Abstract
Transient receptor potential vanilloid 1 (TRPV1) plays a major role in hyperalgesia and allodynia and is expressed both in the peripheral and central nervous systems (CNS). However, few studies have evaluated mechanisms by which CNS TRPV1 mediates hyperalgesia and allodynia after injury. We hypothesized that activation of spinal cord systems releases endogenous TRPV1 agonists that evoke the development of mechanical allodynia by this receptor. Using in vitro superfusion, the depolarization of spinal cord triggered the release of oxidized linoleic acid metabolites, such as 9-hydroxyoctadecadienoic acid (9-HODE) that potently activated spinal TRPV1, leading to the development of mechanical allodynia. Subsequent calcium imaging and electrophysiology studies demonstrated that synthetic oxidized linoleic acid metabolites, including 9-HODE, 13-HODE, and 9 and 13-oxoODE, comprise a family of endogenous TRPV1 agonists. In vivo studies demonstrated that intrathecal application of these oxidized linoleic acid metabolites rapidly evokes mechanical allodynia. Finally, intrathecal neutralization of 9- and 13-HODE by antibodies blocks CFA-evoked mechanical allodynia. These data collectively reveal a mechanism by which an endogenous family of lipids activates TRPV1 in the spinal cord, leading to the development of inflammatory hyperalgesia. These findings may integrate many pain disorders and provide an approach for developing analgesic drugs.
Keywords: inflammation, pain
Transient receptor potential vanilloid 1 (TRPV1) plays a pivotal role in many pain models, leading to the development of hyperalgesia and/or allodynia (1–2). TRPV1 is expressed in the peripheral as well as central nervous systems (CNS) including several areas involved in nociceptive transmission (3). Numerous studies have demonstrated that peripheral TRPV1 is activated by noxious heat and protons and is regulated by endogenous ligands (2), contributing to peripheral mechanisms of heat hyperalgesia (4). However, TRPV1 is also expressed in the CNS, where comparatively little is known about the role of this receptor in mediating central pain mechanisms.
Recent studies have used TRPV1 antagonists to evaluate the role of CNS TRPV1 in inflammatory hyperalgesia. Interestingly, the systemic/spinal administration of TRPV1 antagonists blocks inflammation-induced heat hyperalgesia, as well as mechanical allodynia (5–6). These findings were unexpected since TRPV1 is not activated by mechanical stimuli, suggesting that spinal TRPV1 activation leads to central sensitization to both thermal and mechanical stimuli (7–8). Based upon these observations, we hypothesized that the afferent barrage resulting from peripheral tissue injury leads to generation of endogenous TRPV1 ligands in the spinal cord that activate TRPV1 in the CNS resulting in central sensitization.
Results
Depolarization of Spinal Cord Results in the Release of Endogenous TRPV1 Ligands.
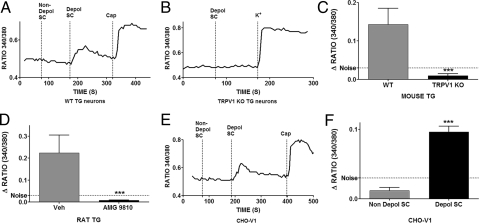
To evaluate the hypothesis that endogenous TRPV1 agonists are released from spinal cord neurons, freshly isolated male rat spinal cords were superfused with Hanks buffer to collect a negative control sample (nondepolarized). The spinal cords were then depolarized by exposure to a buffer containing 50 mM potassium. Both nondepolarized and depolarized samples were passed through C18 SepPak columns to remove salts and the retained lipohilic substances were eluated with 90% acetonitrile/0.05% trifluoroacetic acid. The dried samples were reconstituted in the same volume of Hanks buffer and applied to cultured trigeminal (TG) sensory neurons from wild type mice, with single cell imaging of intracellular calcium concentrations. The application of the eluate from the control, nondepolarized spinal cords had no effect on intracellular calcium levels (Fig. 1A). However, application of the eluate obtained from depolarized spinal cords robustly activated the capsaicin-sensitive subclass of TG neurons (Fig. 1A). Further analysis from multiple experiments indicated that the eluate from depolarized spinal cords activated 92% of capsaicin-sensitive neurons (48/52), suggesting that this response was mediated via activation of TRPV1. Control studies demonstrated that this effect was not due to retained potassium in the buffer since ex vivo addition of 50 mM potassium to the collected control, nondepolarized eluate had no effect after SepPak. To directly test the possible involvement of TRPV1, we applied the eluate from depolarized spinal cords to TG neurons from TRPV1 KO mice (Fig. 1B). The cumulative data obtained from multiple experiments demonstrated that the depolarized spinal cord eluate contains substances that activate sensory neurons in a TRPV1-dependent fashion (Fig. 1C). Additional studies demonstrated that the depolarized spinal cord eluate evoked robust responses in 92% of capsaicin-sensitive TG neurons cultured from rats (71/77) and was also released from spinal cords collected from female rats. Moreover, when the rat TG neurons were pretreated with a TRPV1 antagonist, AMG 9810 (1 μM, 3 min), the response evoked by depolarized spinal cord eluate was abolished (Fig. 1D). To evaluate whether the substances in the depolarized spinal cord eluate directly activate TRPV1, we applied the eluate to CHO cells expressing rat TRPV1. Only the eluate from depolarized spinal cord activated CHO cells expressing TRPV1 (Fig. 1 E and F). In addition, control CHO cells transfected with GFP alone failed to respond to the depolarized spinal cord eluate. Depolarization of the spinal cord with other stimuli, such as noxious heat also resulted in release of endogenous TRPV1 ligands (Fig. S1). Collectively, these data indicate that endogenous TRPV1 agonists (s) are released upon depolarization of spinal cord neurons.
Fig. 1.
Depolarization of the spinal cord releases endogenous TRPV1 agonist(s) (A) Freshly isolated rat spinal cords (9) were washed for 50 min in Hanks buffer and a basal sample (20 min in Hanks, 37 °C, Non- Depol- SC) was collected. Spinal cords were then depolarized with a buffer containing 50 mM potassium (20 min, 37 °C, Depol- SC). Both supernatants were passed through C18 SepPak columns and washed with water/0.05 TFA to remove salts. The substances adsorbed onto the column were eluted with 90% acetonitrile/0.05% TFA. The eluates were dried down under a flow of nitrogen and reconstituted in Hanks buffer. In a fura-2 calcium imaging set up, a representative tracer demonstrates the effect of the application of reconstituted eluates on a TG neuron from WT mice that also responded to capsaicin (100 nM). Ratiometric data are shown. (B) A representative tracer demonstrating the effect of the same eluate (from depolarized spinal cords) applied to a neuron from TRPV1 KO mice. The positive control for neuronal viability was stimulation with 50 mM potassium. (C) Comparison of calcium accumulation evoked by eluate from depolarized spinal cords in TG neurons from WT versus TRPV1 KO mice (n = 48 for WT and 43 for KO neurons,***, = P < 0.001, t-test). (D) Effect of pretreatment with either vehicle or the TRPV1 antagonist, AMG 9810 (1 μM, 3 min) on calcium accumulation in rat TG neurons evoked by eluate from depolarized spinal cords (n = 15 for vehicle and 41 for AMG ***, = P < 0.001, t-test). (E) A representative tracer demonstrating the effect of the application of eluate from depolarized spinal cords in CHO cells expressing rat TRPV1. Capsaicin (100 nM) was used as a positive control for TRPV1 expression. (F) Comparison of calcium accumulation evoked by applying eluates collective from nondepolarized and depolarized spinal cords to CHO cells expressing TRPV1 (n = 57 for nondepol and 47 for depol, ***, = P < 0.001, t-test).
Depolarization of the Spinal Cord Leads To Increased Release of Linoleic Acid Metabolites That Are Endogenous TRPV1 Agonists.
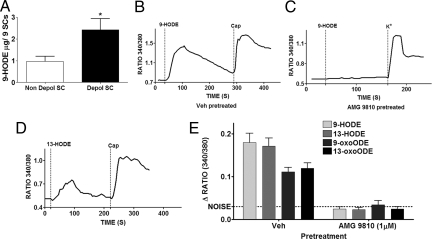
We next analyzed substances elevated in the depolarized spinal cord eluate. After preliminary evaluation of several potential endogenous agonists, we discovered that a linoleic acid metabolite, 9-hydroxyoctadecadienoic acid (9-HODE), was significantly elevated in the depolarized spinal cord eluate (Fig. 2A). Both 9- and 13-HODE and their respective metabolites, 9-oxoODE and 13-oxoODE, are oxidation products of linoleic acid (9), although their actions on TRPV1 are unknown. We next evaluated whether synthetic 9-HODE and the related linoleic acid metabolites activate TRPV1 in rat TG neurons. Application of 9-HODE (100 μM) resulted in a robust increase in intracellular calcium levels in 96% of capsaicin-sensitive rat TG neurons (109/114) (Fig. 2B), with effects observed at concentrations as low as 10 nM. Interestingly, the activity of even the highest concentration of 9-HODE (100 μM) was completely absent in neurons treated with a TRPV1 antagonist AMG9810 (Fig. 2 C and E). 13-HODE, another linoleic acid oxidation metabolite, that is typically formed along with 9-HODE (10) also activated capsaicin-sensitive TG neurons (Fig. 2D). Both 9- and 13-HODE are metabolized into 9- and 13-oxoODE. Both 9- and 13-oxoODE also activated rat TG neurons in a TRPV1 antagonist reversible fashion. The relative activity of all these compounds (100 μM each) on rat TG neurons in the presence and absence of AMG9810 is illustrated in Fig. 2E. Collectively, these data indicate that linoleic acid oxidation metabolites are released from depolarized spinal cords and comprise a family of endogenous TRPV1 ligands.
Fig. 2.
Depolarized spinal cord eluate contains elevated levels of oxidized linoleic acid metabolites that are TRPV1 agonists. (A) ELISA demonstrating the 9-HODE contents in eluates from nondepolarized and depolarized spinal cords (n = 3 separate samples, *, = P < 0.05, t-test). (B) Calcium imaging experiment demonstrating the effect of application of synthetic 9-HODE (100 μM, 1 min) to a rat TG neuron that was pretreated with Vehicle. Capsaicin (100 nM) served as a positive control for the presence of TRPV1 in that neuron. (C) Calcium imaging experiment demonstrating the effect of pre and cotreatment of AMG 9810 (1 μM, 3 min) on stimulatory effects of synthetic 9-HODE (100 μM). Neuronal viability was assessed by response to a buffer containing 50 mM potassium. (D) In a calcium imaging set up, the effect of application of synthetic 13-HODE (100 μM) on a rat TG neuron that also responded to capsaicin (100 nM). (E) Comparison of calcium accumulation evoked by various linoleic acid metabolites in rat TG neurons and the effect of pre and cotreatment with AMG 9810 (1 μM, 3 min) on their activity (n = 43–121 cells per condition).
Linoleic Acid Metabolites Selectively Activate TRPV1.
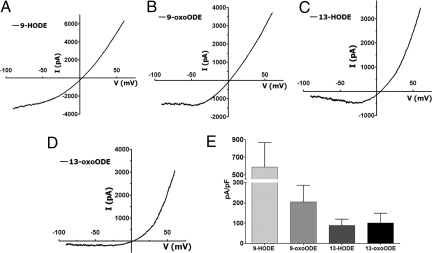
Since the activity of linoleic acid metabolites on rat TG neurons was completely dependent on TRPV1, we evaluated whether these metabolites directly activated rat TRPV1 in an expression system. In CHO cells expressing rat TRPV1, all four of these metabolites demonstrated significant agonist activity. The voltage-current relationship plots for respective compounds are demonstrated in Fig. 3 A–D. A summary of the relative efficacy of these compounds in activation of TRPV1 at holding potential of −60 mV is presented in Fig. 3E. Because both calcium imaging and electrophysiology studies implicated 9-HODE as evoking maximal TRPV1 efficacy, we selected this metabolite as the prototype of the entire family for our behavioral studies.
Fig. 3.
Oxidized linoleic acid metabolites activate recombinant rat TRPV1. (A) A representative tracer demonstrating the I-V plot obtained after 9-HODE (100 μM) application on CHO cells expressing TRPV1. (B, C, and D) Similar tracers obtained for 13-HODE, 9-oxoODE and 13-oxoODE (100 μM each) respectively. (E) Comparison of the current density evoked by various oxidized linoleic acid metabolites at −60 mV.
Spinal Linoleic Acid Metabolites Play an Important Role in Inflammatory Allodynia.
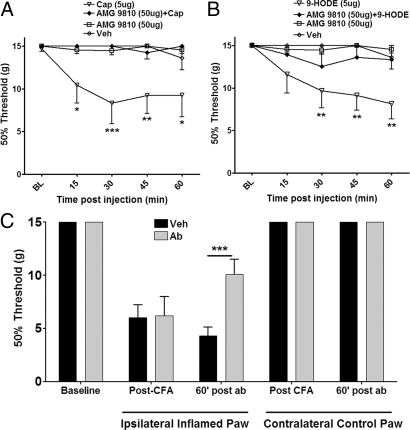
We next evaluated whether intrathecal injection of 9-HODE evoked a TRPV1-dependent mechanical allodynia and whether endogenous HODEs contribute to the development of inflammatory mechanical allodynia. The intrathecal injection of capsaicin (5 μg) resulted in the development of mechanical allodynia in the hindpaw peaking at 30 min post injection (Fig. 4A). This dose of capsaicin was selected based upon previous in vivo studies in the hindpaw hyperalgesia model (11). The capsaicin-induced mechanical allodynia was completely blocked by intrathecal coinjection of the TRPV1 antagonist AMG9810. Importantly, injection of either vehicle alone or AMG9810 alone had no effect on the mechanical withdrawal thresholds (Fig. 4A). Next, we evaluated whether intrathecal injection of 9-HODE (5 μg) also evoked mechanical allodynia. Interestingly, intrathecal 9-HODE produced a profound mechanical allodynia with a time course that appeared to extend somewhat longer than capsaicin. Moreover, the effect was completely absent in animals receiving AMG9810 coinjection, demonstrating a TRPV1 specific effect (Fig. 4B). These studies indicate that intrathecal injection of synthetic HODE rapidly triggers mechanical allodynia in otherwise normal animals. We next evaluated whether peripheral inflammation triggers the release of endogenous linoleic acid metabolites in the spinal cord leading to TRPV1-mediated mechanical allodynia. We tested this hypothesis in the CFA model of inflammation since it is dependent upon spinal TRPV1 activity (6, 8). Intraplantar injection of CFA resulted in the development of mechanical allodynia in the injected paw 24 h post injection (Fig. 4C). Animals then received intrathecal injections of either vehicle or a combination of anti-9-HODE and anti-13-HODE antisera, with subsequent testing of mechanical withdrawal thresholds in both the ipsilateral CFA injected and contralateral uninflamed hindpaws. The antibodies significantly (P < 0.001) reversed CFA-evoked mechanical allodynia in the inflamed paw without affecting the mechanical thresholds in the contralateral paw (Fig. 4C). The controls for the specificity of the antibodies included preimmune goat serum or heat-denatured antibodies. Either of these interventions failed to reverse CFA-evoked allodynia (Fig. S2). The specificity of these antibodies against the respective metabolites was previously established (12).
Fig. 4.
Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites plays a role in CFA-evoked behavioral responses. (A) After habituation, rats were injected with vehicle, AMG 9810 (50 μg), Cap (5 μg) or Cap with AMG (intrathecal, 30 μL volume) under light anesthesia and responses to Von Frey filaments were observed over 1 h post injection. The data are presented as 50% paw withdrawal threshold and analyzed using two way ANOVA with Bonferroni post hoc test (*, = P < 0.05, **, = P < 0.01, ***, = P < 0.001, compared to the vehicle group, n = 6–7 per group). All behavioral studies were conducted by blinded observers. (B) In a similar behavioral set up, comparison of allodynia evoked by intrathecal application of synthetic 9-HODE (5 μg) with or without AMG 9810 (50 μg). The data were analyzed using two way ANOVA with Bonferroni post hoc test (**, = P < 0.01, n = 6–7 per group). (C) After obtaining basal paw withdrawal thresholds, animals were injected with CFA unilaterally (ipsi, 100 μL). The post-CFA withdrawal thresholds in both paws were obtained after 24 h. Then the animals were injected either with vehicle or antibodies against 9- and 13-HODE (30 μg each) and withdrawal thresholds were obtained in both ipsilateral and contralateral paws 60 min post antibody combination injection (***, = P < 0.001, two way ANOVA with Bonferroni post hoc test, n = 5 per group).
Discussion
In the present study we tested the hypothesis that spinal activation of TRPV1 by endogenous ligands contributes to mechanical allodynia. We demonstrated that depolarization of isolated spinal cord leads to the release of a previously unknown family of endogenous TRPV1 ligands related to 9-HODE and 13-HODE. The application of 9-HODE, 13-HODE, and their related linoleic acid metabolites, 9-odoODE and 13-oxoODE, selectively activate TRPV1 in native and expression systems. In behavioral studies, the spinal administration of linoleic acid metabolites evokes mechanical allodynia, and their immunoneutralization reverses inflammatory mechanical allodynia. These studies demonstrate a direct role of endogenous TRPV1 ligands in spinal TRPV1 physiology.
In this study, we used an elevated potassium stimulus to depolarize spinal cord neurons for two reasons. Firstly, potassium selectively depolarizes neurons without directly stimulating glial cells (13). Secondly, potassium chloride being a salt could be easily removed from the supernatant permitting the evaluation of endogenous hydrophobic compounds (lipids, peptides, etc.) released from the spinal cord as potential TRPV1 agonists. Thus, when the eluate was applied to the sensory neurons, the resultant activity could be attributed only to the endogenous substances and not to the buffer. A solution containing elevated potassium will depolarize all neurons. Hence, we used heat as another nonchemical depolarizing agent that will presumably depolarize TRPV1 expressing primary afferents, making it a more selective stimulus. It should be noted that we observed similar generation of endogenous TRPV1 ligands with heat as our depolarizing agent (Fig. S1).
The spinal cord depolarization experiments demonstrated that the biological activity of the substance(s) present in the depolarized spinal cord eluate was completely dependent on the presence of TRPV1. These findings were somewhat surprising given the fact that potassium is a global neuronal depolarizing stimulus and should result in the release of many biologically active substances (14). This selective effect on TRPV1 activities could be due to several factors. First, the concentration of other substances in the depolarized spinal cord eluate may not be sufficient to demonstrate biological activity. Secondly, these substances may have TRPV1 sensitizing activity without evoking detectable calcium accumulation in sensory neurons.
Our studies demonstrated that 9-HODE was elevated in the depolarized spinal cord eluate. However, it is unlikely that 9-HODE alone is responsible for the entire TRPV1 activity in the depolarized spinal cord eluates given the relatively low levels of 9-HODE detected in the sample (Fig. 2A). A more likely scenario is that several linoleic acid metabolites contribute to TRPV1 activation. It is also possible that other factors capable of sensitizing TRPV1 (15–16) were coreleased under these conditions. Thus, the resultant TRPV1 agonist activity could be an entourage effect of several lipids acting in concert. Indeed, such an entourage effect is observed with other lipid families (17).
The mechanism mediating the formation of oxidized linoleic acid metabolites upon depolarization of the spinal cord is unknown. Studies conducted in other tissues have demonstrated that these metabolites can be formed both enzymatically and nonenzymatically (9). In fact, calcium-dependent activation of the enzymes involved in the synthesis of these metabolites is known (18). Thus it can be postulated that calcium influx resulting from a depolarizing barrage activates the enzymes involved in the synthesis of linoleic acid metabolites.
The activation of TRPV1 in the spinal cord results in the development of mechanical allodynia. Such findings have been reported in the past and possible mechanisms have been proposed (7). However, the actual mechanisms leading to activation of spinal TRPV1 are unknown. In the present studies, we demonstrated that immuoneutralization of 9- and 13-HODE resulted in a substantial reversal of CFA-evoked allodynia (Fig. 4C) that was comparable with that observed with intrathecal TRPV1 antagonism (8). Moreover, antioxidants that could block production of these substances are known to have similar antiallodynic effects when injected intrathecally (19). These observations point toward a role for endogenous mediator activation of spinal TRPV1 in central sensitization. Since the activity of TRPV1 antagonists in the periphery results in substantial side effects such as hyperthermia (20), an antagonist that works primarily in the CNS may be devoid of such effects.
Although several other endogenous TRPV1 ligands such as anandamide, leukotrienes, and n-arachidonyl dopamine (21–23) have been proposed, their role in various pathological disorders remains to be established. In contrast, the linoleic acid metabolites are well recognized to be involved in numerous neurological and non-neurological disorders (9, 24–28). The known receptors for these metabolites cannot explain all of their observed effects (29–30). Similarly, the activity of TRPV1 in the CNS is linked with many other disorders apart from pain (31). Thus, activation of TRPV1 may explain some of the biological activity of linoleic acid metabolites and, in turn, these ligands may significantly contribute to the actions of CNS TRPV1.
In summary, this report demonstrates a family of endogenous TRPV1 agonists that is active in the spinal pain pathway and explains the role of TRPV1 in triggering central sensitization and mechanical allodynia. These studies could lead to classes of analgesic compounds that block synthesis or action of linoleic acid metabolites in the spinal cord.
Materials and Methods
Animals and Compounds.
Adult male Sprague–Dawley (Charles River Laboratories) rats weighing 250–300 g or adult C56 B/L mice (both WT and TRPV1 KO, Jackson Laboratory) were used in this Institutional Animal Care and Use Committee (IACUC)-approved study.
The compounds were purchased from the following companies: AMG9810 (Tocris Cookson), capsaicin (Fluka/Sigma-Aldrich), 9- and 13-HODE and 9- and 13-oxoODE (Cayman Chemicals) and antibodies against 9- and 13-HODE (Oxford Biomedical Research). The vehicle for capsaicin, 9-HODE and AMG9810 injections was 5% DMSO and 5% Tween-80. The vehicle for the antibodies was saline.
Spinal Cord Dissection, Depolarization, and Preparation of the Eluates.
Male Sprague-Dawley rats were quickly decapitated and the spinal cord was removed using hydraulic extrusion with modified Hanks buffer. After removal of the dural membranes, 9 spinal cords were washed with modified Hanks buffer (containing 10.9 mM HEPES, 4.2 mM sodium bicarbonate, 10 mM dextrose, and 2 mM calcium chloride, pH 7.4) for 50 min. After collection of a baesline sample (5 mL, 37 °C, 20 min), the spinal cords were exposed to a high potassium Hanks buffer (50 mM potassium chloride and 90 mM sodium chloride, all other ions same) for 30 min at 37 °C. The lipophilic compounds were isolated from the superfusate via C18 SepPak columns (Waters Inc), washed with 20 mL 0.05% TFA and eluted with 90% acetonitrile/0.05% TFA. The eluate was dried under a flow of nitrogen and stored at −80 °C until testing when it was redissolved in the same volume of modified Hanks buffer.
TG Cultures and CHO Cell Preparation.
All experiments were performed on 1-day-old TG cultures grown in the presence of 100 ng/mL NGF (Harlan), as described before (32). CHO cells were transfected with rat TRPV1 (kindly donated by Dr. David Julius), as described before (32) and the experiments were performed 1 day post transfection.
Calcium Imaging and Electrophysiology.
Calcium imaging and electrophysiology was performed using modified Hanks buffer and the protocol was similar to previous studies (32). All compounds/eluates were applied for a duration of 1 min.
9-HODE ELISA.
The total 9-HODE content in the eluates was determined by using a commercially available ELISA kit (Oxford Biomedical Research). The method has been validated (12).
Behavioral Studies.
Animals were habituated to the testing environment in 9.5 × 21 × 25 cm Plexiglas boxes on an elevated perforated plastic surface for a minimum of 30 min before all behavioral tests. A blind observer conducted behavioral testing according to a modification of the up/down method (33), as previously described (34). Baseline mechanical withdrawal thresholds were obtained and unilateral CFA administration followed (50 μL of 1:1 saline and heat-killed and dried Mycobacterium tuberculosis, 0.5 mg/mL, suspended in 85% paraffin oil and 15% mannide monooleate, Sigma). Post-CFA baselines were followed by intrathecal (i.t.) injections administered under brief halothane anesthesia 24 h following CFA. Drugs were administered in a 30 μL volume by lumbar puncture between the L4 and L5 vertebrae and testing occurred at intervals from 5–60 min following drug administration.
Statistical Analyses.
Data are presented as mean ± SEM. Data were analyzed by using GRAPHPAD PRISM software version 4 (GraphPad). Multifactor experimental data were analyzed with two-way ANOVA; single-factor, multiple treatment data were analyzed by one-way ANOVA; and individual groups were compared by using a Bonferroni post hoc test. Experiments examining differences between two groups were analyzed by using Student's t-test.
Supplementary Material
Acknowledgments.
We thank Dr. David Julius (University of California at San Francisco) for kindly providing the clones for WT and mutant TRPV1, Dr. Narender Gavva (Amgen) for AMG 8562, and Dr. Nathaniel Jeske (University of Texas Health Science Center at San Antonio) for providing CHO cells. This work was supported by National Institutes of Health Grant DA019585 and Clinical and Translational Science Awards U54RR02438 (to K.M.H.) and DE017696 and DE019311 (to A.N.A.).
Footnotes
Conflict of interest statement: The University of Texas has claimed intellectual property on this discovery.
This article is a PNAS Direct Submission.
See Commentary on page 18435.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905415106/DCSupplemental.
References
- 1.Caterina MJ, et al. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 2.Cortright DN, Szallasi A. Biochemical pharmacology of the vanilloid receptor TRPV1. An update. Eur J Biochem. 2004;271:1814–1819. doi: 10.1111/j.1432-1033.2004.04082.x. [DOI] [PubMed] [Google Scholar]
- 3.Mezey E, et al. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci USA. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang J, Zhang X, McNaughton PA. Inflammatory pain: The cellular basis of heat hyperalgesia. Curr Neuropharmacol. 2006;4:197–206. doi: 10.2174/157015906778019554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang L, et al. Antinociceptive pharmacology of N-(4-chlorobenzyl)-N′-(4-hydroxy-3-iodo-5-methoxybenzyl) thiourea, a high-affinity competitive antagonist of the transient receptor potential vanilloid 1 receptor. J Pharmacol Exp Ther. 2007;321:791–798. doi: 10.1124/jpet.106.117572. [DOI] [PubMed] [Google Scholar]
- 6.Kanai Y, Hara T, Imai A, Sakakibara A. Differential involvement of TRPV1 receptors at the central and peripheral nerves in CFA-induced mechanical and thermal hyperalgesia. J Pharm Pharmacol. 2007;59:733–738. doi: 10.1211/jpp.59.5.0015. [DOI] [PubMed] [Google Scholar]
- 7.Pitcher MH, Price TJ, Entrena JM, Cervero F. Spinal NKCC1 blockade inhibits TRPV1-dependent referred allodynia. Mol Pain. 2007;3:17. doi: 10.1186/1744-8069-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui M, et al. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J Neurosci. 2006;26:9385–9393. doi: 10.1523/JNEUROSCI.1246-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida Y, Niki E. Bio-markers of lipid peroxidation in vivo: Hydroxyoctadecadienoic acid and hydroxycholesterol. Biofactors. 2006;27:195–202. doi: 10.1002/biof.5520270117. [DOI] [PubMed] [Google Scholar]
- 10.Spiteller G. Linoleic acid peroxidation–the dominant lipid peroxidation process in low density lipoprotein–and its relationship to chronic diseases. Chem Phys Lipids. 1998;95:105–162. doi: 10.1016/s0009-3084(98)00091-7. [DOI] [PubMed] [Google Scholar]
- 11.Gilchrist HD, Allard BL, Simone DA. Enhanced withdrawal responses to heat and mechanical stimuli following intraplantar injection of capsaicin in rats. Pain. 1996;67:179–188. doi: 10.1016/0304-3959(96)03104-1. [DOI] [PubMed] [Google Scholar]
- 12.Spindler SA, Clark KS, Callewaert DM, Reddy RG. Significance and immunoassay of 9- and 13-hydroxyoctadecadienoic acids. Biochem Biophys Res Commun. 1996;218:187–191. doi: 10.1006/bbrc.1996.0033. [DOI] [PubMed] [Google Scholar]
- 13.Bonnington JK, McNaughton PA. Signaling pathways involved in the sensitisation of mouse nociceptive neurones by nerve growth factor. J Physiol. 2003;551:433–446. doi: 10.1113/jphysiol.2003.039990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saria A, et al. Simultaneous release of several tachykinins and calcitonin gene-related peptide from rat spinal cord slices. Neurosci Lett. 1986;63:310–314. doi: 10.1016/0304-3940(86)90376-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, et al. Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKCepsilon: A novel pathway for heat hyperalgesia. J Neurosci. 2007;27:12067–12077. doi: 10.1523/JNEUROSCI.0496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dirig DM, Konin GP, Isakson PC, Yaksh TL. Effect of spinal cyclooxygenase inhibitors in rat using the formalin test and in vitro prostaglandin E2 release. Eur J Pharmacol. 1997;331:155–160. doi: 10.1016/s0014-2999(97)01053-4. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Shabat S, et al. An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol. 1998;353:23–31. doi: 10.1016/s0014-2999(98)00392-6. [DOI] [PubMed] [Google Scholar]
- 18.Brinckmann R, et al. Membrane translocation of 15-lipoxygenase in hematopoietic cells is calcium-dependent and activates the oxygenase activity of the enzyme. Blood. 1998;91:64–74. [PubMed] [Google Scholar]
- 19.Kim HK, et al. Analgesic effect of vitamin E is mediated by reducing central sensitization in neuropathic pain. Pain. 2006;122:53–62. doi: 10.1016/j.pain.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Steiner AA, et al. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J Neurosci. 2007;27:7459–7468. doi: 10.1523/JNEUROSCI.1483-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang SW, et al. Direct activation of capsaicin receptors by products of lipoxygenases: Endogenous capsaicin-like substances. Proc Natl Acad Sci USA. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang SM, et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zygmunt PM, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida Y, et al. Hydroxyoctadecadienoic acid and oxidatively modified peroxiredoxins in the blood of Alzheimer's disease patients and their potential as biomarkers. Neurobiol Aging. 2007;30:174–185. doi: 10.1016/j.neurobiolaging.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Jira W, Spiteller G, Richter A. Increased levels of lipid oxidation products in low density lipoproteins of patients suffering from rheumatoid arthritis. Chem Phys Lipids. 1997;87:81–89. doi: 10.1016/s0009-3084(97)00030-3. [DOI] [PubMed] [Google Scholar]
- 26.Goodman Y, Steiner MR, Steiner SM, Mattson MP. Nordihydroguaiaretic acid protects hippocampal neurons against amyloid beta-peptide toxicity, and attenuates free radical and calcium accumulation. Brain Res. 1994;654:171–176. doi: 10.1016/0006-8993(94)91586-5. [DOI] [PubMed] [Google Scholar]
- 27.van Heuven-Nolsen D, et al. Hypotensive effect of 13-hydroxylinoleic acid in the rat: Mediation via the release of a CGRP-like mediator from capsaicin-sensitive nerves. Br J Pharmacol. 1995;115:835–839. doi: 10.1111/j.1476-5381.1995.tb15008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camp RD, et al. The identification of hydroxy fatty acids in psoriatic skin. Prostaglandins. 1983;26:431–447. doi: 10.1016/0090-6980(83)90178-8. [DOI] [PubMed] [Google Scholar]
- 29.Obinata H, Hattori T, Nakane S, Tatei K, Izumi T. Identification of 9-hydroxyoctadecadienoic acid and other oxidized free fatty acids as ligands of the G protein-coupled receptor G2A. J Biol Chem. 2005;280:40676–40683. doi: 10.1074/jbc.M507787200. [DOI] [PubMed] [Google Scholar]
- 30.Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): Nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000;49:497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- 31.Starowicz K, Cristino L, Di Marzo V. TRPV1 receptors in the central nervous system: Potential for previously unforeseen therapeutic applications. Curr Pharm Des. 2008;14:42–54. doi: 10.2174/138161208783330790. [DOI] [PubMed] [Google Scholar]
- 32.Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol. 2007;583:175–193. doi: 10.1113/jphysiol.2007.133231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 34.Scotland PE, Coderre TJ. Enhanced 3,5-dihydroxyphenylglycine-induced sustained nociceptive behaviors in rats with neuropathy or chronic inflammation. Behav Brain Res. 2007;184:150–156. doi: 10.1016/j.bbr.2007.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.