Summary
H. pylori genomes typically contain 8 or 9 genes that code for secreted and highly disulfide-bridged proteins designated Helicobacter cysteine-rich proteins (Hcp). Here we show that HcpA (hp0211) but not HcpC (hp1098) triggers the differentiation of human myeloid Thp1 monocytes into macrophages. Small amounts of HcpA cause the transition of round-shaped monocytes into cells with star-like morphologies, adherence to the culture dish surface, phagocytosis of opsonized fluorescent microspheres, and expression of the surface marker protein CD11b, all of which are indicative of a macrophage-like phenotype. We conclude that HcpA acts as a bacterial immune modulator similar to a eukaryotic cytokine.
Keywords: innate immunity, inflammation, H. pylori, cytokines, Thp1, monocytesa, macrophage, pathogen/host interactions, Sel1-like repeat, TPR
Introduction
The gram-negative ε-proteobacterium Helicobacter pylori chronically infects the gastric mucosa of humans and certain other vertebrates, a niche that is hostile to all other microbes. H. pylori infections tend to persist for life if not treated, implying effective adaptation to this harsh ecological niche [1]. The interactions between H. pylori and the host innate immune system seem to be of particular importance in the persistence of infection and development of pathological states such as peptic ulcer disease and gastric cancer [2–6].
The genome sequences of six H. pylori strains reveal a family of 8 or 9 conserved hypothetical proteins with a high content of disulfide bridges [7–10] that accordingly were designated Helicobacter cysteine-rich proteins (Hcp) [11,12]. HcpA and most other family members carry N-terminal leader sequences for periplasmic expression and HcpA was found to be secreted into the supernatant of H. pylori cultures [11], which would allow it to directly contact eukaryotic host cells. Several HcpAs were detected based on high antibody titres against them in H. pylori infected patients ((HcpA and HcpC studied here, and HcpE), showing that these proteins, in particular, are well expressed in vivo [13].
The crystal structures of HcpC and HcpB revealed modular architectures consisting of four and seven Sel1-like repeats (SLR), respectively. The SLR motif is 36 to 44 amino acids long and folds into pairs of anti-parallel α-helices. The structural similarity between SLR proteins and tetratricopeptide repeat (TPR) proteins suggests that Hcps might act as protein-protein interaction modules in signal transduction pathways [14]. This structure-based hypothesis is supported by the observation that recombinant HcpA elicits high titres of pro-inflammatory cytokines, such as IFN-γ, TNF-α, IL-6, IL-10, and IL-12, in a cellular assay of naïve mouse splenocytes. [15].
Additional evidence that Hcps might modulate interactions between H. pylori and its host comes from an analysis of phase variable H. pylori genes. Phase variation by frameshift mutations in repetitive DNA sequences is a common mechanism used by pathogenic bacteria to generate intra-strain diversity and to evade specific adaptive immune system responses. The hcpA and hcpB genes undergo phase variation, suggesting that their gene products are important for niche adaptation [16]. A recent statistical analysis of Hcp sequence data supports this inference. Several Hcps show signatures of selection for amino acid change, implying adaptive evolution in different human populations [17].
The differentiation of monocytes into macrophages is a crucial step in the early immune response, and the human monocytic leukemia cell lines Thp1 and U937 have been frequently used as in vitro models to investigate this process. Several compounds such as phorbol 12-myristate 13-acetate (PMA), 1,25-dihydroxyvitamin D3, retinoic acid, or eukaryotic cytokines have been used to trigger this differentiation process, which can be monitored by changes in morphology, adherence, phagocytosis, the expression of surface markers or the release of prostaglandine E and TNF-α [18,19]. The magnitude of the signal obtained depends on the stimulating agent; PMA has one of the strongest effects.
Here we investigated the effects of recombinant HcpA and HcpC on Thp1 and U937 cell-lines. We found that small amounts of HcpA stimulate adherence of Thp1 cells to the culture dish surface, foster phagocytosis of fluorescent microspheres, trigger a transition of round-shaped monocytes into cells with star-like morphologies, and stimulate expression of surface markers that are indicative for macrophage-like phenotypes, such as CD11b. We conclude that HcpA shows cytokine-like properties and - at least transiently - provokes a human monocyte to macrophage differentiation.
Material and Methods
Expression of recombinant HcpA and HcpC
We expressed HcpA and -C as soluble Mbp-fusion proteins in the periplasm of E. coli, thereby avoiding the need to refold them from inclusion bodies that complicated previous studies [12,20]. HcpA and -C were purified by exactly the same strategies, making use of two affinity chromatography steps on amylose- and Ni-NTA-agarose resins (Supplementary experimental procedures). After deletion of the bulky N-terminal Mbp-tags, cleaved HcpA-His6 and HcpC-His6 where concentrated by ultra-filtration and finally filtered through 0.1 μm spin-filters to prevent bacterial contaminations. Recombinant HcpA and -C were > 95 % pure as judged by SDS-Page and reversed-phase HPLC analysis (Supplementary Fig. 1). The flow-through of the final ultra-filtration step was filtered and kept as a negative control for subsequent cell stimulation experiments. Since Thp1 and U937 cells are sensitive to bacterial endotoxins, such as lipopolysaccarides (LPS), the possibility of contamination of recombinant Hcp preparations with endotoxins was investigated using the Limulus amebocyte lysate test. Endotoxin concentrations were below 1.25 endotoxin units (EU)/ml in flow-through buffers of HcpA and -C preparations, which corresponds to less than 0.3 ± 0.1 EU/mg HcpA.
Stimulation of cells and adherence
Thp1 and U937 cells were maintained under standard conditions. To estimate cell adherence cultures were cooled to 4°C approximately 5 to 24 hours after stimulation. Loosely attached cells were re-suspended in the medium and the supernatant was withdrawn. Adherent cells were washed with PBS and the washing solution was added to the culture supernatant. Adherent cells were detached with 150 μL trypsin/EDTA solution (Sigma) and the culture dish was rinsed with 850 μL PBS, which was later combined with the suspension of detached cells. After gentle mixing 25 μL to 300 μL of cell suspension was diluted with 10 mL isotonic buffer and cells were counted in a CASY cell counter (Schärfe System). For statistical analysis, Student's t-test function, assuming unequal variances, was applied.
Phagocytosis
Thp1 cells were stimulated with varying concentrations of HcpA, HcpC or PMA and allowed to differentiate for 21 hours. Opsonized polystyrene particles were prepared by incubating 3 μL green fluorescent microspheres (2.5 % suspension of 1 μm beads, Sigma) in 300 μL 10 % foetal calf serum for one hour at 37°C. Opsonized microspheres were added to Thp1 cell cultures (approximately 0.6·106 cells/ml) at a ratio of 10 microspheres per cell. Cultures were maintained at 37°C for one hour and cooled to 4°C to harvest the cells by trypsinisation. After washing the mixture of suspended and adherent cells once with 12 mL FACS buffer (0.1 % BSA in PBS), cells were re-suspended in 300 μL FACS buffer and analysed by flow-cytometry (BD FACScanto II).
Expression of surface markers
The expression of the β2 integrin CD11b was estimated by flow-cytometry. Thp1 cell cultures (2 mL) in either 3.5 cm culture dishes or 12-well plates were stimulated with varying concentrations of HcpA, HcpC or PMA and maintained over night. Cells were harvested by trypsinisation and washed once with 10 mL FACS buffer. After adding 1 μL FITC labelled rat anti-CD11b antibody (BD Pharmingen) to 100 μL cells in FACS buffer the mixture was kept on ice for one hour. Cells were washed once with 10 mL FACS buffer and analysed by flow-cytometry.
In vivo analysis
To analyse the contribution of HcpA in vivo we compared the colonization behaviour of H. pylori strain X47 with an isogenic hcpA knockout mutant. This mutant was generated by replacing the hcpA gene with an rpsL,erm (streptomycin susceptibility, erythromycin resistance) cassette in a two-step PCR protocol as described [21]. The X47 ΔhcpA transformants were then tested in C57BL/6 mice (Supplementary experimental procedures).
Results
Morphology
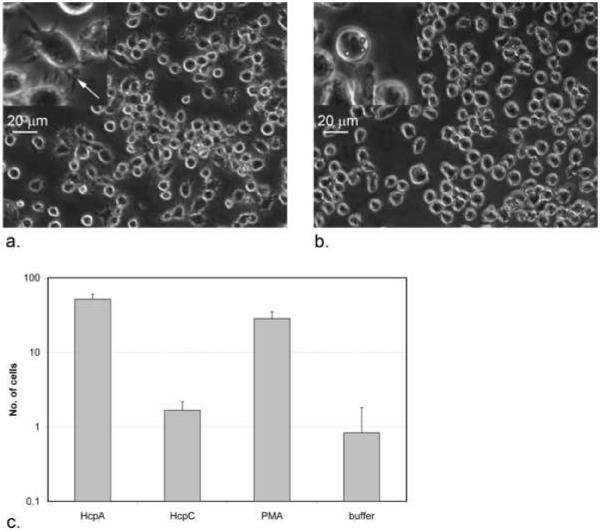
We investigated the effects of recombinant HcpA and -C on the morphology of human Thp1 monocytes. Under normal culture conditions Thp1 cells had round morphologies, which was significantly affected by recombinant HcpA addition. HcpA concentrations between 10 nM and 100 nM stimulated the transition from round to star-like morphologies (Fig. 1a and b). To quantify this effect cells with star-like morphologies were counted after stimulation with varying concentrations of HcpA, HcpC, flow-through buffer or PMA. A p-value of 1.9·10−5 for HcpA at 120 nM indicates that HcpA had an equally significant effect on the morphology of Thp1 cells compared to PMA (Fig. 1c). In contrast, no such effect was seen for the stimulation of U937 cells with HcpA (data not shown). When HcpC was used for stimulation, most cells were visually indistinguishable from buffer-treated cells.
Figure 1.
Phase contrast light microscopy images of Thp1 cells. (a) Cells after adding 460 nM HcpA. An arrow in the magnified insert indicates filopodia. (b) Cells after adding flow-through buffer at an equal dilution. (c) Given are the numbers of cells with star-like morphologies after treatment with 120 nM HcpA, HcpC, PMA, or flow-through buffer. P-values for the comparison between buffer-treated cells and cells treated with HcpA, HcpC and PMA are 1.9·10−5, 0.10, and 1.2·10−4, respectively. Cells with star-like morphologies were counted at six representative surface areas (0.64 mm2 each).
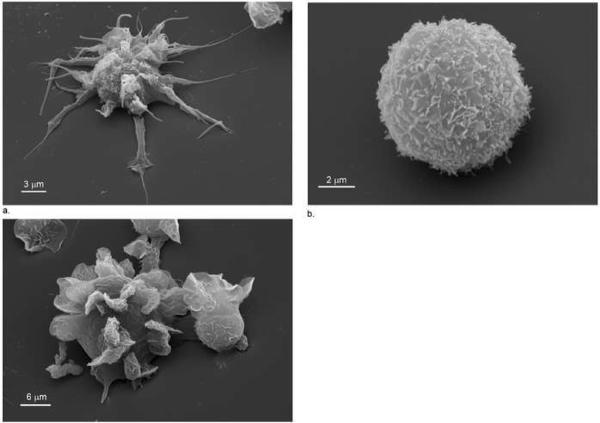
Scanning electron microscopy was used to investigate these effects at higher resolution. After treatment with HcpA, flow-through buffer and PMA, Thp1 cells that adhered to glass cover slides were fixed with glutaraldehyde and osmium tetroxide and covered with gold. These images revealed that HcpA treated Thp1 cells develop approximately 20 needle-like protrusions (filopodia) per cell, which correspond to the star-like morphology seen by phase-contrast light microscopy. These protrusions were 10 to 20 μm long and had a typical width of less than 1 μm (Fig. 2a). In contrast to this, buffer treated Thp1 cells were round shaped (8 to 12 μm diameter) with almost no protrusions (Fig. 2b), whereas treatment with PMA caused Thp1 cells to develop leaf-like protrusions (lamelopodia) that tightly interacted with the glass cover slide (Fig. 2c), and were distinct from the protrusions induced by HcpA.
Figure 2.
Scanning electron microscopy images of typical Thp1 cells 7 hours after treatment with (a) 230 nM HcpA (1:10'000 magnification), (b) with an equal volume of flow-through buffer (1:21'000 magnification), and (c) with 230 nM PMA (1:6'000 magnification).
Adherence
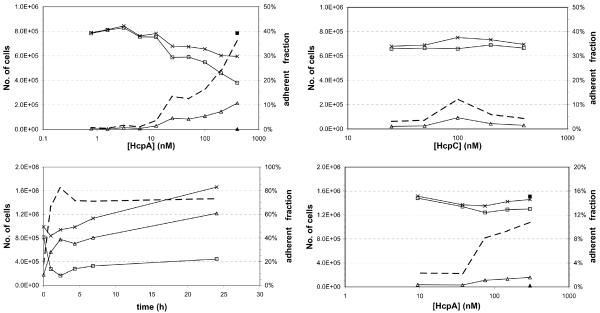
In untreated Thp1 and U937 cell cultures the majority of cells float freely in the medium. Treatment with HcpA stimulates the development of filopodia that enable the cells to attach to the bottom of the culture dishes. To quantify this effect, adherent cells and cells in suspension were counted after stimulation with varying concentrations of HcpA. Depending on the age of the cell culture and the surface coating of the culture dish between 0.1 % and 20 % of cells are loosely attached to the plastic surface even in the absence of stimulating agents, such as HcpA or PMA. Typically the amount of loosely attached cells increases with the age of the cell culture. Addition of HcpA causes a dose-dependent increase in Thp1 cell adherence. With young Thp1 cell cultures (less than 1 % of cells adhered) HcpA addition at a concentration of 400 nM caused 36 % of cells to adhere (Fig. 3a); with old Thp1 cell cultures (approximately 15 % spontaneously adhered) HcpA addition caused 90 % of cells to adhere (data not shown). Neither the flow-through buffer of the final HcpA concentration step (Fig. 3a) nor HcpC that was purified in the same way as HcpA stimulated adherence. Less than 10 % of Thp1 cells adhered in tests with HcpC, independent of the concentration used (Fig. 3b).
Figure 3.
Adherence of cells after stimulation. Total number of cells (crosses), number of cells in suspension (open squares), number of adherent cells (open triangles), and fraction of adherent cells (broken line). Adherent cells and cells in suspension after treatment with a corresponding volume of flow-through buffer are indicated by filled squares and triangles, respectively. (a) Dose-response curve for the stimulation of Thp1 cells with HcpA. (b) Dose-response curve for the stimulation of Thp1 cells with HcpC. (c) Time-course for Thp1 cell adherence after stimulation with 123 nM HcpA. (d) Dose-response curve for the stimulation of U937 cells with HcpA.
Cell adherence started within one hour after the addition of HcpA and reached its maximum of 70 % within four to six hours. The high level of cell adherence persisted for at least 24 hours and cells continued to proliferate with approximately one cell division per day (Fig. 3c). Although U937 cells did not show HcpA-induced morphological changes, their adherence was also stimulated by HcpA addition, albeit less dramatically (Fig. 3d): from 2 % of cells (no HcpA) to more than 10 % (300 nM HcpA), with a large increase between 50 nM and 100 nM HcpA. An equal volume of flow-through buffer had no effect on the adherence of U937 cells.
Phagocytosis
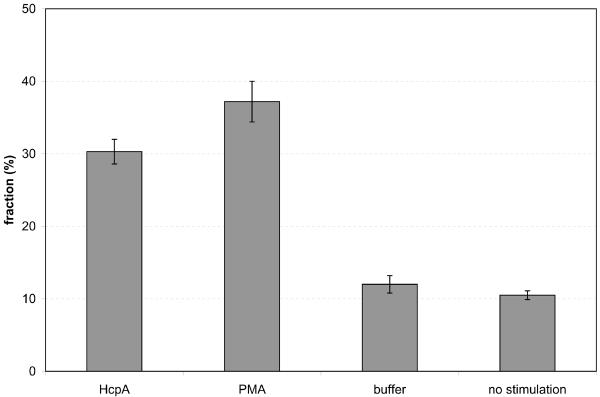
Morphological changes and the onset of cellular adherence are hallmarks for the differentiation of monocytes into macrophages. Further signs of this differentiation process are increased phagocytosis of opsonized particles, due to the expression of Fc-receptors and other surface markers. Therefore we investigated the uptake of fluorescent microspheres by Thp1 cells that were stimulated with HcpA, PMA, or flow-through buffer, using flow-cytometry. When flow-through buffer was used for the stimulation 12.0 ± 1.2 % of living Thp1 cells contained phagocytosed particles, which is almost identical to the fraction of cells in a control where no stimulus was added (10.5 ± 0.6 %). One day after the stimulation with 283 nM HcpA or 333 nM PMA the fractions of Thp1 cells with phagocytosed particles had increased to 30.3 ± 1.7 % and 37.2 ± 2.8 %, respectively (Fig. 4a and supplementary Fig. 2). These results showed that HcpA and PMA have almost equal effects on the ability of Thp1 cells to phagocytose opsonized microspheres.
Figure 4.
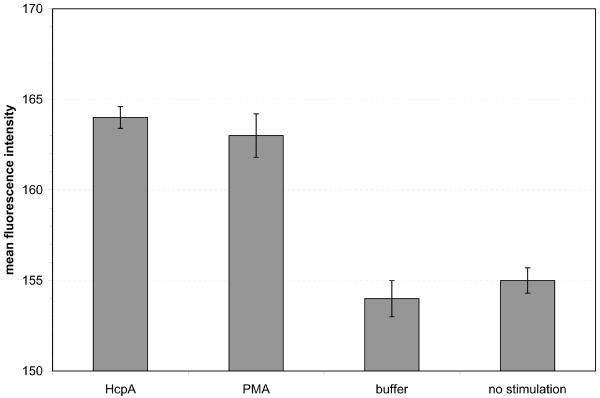
Flow-cytometry analysis of Thp1 cells. (a) Fraction of cells with phagocytosed fluorescent microspheres. P-values for the stimulation with HcpA and PMA are 2.6·10−3 and 1.0·10−3, respectively. (b) Mean fluorescence intensity of cells stained with a fluorescent anti-CD11b antibody. P-values for the stimulation with HcpA and PMA are 4.1·10−4 and 5.0·10−4, respectively.
Molecular surface markers
The expression of specific molecular markers, such as the β2 integrin CD11b, is indicative of the maturation of monocytes into macrophages. 24 hours after stimulation the expression of CD11b was quantified by flow-cytometry. Thp1 cells that were treated with either 340 nM HcpA or 250 nM PMA revealed average fluorescence intensities of 164 ± 0.6 fluorescence units (FU) and 163 ± 1.2 FU, respectively. In contrast, flow-through buffer treated cells showed an average fluorescence intensity of 154 ± 1.0 FU, which is identical to the background intensity of 155 ± 0.7 FU (Fig. 4b). Thp1 cells that were stimulated with 240 nM HcpC did not show an elevated expression of CD11b (Supplementary Fig. 3). Thus, HcpA, like PMA, caused up-regulation of CD11b expression, whereas buffer- or HcpC treatment had no effect.
In vivo analysis
To correlate the in vitro data with the colonization behaviour of H. pylori, an hcpA knockout derivative of strain X47 was in parallel with isogenic X47 wild type tested by oral gavage of C57BL/6 mice (n=3). Colonization was estimated by culture of gastric mucosa from mice sacrificed two weeks after inoculation. The hcpA knockout mutant colonized the stomachs of each of several mice tested at approximately the same level as the isogenic X47 wildtype parent. This showed that hcpA is not essential for H. pylori in vivo.
Discussion
Here we show that the immunogenic H. pylori protein HcpA has an effect like that of the tumor promoter PMA on the differentiation of Thp1 monocytes. HcpA causes the development of filopodia and adherence within a few hours of addition to cell cultures, induces the ability to phagocytose opsonized microspheres, and causes elevated expression of the macrophage marker CD11b. These results agree with and extend an earlier report that HcpA induces the release of cytokines, such as INF-γ, TNF-α, IL-6, IL-10, and IL-12 in a cellular assay of naïve mouse splenocytes [15]. Therefore we conclude that HcpA induces the differentiation of freely circulating monocytes into sessile macrophages. Although HcpA and -C share 56 % sequence identity HcpC does not exert such effects (Supplementary Fig. 4), and thus it likely has other roles during H. pylori infection. The intensity of the HcpA-induced differentiation is cell line dependent. Thp1 and U937 cells each belong to the mononuclear phagocyte lineage, but HcpA has a much stronger effect on Thp1 cells than on U937 cells. We suggest this is because U937 cells are promonocytic cells that are less differentiated than Thp1 cells [22].
Both cell lines are sensitive to contamination with living bacteria and bacteria derived endotoxins. Control experiments with flow-through buffers and with HcpC ruled out contamination-based explanations for our results. In addition, endotoxin concentrations of protein preparations and flow-through buffers, determined by the extremely sensitive Limulus amebocyte lysate test, were found to be extremely low (<1.25 EU/ml), and proteolytic digestion with Pronase and subsequent heat denaturation abrogated the ability of HcpA preparations to induce the star-like phenotype and adherence (data not shown). We conclude that Thp1 differentiation is induced by a temperature and protease sensitive macromolecule, most likely HcpA.
To test for possible HcpA essentiality in vivo, we compared colonization densities of an hcpA deletion knockout mutant strain and its isogenic wild type parent in a two-week C57BL/6 mouse infection model. No difference in was observed. This result shows that HcpA is not essential during the critical establishment phase of infection. Whether it shares overlapping functions with other H. pylori-encoded immune modulators, and how it might affect bacterial persistence and host responses during long term mouse infection, or in models with more severe pathologic effects (e.g., Mongolian gerbils, or specific types of mutant mice) merit testing in the future. Our results are consistent with a previous report on the hcpA knockout H. pylori strain 2802, which displayed a similar cytokine profile like the wildtype when used to challenge splenic cells of naïve BALB/c mice [15].
Why should H. pylori foster monocyte maturation? Monocytes are the precursors for macrophages and dendritic cells and both cell types are crucial for triggering the innate immune response and combating infection. Although the influx of myeloid dendritic cells (CD11b positive) into the lamina propria of the gastric mucosa in response to H. pylori infection is well known [23], the possibility of H. pylori susceptibility to phagocytosis is controversial. Invading macrophages have seemed to be dysfunctional, and H. pylori resisted phagocytosis in one study [24], whereas H. pylori stimulated its own uptake into specific vacuoles called megasomes through transient activation of the protein kinase-C (PKC) isoenzymes ζ in another study [25].
If stimulating monocyte to macrophage differentiation does not help clear H. pylori infection, we propose that it could actually promote H. pylori infection of the gastric mucosa by any of several means: (i) The strong immune response causes mammalian cell lysis and liberation of nutrients upon which H. pylori may feed. (ii) Activating the maturation of monocytes could eliminate microorganisms that would compete with H. pylori in its gastric niche, but that are less resistant against macrophage attack [26]. This could be especially important after H. pylori-elicited suppression of gastric acidity. Finally (iii) HcpA could penetrate deeply into host tissue, due to its extraordinary stability, where it could arrest circulating monocytes before they enter the site of infection. In this case host immune cells would be distracted from their normal function to attack the invading microorganism.
The details of the HcpA signalling pathway remain to be examined. One possibility might be that HcpA serves as a pathogen-associated molecular pattern (PAMP). The HcpA homologue Hsp12 is up-regulated under iron-, pH- and temperature-stress conditions [27]. Thus, Hsp12 and HcpA are regarded as heat-shock proteins, which often serve as PAMPs (reviewed in [28]). Eukaryotic cells sense bacterial PAMPs, such as bacterial lipoproteins, LPS, and flagellin, by means of membrane-bound and cytoplasmic pattern recognition receptors (PRR) such as the Toll-like receptors (TLR), the best-studied members of the PRR family. However, H. pylori LPS and flagellin are virtually inactive in binding to human TLRs; this illustrates one of H. pylori's adaptations for evasion of the innate immune response [29,30].
On the other hand TLRs are weakly expressed in Thp1 monocytes and PMA-treatment is generally used as an in vitro tool to trigger TLR up-regulation and to prime Thp1 cells for PAMP recognition [31]. Therefore un-stimulated Thp1 monocytes are rather insensitive to PAMPs, such as LPS. Considering the low TLR expression level in un-stimulated Thp1 cells, it remains questionable whether HcpA acts through a PRR-dependent pathway. However, HcpA could still act through an autocrine feedback loop, involving the recognition of HcpA by a weakly expressed PRR and subsequent release of cytokines that trigger the morphological changes described above.
Other signalling pathways should also be considered. The observation that HcpA, like cancer-promoting agent PMA, elicits adherence and differentiation within a few hours of administration suggests that they might function by similar mechanisms. PMA induces differentiation and TNF-α release from monocytes by the activation and subsequent translocation of PKC isoenzymes (reviewed in [32,33]). Other SLR proteins such as ExoR from Sinorhizobium melioti, PodJ from Caulobacter crescentus, Nif1 from Schizosaccharomyces pombe, and Ack1 form Saccharomyces cerevisiae recognize the endogenous protein kinases ExoS, PleC, Nim1, and PKC1 respectively [34–37]. The functional analogy between HcpA and PMA and the structural homology between HcpA and other SLR proteins suggest that HcpA could transmit a signal for monocyte maturation through activation of a yet unidentified protein kinase.
We conclude that in vitro HcpA shows molecular and functional hallmarks of a bacterial immune modulator similar to a eukaryotic cytokine. HcpA is secreted by H. pylori cells and folds into a stable three-dimensional structure, consisting of six disulfide-bridged SLRs (Supplementary Fig. 4) [12,17]. Its close homologue HcpC does not show any of these effects and the functional properties of the other HcpA orthologues in H. pylori genomes are unknown. It is tempting to speculate that they might have similar or complementary molecular functions, and altogether constitute a well balanced molecular toolbox that enables H. pylori to fine-tune eukaryotic signalling mechanisms according to its own needs. Closely related HcpA homologues have been identified in the genome sequences of other pathogens and symbionts from the Helicobacter, Campylobacter, Wolinella, and Arcobacter lineages, suggesting that these microorganisms possess similar capacities for managing eukaryotic immune responses.
Supplementary Material
Acknowledgement
We would like to thank Klaus Marquardt for electron microscopy sample preparation and Prof. J. Fritz-Steuber for fruitful discussions. This work was supported by grants from the Velux Foundation (Zürich, Switzerland) and the US National Institutes of Health (RO1 DK63041). Author contribution: CD, flow-cytometry; LS, light microscopy; UZ, scanning electron microscopy; SSC, AK, DB, prepared mutant; AF, WL, MB, BM, analysed mutant in mice; PREM, designed study, protein and cell biology, wrote manuscript.
Abbreviations
- Mbp
maltose binding protein
- NTA
nitrilotriacetic acid
- FACS
fluorescence-activated cell sorting
- BSA
bovine serum albumin
- PBS
phosphate buffer saline
- FITC
Fluorescein isothiocyanate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–5. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- [2].Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19:449–90. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mohammadi M, Czinn S, Redline R, Nedrud J. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156:4729–38. [PubMed] [Google Scholar]
- [4].Haeberle HA, et al. Differential stimulation of interleukin-12 (IL-12) and IL-10 by live and killed Helicobacter pylori in vitro and association of IL-12 production with gamma interferon-producing T cells in the human gastric mucosa. Infect Immun. 1997;65:4229–35. doi: 10.1128/iai.65.10.4229-4235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sommer F, Faller G, Rollinghoff M, Kirchner T, Mak TW, Lohoff M. Lack of gastritis and of an adaptive immune response in interferon regulatory factor-1-deficient mice infected with Helicobacter pylori. Eur J Immunol. 2001;31:396–402. doi: 10.1002/1521-4141(200102)31:2<396::aid-immu396>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- [6].D'Elios MM, Amedei A, Benagiano M, Azzurri A, Del Prete G. Helicobacter pylori, T cells and cytokines: the “dangerous liaisons”. FEMS Immunol Med Microbiol. 2005;44:113–9. doi: 10.1016/j.femsim.2004.10.013. [DOI] [PubMed] [Google Scholar]
- [7].Tomb JF, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–47. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- [8].Alm RA, et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–80. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- [9].Oh JD, et al. The complete genome sequence of a chronic atrophic gastritis Helicobacter pylori strain: evolution during disease progression. Proc Natl Acad Sci U S A. 2006;103:9999–10004. doi: 10.1073/pnas.0603784103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Baltrus DA, et al. The Complete Genome Sequence of Helicobacter pylori strain G27. J Bacteriol. 2009;191:447–8. doi: 10.1128/JB.01416-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cao P, McClain MS, Forsyth MH, Cover TL. Extracellular release of antigenic proteins by Helicobacter pylori. Infect Immun. 1998;66:2984–6. doi: 10.1128/iai.66.6.2984-2986.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mittl PR, Lüthy L, Hunziker P, Grütter MG. The cysteine-rich protein A from Helicobacter pylori is a beta-lactamase. J Biol Chem. 2000;275:17693–9. doi: 10.1074/jbc.M001869200. [DOI] [PubMed] [Google Scholar]
- [13].Mittl PR, Lüthy L, Reinhardt C, Joller H. Detection of high titers of antibody against Helicobacter cysteine-rich proteins A, B, C, and E in Helicobacter pylori-infected individuals. Clin Diagn Lab Immunol. 2003;10:542–5. doi: 10.1128/CDLI.10.4.542-545.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mittl PR, Schneider-Brachert W. Sel1-like repeat proteins in signal transduction. Cell Signal. 2007;19:20–31. doi: 10.1016/j.cellsig.2006.05.034. [DOI] [PubMed] [Google Scholar]
- [15].Deml L, et al. Characterization of the Helicobacter pylori cysteine-rich protein A as a T-helper cell type 1 polarizing agent. Infect Immun. 2005;73:4732–42. doi: 10.1128/IAI.73.8.4732-4742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Salaun L, Linz B, Suerbaum S, Saunders NJ. The diversity within an expanded and redefined repertoire of phase-variable genes in Helicobacter pylori. Microbiology. 2004;150:817–30. doi: 10.1099/mic.0.26993-0. [DOI] [PubMed] [Google Scholar]
- [17].Ogura M, et al. Helicobacter pylori evolution: lineage- specific adaptations in homologs of eukaryotic Sel1-like genes. PLoS Comput Biol. 2007;3:e151. doi: 10.1371/journal.pcbi.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schwende H, Fitzke E, Ambs P, Dieter P. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J Leukoc Biol. 1996;59:555–61. [PubMed] [Google Scholar]
- [19].Kohro T, Tanaka T, Murakami T, Wada Y, Aburatani H, Hamakubo T, Kodama T. A comparison of differences in the gene expression profiles of phorbol 12-myristate 13-acetate differentiated THP-1 cells and human monocyte-derived macrophage. J Atheroscler Thromb. 2004;11:88–97. doi: 10.5551/jat.11.88. [DOI] [PubMed] [Google Scholar]
- [20].Luthy L, Grutter MG, Mittl PR. The crystal structure of Helicobacter cysteine-rich protein C at 2.0 A resolution: similar peptide-binding sites in TPR and SEL1-like repeat proteins. J Mol Biol. 2004;340:829–41. doi: 10.1016/j.jmb.2004.04.055. [DOI] [PubMed] [Google Scholar]
- [21].Dailidiene D, Dailide G, Kersulyte D, Berg DE. Contraselectable streptomycin susceptibility determinant for genetic manipulation and analysis of Helicobacter pylori. Appl Environ Microbiol. 2006;72:5908–14. doi: 10.1128/AEM.01135-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cassol E, Alfano M, Biswas P, Poli G. Monocyte-derived macrophages and myeloid cell lines as targets of HIV-1 replication and persistence. J Leukoc Biol. 2006;80:1018–30. doi: 10.1189/jlb.0306150. [DOI] [PubMed] [Google Scholar]
- [23].Nishi T, et al. Involvement of myeloid dendritic cells in the development of gastric secondary lymphoid follicles in Helicobacter pylori-infected neonatally thymectomized BALB/c mice. Infect Immun. 2003;71:2153–62. doi: 10.1128/IAI.71.4.2153-2162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ramarao N, Meyer TF. Helicobacter pylori resists phagocytosis by macrophages: quantitative assessment by confocal microscopy and fluorescence-activated cell sorting. Infect Immun. 2001;69:2604–11. doi: 10.1128/IAI.69.4.2604-2611.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Allen LA, Allgood JA. Atypical protein kinase C-zeta is essential for delayed phagocytosis of Helicobacter pylori. Curr Biol. 2002;12:1762–6. doi: 10.1016/s0960-9822(02)01216-2. [DOI] [PubMed] [Google Scholar]
- [26].Stecher B, et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5:2177–89. doi: 10.1371/journal.pbio.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].de Vries N, van Ark EM, Stoof J, Kuipers EJ, van Vliet AH, Kusters JG. The stress-induced hsp12 gene shows genetic variation among Helicobacter pylori strains. FEMS Immunol Med Microbiol. 2003;38:45–51. doi: 10.1016/S0928-8244(03)00105-6. [DOI] [PubMed] [Google Scholar]
- [28].Osterloh A, Breloer M. Heat shock proteins: linking danger and pathogen recognition. Med Microbiol Immunol. 2008;197:1–8. doi: 10.1007/s00430-007-0055-0. [DOI] [PubMed] [Google Scholar]
- [29].Backhed F, et al. Gastric mucosal recognition of Helicobacter pylori is independent of Toll-like receptor 4. J Infect Dis. 2003;187:829–36. doi: 10.1086/367896. [DOI] [PubMed] [Google Scholar]
- [30].Andersen-Nissen E, Smith KD, Strobe KL, Barrett SL, Cookson BT, Logan SM, Aderem A. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci U S A. 2005;102:9247–52. doi: 10.1073/pnas.0502040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–61. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- [32].Blumberg PM. Protein kinase C as the receptor for the phorbol ester tumor promoters: sixth Rhoads memorial award lecture. Cancer Res. 1988;48:1–8. [PubMed] [Google Scholar]
- [33].Liu WS, Heckman CA. The sevenfold way of PKC regulation. Cell Signal. 1998;10:529–42. doi: 10.1016/s0898-6568(98)00012-6. [DOI] [PubMed] [Google Scholar]
- [34].Chen EJ, Sabio EA, Long SR. The periplasmic regulator ExoR inhibits ExoS/ChvI two-component signalling in Sinorhizobium meliloti. Mol Microbiol. 2008;69:1290–303. doi: 10.1111/j.1365-2958.2008.06362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Viollier PH, Sternheim N, Shapiro L. Identification of a localization factor for the polar positioning of bacterial structural and regulatory proteins. Proc Natl Acad Sci U S A. 2002;99:13831–6. doi: 10.1073/pnas.182411999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wu L, Russell P. Nif1, a novel mitotic inhibitor in Schizosaccharomyces pombe. Embo J. 1997;16:1342–50. doi: 10.1093/emboj/16.6.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Krause SA, Xu H, Gray JV. The synthetic genetic network around PKC1 identifies novel modulators and components of protein kinase C signaling in Saccharomyces cerevisiae. Eukaryot Cell. 2008;7:1880–7. doi: 10.1128/EC.00222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.