treated as a continuous variable); extent of tumor resection (biopsy, subtotal or gross total resection); steroid use (yes/no) at baseline; and the clinical trial (NCCTG 937252, 987252, or N0074).
Comparisons of variable distributions among groups were performed with a χ2 test for categorical variables, the Wilcoxon rank sum (for comparisons of 2 groups), or the Kruskal-Wallis test (comparisons of 3 groups) for continuous variables.5 Specific groups were defined by treatment protocol, anticonvulsant use (yes vs no), EIAC use (yes vs no), and baseline history of seizures (yes/no). Distributions of time-to-event data (survival and PFS) were estimated with the Kaplan-Meier estimator.6 Comparisons of PFS and survival experiences among defined groups of patients were performed with the log-rank test.7 Univariable and multivariable Cox proportional hazards models were used to assess the association between EIAC, history of seizures, and outcome (PFS and survival).8,9 Immediate progression rates were compared univariably by a χ2 test and odds ratios (OR). Multivariable logistic regression models were used to assess the relationship of EIAC use to immediate progression in the presence of other important clinical factors.5
Two different multivariable analyses were conducted. In the first analysis, all known prognostic variables for survival and PFS were entered into the model as adjusting variables, along with the variable of interest (EIAC use). Because the choice to use or not use EIAC was made totally by the treating physician, we considered that bias, which potentially affected estimates of EIAC association with the outcome variable (immediate progression, survival, or PFS), might have been introduced. Thus, a second multivariable analysis examined the association that remained when all prognostic variables were added as individuals with adjustment for covariates, using propensity scores.10,11 Propensity score, defined as the probability of being treated with EIAC (given all other prognostic variables or covariates), was used to balance these covariates in the 2 groups, similar to what would be achieved by randomization. A propensity score for EIAC treatment was estimated using all patients and treatment variables in a logistic regression model, and used as an adjusting variable in the Cox proportional hazards and logistic regression models to determine the association of EIAC with the outcome variable (survival or PFS). All statistical tests were 2-sided, and the a priori level for declaring statistical significance was 0.05.
Standard protocol approvals, registrations, and patient consents.
This database study (NCCTG N0475) was approved by the Cancer Treatment and Evaluation Program of the National Cancer Institute. This study, as well as the 3 clinical trials from which the database was generated (NCCTG 937252, 987252, and N0074), were approved by the Mayo institutional review board. Informed, written consent was obtained from all patients or guardians for participation in one of these trials. The ClinicalTrials.gov identifiers were NCT00003996 (NCCTG 987252).and NCT00014170 (NCCTG N0074). There was no identifier for NCCTG 937252, which was completed before institution of regulatory requirements for identifiers.
RESULTS
Baseline patient and treatment characteristics.
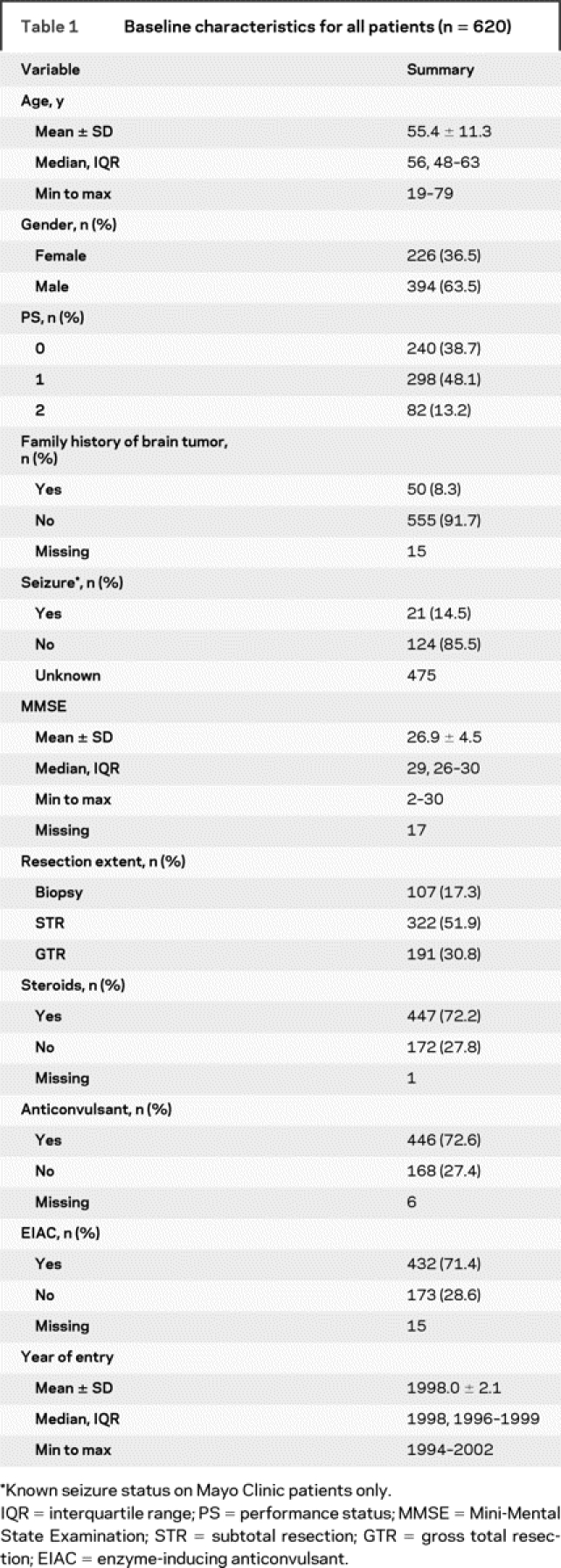
A total of 620 patients were enrolled from 1994 to 2002; 70% were accrued before 2000. Baseline patient and treatment characteristics are summarized in table 1. At the time of analysis, 48 of 620 patients (8%) were alive at last follow-up, and 19 (3%) had no documented disease progression. Anticonvulsant use information was available on 605 patients; 432 (71.4%) were receiving an EIAC, 14 (2.3%) were receiving a non–enzyme-inducing anticonvulsant, and 159 (26.2%) were not receiving any anticonvulsant. There was no difference in the percent of Mayo patients who were taking EIAC (70.1%) vs non-Mayo patients (69.6%). At baseline, most patients were receiving steroids (72.2%).
Table 1 Baseline characteristics for all patients (n = 620)
To identify potential changes in practice patterns over time, we looked at variation in patient characteristics among the 3 sequential studies. Most characteristics remained similar. The proportion of patients using an EIAC did not differ among the studies (p = 0.28). A baseline history of seizures was more common in earlier trials (chronologically, 33.3%, 6.5%, and 14.3% incidence, p = 0.0004). Biopsies were more common in earlier studies (chronologically, 19.6%, 15.2%, and 8.4%), and gross total resections were more frequent in later studies (chronologically, 26.3%, 35.9%, and 46.3%, p = 0.001). Baseline steroid administration was also more common in the earlier studies (chronologically, 77.3%, 63.0%, and 57.9%, p < 0.0001).
Comparison of patient/treatment characteristics by EIAC use.
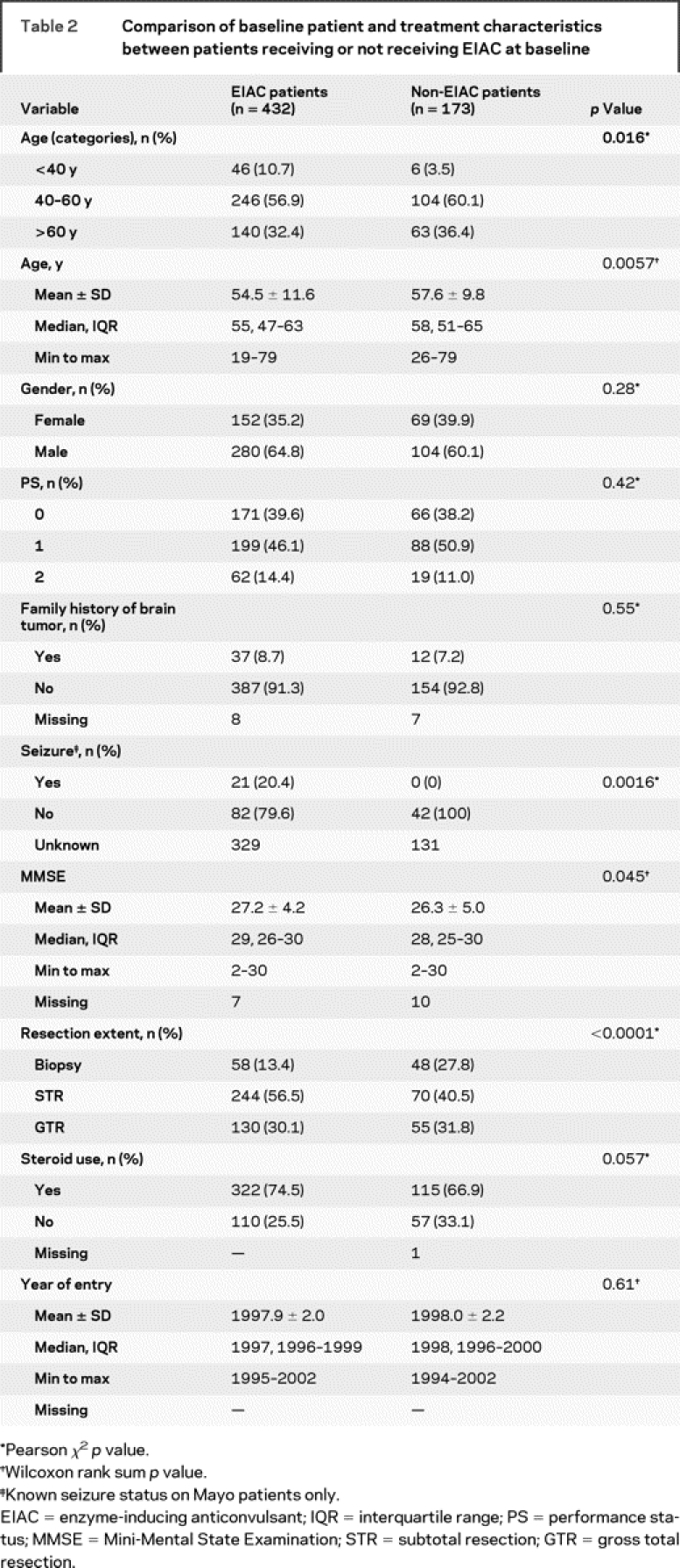
The EIAC-treated patients were slightly younger than non-EIAC patients (median 55 vs 58 years, p = 0.0057), were less likely to have had a biopsy only (13.4% vs 27.8%, p < 0.0001), and had slightly higher Mini-Mental State Examination scores (median 29 vs 28, p = 0.045). The frequency of use of steroids at baseline did not differ (74.5% vs 66.9%, p = 0.057). The patients did not differ on any other defined baseline characteristics.
Association between EIAC use and immediate progression.
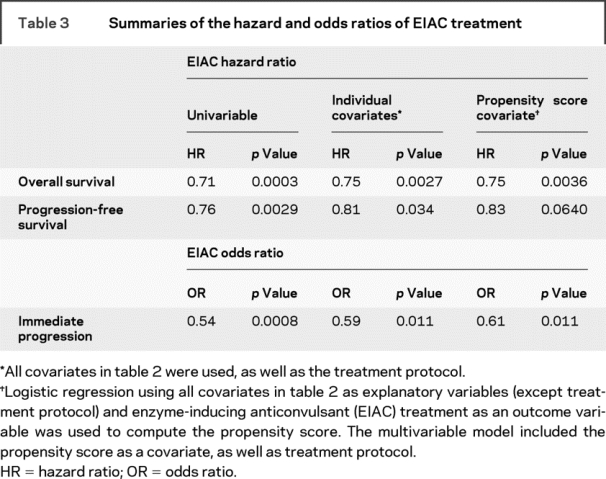
Immediate progression rates were higher in patients not treated with an EIAC than in those treated with an EIAC (45.1% vs 30.6%, p = 0.0007). The univariable OR for EIAC treatment and immediate progression was 0.54 (95% confidence interval [CI] 0.37–0.77). A multivariable logistic regression model was generated for immediate progression, adjusted for known prognostic factors (table 2) as well as treatment protocol. The OR for immediate progression for EIAC use was 0.59 (95% CI 0.39–0.89). An OR of 0.61 (95% CI 0.41–0.89) for EIAC use was observed in the multivariable logistic regression model, when adjusted for treatment protocol and propensity score. The univariable and multivariable estimates indicated that EIAC-treated patients were approximately half as likely to have an immediate progression.
Table 2 Comparison of baseline patient and treatment characteristics between patients receiving or not receiving EIAC at baseline
Association between EIAC use and overall survival.
EIAC-treated patients had longer OS compared with non-EIAC patients (p = 0.0002) (figure 1). The median survival for EIAC-treated patients was 12.3 months, compared with a median survival of 10.7 months for patients not treated with an EIAC. The univariable Cox proportional hazards ratio for EIAC use was 0.71 (95% CI 0.59–0.85) (table 3). In a multivariable Cox proportional model that adjusted for the known prognostic factors and treatment protocol, the hazard ratio for EIAC use was 0.75 (95% CI 0.62–0.90). The multivariable Cox proportional hazards model with adjustment for propensity score and treatment protocol identified a hazards ratio of 0.75 (95% CI 0.62–0.91) for EIAC use. We did not find a survival difference between the Mayo patients (n = 87) and the non-Mayo patients (n = 533) (11.8 months [CI 11.1–12.5]) vs 11.4 months [CI 9.8–14.4], p = 0.431).
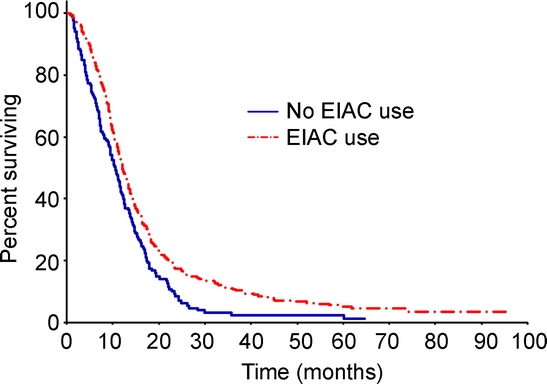
Figure 1 Comparison of overall survival between EIAC and non-EIAC patients
Median overall survival was 12.3 months in enzyme-inducing anticonvulsant (EIAC) patients and 10.7 months in non-EIAC patients (p = 0.0002).
Table 3 Summaries of the hazard and odds ratios of EIAC treatment
Association between EIAC use and progression-free survival.
EIAC-treated patients also had longer PFS than patients not treated with an EIAC (5.6 vs 4.8 months, p = 0.003) (figure 2). The univariable and multivariable estimates were consistent with the estimate produced by the multivariable model using a propensity score adjuster.
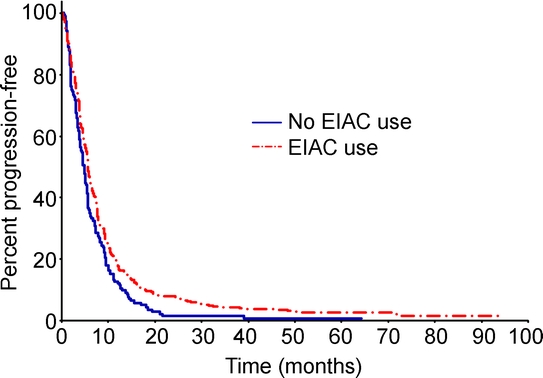
Figure 2 Comparison of progression-free survival between EIAC and non-EIAC patients
Median progression-free survival was 5.6 months in enzyme-inducing anticonvulsant (EIAC) patients and 4.8 months in non-EIAC patients (p = 0.003).
Relationship of seizure history with outcome.
Of the 145 Mayo Clinic patients, 21 had a baseline seizure history, all of whom were receiving EIAC. Of the remaining patients without a history of seizures, 82 (66.1%) were receiving prophylactic EIAC. No significant differences in defined characteristics were observed between those with or without a seizure history. Immediate progression rates did not differ between these groups (37.9% vs 23.8%, p = 0.32). The univariable OR for immediate progression for those with a baseline history of seizures was 0.51 (95% CI 0.18–1.49), and that for those without a history of seizures was 1.15 (95% CI 0.53–2.49). Immediate progression rates for patients without a history of seizures were 39.2% if EIAC treated and 35.7% if not receiving EIAC (p = 0.72). With propensity score adjustment, the immediate progression OR for EIAC patients with a seizure history was 1.08 (95% CI 0.48–2.46). This differed from the overall data set, in which EIAC use was associated with reduced odds of immediate progression.
The median survival for patients with seizures (all receiving EIAC) was 16.4 months, compared with 12.4 months for EIAC patients without a seizure history and 9.9 months for those non-EIAC patients without a seizure history. However, OS did not differ between those with or without a history of seizures, regardless of EIAC status (median survival 16.4 months vs 10.9 months, p = 0.20). Although there was a trend for the EIAC patients without baseline seizure history to live longer, it did not achieve significance (p = 0.079). Similarly, there was no difference in PFS between EIAC patients with a history of seizures, EIAC patients without seizures, and non-EIAC patients without a history of seizures (4.3, 6.0, and 5.0 months, p = 0.14). No difference in PFS was observed between patients with or without a history of seizures (p = 0.19) or between EIAC and non-EIAC patients without a seizure history (p = 0.11).
Models were used to compare the association between EIAC use and outcome (OS and PFS) for patients without a history of seizures. The univariable hazards ratio of EIAC use for survival was 0.71 (95% CI 0.48–1.04). With propensity score adjustment, the survival hazards ratio for EIAC use was 0.79 (95% CI 0.52–1.22).
DISCUSSION
The enzyme-inducing anticonvulsants phenobarbital, phenytoin, carbamazepine, and primidone are potent P450 microsomal enzyme inducers. Of the 6 major human hepatic cytochrome P450 (CYP) isoenzymes (CYP 1A2, 2C9, 2C19, 2D6, 2E1, and 3A4), induction of CYP 3A4 and CYP 2D6 are probably the most clinically relevant in patients receiving chemotherapy. For example, the clearance of irinotecan differs in those receiving EIAC (29.7 ± 9.0 L/hour/m2) and those not receiving EIAC (18.8 ± 10.6 L/hour/m2, p = 0.033), although there is no difference in area under the curve of the active metabolites SN-38 (p = 0.4) and SN-38 glucuronide (p = 0.55).12 The maximum tolerated dose (MTD) of IV irinotecan administered to patients with GBM in an every-3-week schedule was 750 mg/m2 in those receiving EIAC, compared with 350 mg/m2 in the non-EIAC group.13 Wide differences in the MTD of imatinib, tipifarnib, gefitinib, temsirolimus, taxanes, vinca alkaloids, methotrexate, and teniposide have also been observed in EIAC vs non-EIAC patients.14–17
Recently, there has been a decrease in the use of EIAC in favor of the newer non–enzyme-inducing anticonvulsants, which do not have potent P450 enzyme-inducing or inhibiting activity. It should be pointed out that the pharmacokinetic interactions of newer non-EIAC with chemotherapy have been less well studied. Gabapentin, vigabatrin, levetiricam, and lamotrigine are relatively devoid of P450-inducing or inhibiting properties. Topiramate and oxcarbazepine are weak inducers of CYP 3A4 and weak inhibitors of CYP 2C19, and zonisamide also has variable but weak inducing or inhibiting effects.18 In contrast, valproic acid is a potent inhibitor of microsomal enzymes and may actually increase the toxicity of chemotherapy.
There are limited prior data correlating outcome of patients with cancer with EIAC use. In a review of children with B-cell acute lymphoblastic leukemia who were receiving active chemotherapy, those receiving EIAC for >30 days had shorter event-free survival and an increase in the rate of hematologic and CNS relapse.1 In a phase 2 study of tipifarnib in for recurrent GBM, EIAC patients had more hematologic toxicities and lower PFS at 6 months than those not receiving EIAC.15 Finally, a prior study of 168 patients with GBM found differences in survival between those receiving vs not receiving EIAC (10.8 vs 13.9 months) and increased hematologic toxicity in the non-EIAC group.19 This study differed in several ways from our current analysis, in that it was a retrospective review of patients treated with a variety of regimens, the study did not include adjustments for known prognostic covariables, and most non-EIAC patients were receiving the P450 inducer valproic acid. In contrast, our current analysis was derived from patients treated on prospective clinical trials with a limited number of regimens, prognostic variables were studied for covariation via multivariable analyses, and we primarily compared patients receiving EIAC vs those not receiving any anticonvulsants.
Other prior studies have reported improved outcome in EIAC patients.20,21 In a prospective study of patients with recurrent GBM receiving imatinib and hydroxyurea, EIAC use was an independent predictor of longer PFS, even though there were lower imatinib plasma levels in the EIAC group.20 EIAC use also correlated with improved outcome in a prospective phase 2 single-arm trial of temozolomide and marimastat in treatment of patients with anaplastic glioma.21
A potential limitation of the current study is that most patients were treated in an era of widespread use of prophylactic EIAC (phenytoin, phenobarbital, carbamazepine, and others). These trials were conducted before publication of the meta-analysis results, which did not find a role for prophylactic use of anticonvulsants in patients who had brain tumors without seizures22; also, before the general availability of newer non-EIAC; and finally, before the impact of EIAC on chemotherapy metabolism was well recognized. In the past decade, EIAC use has dropped considerably because of more discriminate use of anticonvulsants and the more widespread use of non–enzyme-inducing anticonvulsants. Another limitation is that we did not perform a separate analysis that correlated outcome with changes in EIAC administration during the actual treatment on the clinical trials.
Nevertheless, we found that patients receiving EIAC at baseline had longer OS and PFS and lower immediate progression frequency than the group of patients who were not receiving EIAC. A cause-and-effect relationship for this association cannot be derived from this analysis. We could not identify physician selection bias prompting use of EIAC in patients expected to have a favorable outcome. If anything, one would expect that nonneurologic physicians (most NCCTG accruals originate from general medical oncologists) might administer prophylactic EIAC to patients expected to have poorer outcome, e.g., lower performance status and steroid dependence, but these prognostic factors did not account for the observed differences in the multivariable analysis. One would expect the use of EIAC in patients with a baseline history of seizures, which has been associated with more indolent progression of gliomas,23 but the presence of seizures at baseline did not account for the observed survival differences between patients receiving or not receiving EIAC. In fact, in the multivariable analysis, the association of improved survival with EIAC use also remained significant even after adjustment for age, neurocognitive and performance status, baseline steroid use, and extent of resection, and despite adjustment for variations in the distribution of known prognostic variables between EIAC and non-EIAC patients using propensity scores. It is possible that EIAC affected metabolism of steroids, increasing survival in this group. Such an association was not identified, and we do not have data on steroid requirements after study entry. Steroid administration has not been shown to have a significant effect on prolongation of survival in patients with GBM when administered in the absence of radiation therapy.23
We can only speculate on the cause for the observed association. It is possible that a selection bias that we did not identify was introduced by treating physicians. Although small differences in age favoring EIAC patients were observed in the univariable analysis, age was not correlated with EIAC use in the multivariable analysis. It is possible that non-EIAC patients developed greater toxicity, contributing to morbidity or shorter survival. It is conceivable that more patients with prognostically more favorable secondary GBM were receiving EIAC. However, these potential variables were not retrievable from the database.
It is unclear whether EIAC have direct antitumor effects. Direct exposure of C6 glioma cell lines to phenytoin increased the doubling time.24 In contrast, pretreatment of rats bearing intracranial RG-12 gliomas with phenobarbital reduced tumoricidal activity and toxicity of the alkylator amino-methyl-pyrimidinyl-methyl-chlorethyl nitrosourea (ACNU), but only when compared with survival after exposure to the CYP 3A4 inducer sodium valproate.25 Carbamazepine, phenytoin, and valproic acid do not seem to reduce or increase glioma cell viability, proliferation, or clonogenicity at clinically relevant concentrations.26
Finally, it is possible that EIAC provide a protective effect, via a mechanism that is not yet recognized. For example, folinic acid, an important factor for neoplastic cell growth, is depleted via induction of metabolism by phenytoin. However, the reason for the observation of increased survival of EIAC patients remains a mystery.
AUTHOR CONTRIBUTIONS
Biostatistical analysis was performed by K.B. and A.F.
DISCLOSURE
Dr. Jaeckle has received speaker honoraria from Educational Concepts, Inc. Dr. Ballman receives research support from the NIH [P30 CA 15083 (Statistician), P50 CA 108961 (Director of Biostatistics Core), U10 CA25224 (Statistician), and U10 CA 076001 (Director of the Statistics and Data Center)]. Dr. Furth reports no disclosures. Dr. Buckner has received funding for travel and honoraria for non–industry-sponsored activities.
Address correspondence and reprint requests to Dr. Kurt A. Jaeckle, Mayo Clinic, 4500 San Pablo Rd., Jacksonville, FL 32224 jaeckle.kurt@mayo.edu
Supported by grants NIH/NCI U10 CA25224 and U24 CA114740.
Disclosure: Author disclosures are provided at the end of the article.
Received November 10, 2008. Accepted in final form July 21, 2009.
REFERENCES
- 1.Relling MV, Pui CH, Sandlund JT, et al. Adverse effect of anticonvulsants on efficacy of chemotherapy for acute lymphoblastic leukaemia. Lancet 2000;356:285–290. [DOI] [PubMed] [Google Scholar]
- 2.Buckner JC, Ballman KV, Michalak JC, et al. Phase III trial of carmustine and cisplatin compared with carmustine alone and standard radiation therapy or accelerated radiation therapy in patients with glioblastoma multiforme: North Central Cancer Treatment Group 93–72-52 and Southwest Oncology Group 9503 Trials. J Clin Oncol 2006;24:3871–3879. [DOI] [PubMed] [Google Scholar]
- 3.Moynihan TJ, O’Fallon JR, Krook JE, et al. A phase II trial of pre-irradiation chemotherapy with BCNU, cisplatin and oral etoposide combined with radiation therapy in the treatment of glioblastoma (GBM). Program/Proceedings of the American Society of Clinical Oncology 2002;21:77a. Abstract 306.
- 4.Uhm JH, Ballman KV, Krause JC, et al. Phase II study of ZD1839 in patients with newly diagnosed grade 4 astrocytoma. Program/Proceedings of the American Society of Clinical Oncology 2004;22:108s. Abstract 1505.
- 5.Agresti A. Categorical Data Analysis. New York: John Wiley & Sons; 1990. [Google Scholar]
- 6.Moses LE, Emerson JD, Hosseini H. Analyzing data from ordered categories. N Engl J Med 1984;311:442–448. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Statist Assoc 1958;53:457–481. [Google Scholar]
- 8.Peto R, Peto J. Asymptotically efficient rank invariant procedures (with discussion). J R Stat Soc Ser A 1972;135:185–207. [Google Scholar]
- 9.Cox DR. Regression models and life-tables (with discussion). J R Stat Soc Ser B 1972;34:187–220. [Google Scholar]
- 10.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41–55. [Google Scholar]
- 11.D’Agostino RB. Propensity score method for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265–2281. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert MR, Supko JG, Batchelor T, et al. Phase I clinical and pharmacokinetic study of irinotecan in adults with recurrent malignant glioma. Clin Cancer Res 2003;9:2940–2949. [PubMed] [Google Scholar]
- 13.Prados MD, Lamborn K, Yung WK, et al; North American Brain Tumor Consortium. A phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study. Neuro Oncol 2006;8:189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen PY, Yung WK, Lamborn KR, et al. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99-08. Clin Cancer Res 2006;12:4899–4907. [DOI] [PubMed] [Google Scholar]
- 15.Cloughesy TF, Wen PY, Robins HI, et al. Phase II trial of tipifarnib in patients with recurrent malignant glioma either receiving or not receiving enzyme-inducing antiepileptic drugs: a North American Brain Tumor Consortium Study. J Clin Oncol 2006;4:3651–3656. [DOI] [PubMed] [Google Scholar]
- 16.Reardon DA, Quinn JA, Vredenburgh JJ, et al. Phase 1 trial of gefitinib plus sirolimus in adults with recurrent malignant glioma. Clin Cancer Res 2006;12:860–868. [DOI] [PubMed] [Google Scholar]
- 17.Armijo JA, Sanchez MB, Campos C, et al. The interactions of antiepileptic drugs in oncology practice. Rev Neurol 2006;42:681–690. [PubMed] [Google Scholar]
- 18.Benedetti MS. Enzyme induction and inhibition by new antiepileptic drugs: a review of human studies. Fundam Clin Pharmacol 2000;14:301–319. [DOI] [PubMed] [Google Scholar]
- 19.Oberndorfer S, Piribauer M, Marosi C, et al. P450 enzyme inducing and non-enzyme inducing antiepileptics in glioblastoma patients treated with standard chemotherapy. J Neurooncol 2005;72:255–260. [DOI] [PubMed] [Google Scholar]
- 20.Reardon DA, Egorin MJ, Quinn JA, et al. Phase II study of imatinib mesylate plus hydroxyurea in adults with recurrent glioblastoma multiforme. J Clin Oncol 2005;23:9359–9368. [DOI] [PubMed] [Google Scholar]
- 21.Groves MD, Puduvalli VK, Conrad CA, et al. Phase II trial of temozolomide plus marimastat for recurrent anaplastic gliomas: a relationship among efficacy, joint toxicity and anticonvulsant status. J Neurooncol 2006;80:83–90. [DOI] [PubMed] [Google Scholar]
- 22.Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors—report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000;54:1886–1893. [DOI] [PubMed] [Google Scholar]
- 23.Green SB, Byar DP, Walker MD, et al. Comparisons of carmustine, procarbazine, and high-dose methylprednisolone as additions to surgery and radiotherapy for the treatment of malignant glioma. Cancer Treat Rep 1983;67:121–132. [PubMed] [Google Scholar]
- 24.Lordo CD, Stroude EC, Del Maestro RF. The effects of diphenylhydantoin on murine astrocytoma radiosensitivity. J Neurooncol 1987;5:339–350. [DOI] [PubMed] [Google Scholar]
- 25.Nagashima T, Matsutani M, Kohono T. Effects of phenobarbital on the metabolism of ACNU in vivo. No To Shinkei 1983;35:677–682. [PubMed] [Google Scholar]
- 26.Stander M, Dichgans J, Weller M. Anticonvulsant drugs fail to modulate chemotherapy-induced cytotoxicity and growth inhibition of human malignant glioma cells. J Neurooncol 1998;37:191–198. [DOI] [PubMed] [Google Scholar]


