Abstract
Polyaromatic hydrocarbons, including benzo[a]pyrene (BP), are major tobacco carcinogens. Their carcinogenic effects require metabolic activation by cytochrome p450 (CYP) enzymes. Relative CYP isoform expression is related to tissue-specific tobacco-related squamous cell carcinoma (SCC) susceptibility. There have been conflicting reports regarding relative CYP1A1 and CYP1B1 oral expression, and information regarding CYP1B1 expression in oral tissues is limited.
OBJECTIVE
To quantify BP- and tobacco-induced CYP1A1 and CYP1B1 expression in oral SCC cells and oral mucosa.
STUDY DESIGN
Real-time qPCR was performed to measure 1) BP-induced CYP1A1 and CYP1B1 mRNA expression in seven oral/other head and neck SCC cell lines 2) CYP1A1 and CYP1B1 mRNA expression in gingiva from 22 smokers and 24 nonsmokers.
RESULTS
SCC lines exhibited either similar induction of both isoforms or preferential CYP1A1 induction (CYP1A1-to-CYP1B1 ratios 0.8-4.3). In contrast, gingival tissues from smokers exhibited preferential CYP1B1 induction. Marked interindividual variation in CYP1A1 and CYP1B1 expression was observed among smokers.
CONCLUSIONS
In vitro conditions may not account for factors that modulate expression in vivo. Interindividual variation in inducible CYP1A1 and CYP1B1 expression may account in part for variation in tobacco-related oral SCC risk.
Keywords: oral cancer, CYP1A1, CYP1B1, tobacco, squamous cell carcinoma
INTRODUCTION
Tobacco use is a major risk factor for oral squamous cell carcinoma (SCC).1 Chief among the >60 carcinogens in cigarette smoke are the polycyclic aromatic hydrocarbons (PAH)--including the prototype of this chemical class benzo[a]pyrene (BP).2, 3 BP exerts its carcinogenic effects after metabolic activation.4, 5 Activation involves monooxygenation by cytochrome p450 (CYP) isoforms CYP1 and hydration by microsomal epoxide hydrolase. These reactions produce reactive species that form DNA adducts.5, 6 DNA adducts may lead to cancer by causing mutations in genes essential for key functions, including apoptosis, proliferation, and differentiation.
The major CYP isoforms involved in BP bioactivation are CYP1A1 and CYP1B1. CYP1A1 is considered the classical isoform for PAH activation.7 The discovery of CYP1B1 in 1994 has led to recent studies suggesting CYP1B1 is important in tobacco-related head and neck carcinogenesis as well. Some have suggested CYP1B1 may play an even more important role than CYP1A1 in this process8, 9, and the CYP1B1*3 polymorphism has been identified as a susceptibility factor for head and neck SCC.10, 11
The literature regarding CYP1B1 expression in human oral cell lines is limited and variable. In previous studies, Wen and Walle reported preferential induction by BP of CYP1B1 over CYP1A1 in SCC-9 cells and bioengineered human gingiva.8, 9 In contrast, in a microarray profiling study with validation by qRT-PCR to assess gene expression in response to cigarette smoke condensate, Nagaraj et al. reported similar CYP1A1 and CYP1B1 induction in oral dysplasia lines (Leuk1, Leuk2) and greater induction of CYP1A1 than CYP1B1 in oral SCC line 101A.12
Reports of oral CYP1B1 expression in humans also are limited. Spivack et al. demonstrated CYP1B1 mRNA induction using real-time qPCR on exfoliated buccal mucosa cells obtained from a tobacco-naïve individual who smoked four cigarettes. They also observed a positive relationship between smoking and CYP1B1 (but not CYP1A1) mRNA levels in exfoliated buccal epithelial cells from a small group of human subjects.13
Our underlying hypothesis is that CYP1B1 is a biomarker of tobacco use with biologic relevance to smoking-induced oral carcinogenesis. In this study, we quantified inducible CYP1A1 and CYP1B1 expression to assess whether there is preferential CYP1B1 induction. CYP1A1 and CYP1B1 mRNA expression was evaluated in oral SCC lines exposed to BP and in normal oral tissues from smokers and nonsmokers.
MATERIALS AND METHODS
Cell culture
Oral SCC lines UM-SCC-1 and UM-SCC-14A, hypopharyngeal SCC line UM-SCC-22A, and laryngeal SCC line UM-SCC-12 were provided by Prof. T. Carey (University of Michigan, MI, USA). SCC-9 (derived from oral tongue) was obtained from ATCC (Bethesda, MD, USA). These lines were maintained in DMEM/F12 with 10% fetal bovine serum (FBS) (Hyclone), hydrocortisone, and penicillin/streptomycin. Oral SCC lines UPCI:SCC040 and UPCI:SCC081 (courtesy of Dr. Susanne Gollin, University of Pittsburgh, PA, USA) were maintained in MEM with 1% non-essential amino acids, 1% L-glutamine, 50μg/ml gentamicin and 15% FBS. Cell lines were grown in a humidified incubator (37°C, 5% CO2).
BP treatment and CYP1A1/CYP1B1 mRNA quantification for SCC lines
Cell lines were exposed to 2μM BP or vehicle for 24h. Additionally, to demonstrate a dose response, UMSCC-14A was treated for 48h with varying BP concentrations (.01, .1, 1, 10μM) or vehicle.
Total RNA was isolated using the RNAqueous-4PCR Kit (Ambion, Austin, TX); cDNA was synthesized from 1μg total RNA using the Accuscript High-Fidelity 1st Strand cDNA Synthesis Kit (Stratagene, La Jolla, CA). Real-time qPCR was performed using an iCycler (Bio-Rad, Austin, TX). Each 20μL reaction included 10μL iQ SYBR-Green Supermix (Bio-Rad), 20pmol primers, and 1μL cDNA template. Primers were as follows:
- CYP1A1: (forward)5′-GCTGACTTCATCCCTATTCT-3′
- (reverse)5′-GCTCCAGGAGATAGCAGTTG-3′
- CYP1B1: (forward)5′-GGCCACTATCACTGACATCT-3′
- (reverse)5′-ACAGTGTCCTTGGGAATGTG-3′
- β-actin: (forward)5′-GACGAGGCCCAGAGCAAGAG-3′
- (reverse)5′-GTGGTGGTGAAGCTGTAGCC-3′
Samples were run in triplicate. Parameters included 95°C 1min followed by 45 amplification cycles (95°C 20s, 55°C 30s, 70°C 30s). Dissociation curve analysis was verified for each run. Products were visualized by 2% agarose-ethidium-bromide electrophoresis.
Changes in gene expression between BP-treated and untreated cells were calculated using the comparative threshold cycle method,14 with normalization to β-actin. The relative quantification (RQ) (i.e., fold-change in expression) was expressed as 2-Δ ΔCt. The Wilcoxon two-sample rank sum test15 (SAS, v9.1, SAS Institute, Cary, NC) was applied to compare fold-changes in CYP1A1 and CYP1B1 mRNA expression in BP-treated versus vehicle-treated controls (alpha<0.05).
Western blot analysis
UPCI:SCC040 cells treated with 2μM BP or vehicle were harvested at 24h. Microsome isolation and Western blot analysis [with primary antibodies to CYP1A1 (1:500, rabbit anti-fish 1A, Biosense Laboratories, Bergen, Norway, specific for human 1A1) and CYP1B1 (1:1000, rabbit anti-human, gift from Dr. Craig Marcus, University of New Mexico, Albuquerque, USA)] were performed using protocols described previously.9
Gingival samples
Gingival tissues were obtained from patients in the periodontics and oral surgery clinics at the Medical University of South Carolina College of Dental Medicine. Subjects were receiving surgical procedures during which tissue that otherwise would have been discarded was removed. The protocol was approved by the Institutional Review Board, and patient consent was obtained. Demographic (age/gender/ethnicity), tobacco, alcohol, medication, and occupational exposure information was recorded. Upon clinical and histopathologic examination, the tissues exhibited either no pathology or nonspecific inflammation. The smoking group reported current cigarette use >1 pack/day. The nonsmoking group consisted of never smokers. Gingival samples (10-20mg) were immersed in 10 volumes RNAlater (Ambion) and stored (-20°C).
Salivary cotinine assay
Patient current smoking status was verified by Cotinine Saliva Micro-Plate EIA (OraSure Technologies, Bethlehem, PA). 1mL unstimulated whole saliva was collected and stored at 4°C for <21 days. Salivary cotinine concentrations were analyzed in duplicate per manufacturer’s protocol (>10ng/mL positive for smoking).
RNA isolation and cDNA synthesis from tissue samples
Frozen tissues were thawed (37°C), placed in RLT-βME lysis buf fer, pulverized, and centrifuged (6,000g, 3min). RNA was isolated from supernatants using the RNeasy Plus Micro Kit (Qiagen, Valencia, CA). RNA purity and quantity was evaluated by A260/A280 measurement (>1.8). cDNA synthesis was performed from 1μg RNA using SuperScript III First-Strand Synthesis Supermix (Invitrogen, Carlsbad, CA) per manufacturer’s protocol.
CYP1A1/CYP1B1 mRNA quantification in tissue samples by real-time qPCR
Real-time qPCR was performed using the StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA). Reactions were prepared as described above with primers as follows:
- CYP1A1: (forward)5′-GCTGACTTCATCCCTATTCT-3′
- (reverse)5′-GCTCCAGGAGATAGCAGTTG-3′
- CYP1B1: (forward)5′-GGCCACTATCACTGACATCT-3′
- (reverse)5′-CCACGACCTGATCCAATTCT-3′
- TATA-box binding protein(TBP):
- (forward)5′-TTCGGAGAGTTCTGGGATTGTA-3′
- (reverse)5′-TGGACTGTTCTTCACTCTTGGC-3′
Samples were run in duplicate. Along with each patient sample, a reaction was run using a constant amount UM-SCC-22A cDNA template (1μL from a single cDNA synthesis). Parameters included 95°C 10min and 50 amplification cycles (95°C 20s, 55°C 30s, 70°C 30s). Dissociation curve analysis was verified for each run. Products were visualized by 2% agarose-ethidium-bromide gel electrophoresis.
RQ was calculated using the comparative threshold cycle method14 with normalization to TBP (a housekeeping gene) and comparison to UM-SCC-22A. Wilcoxon two-sample rank sum15 and Kruskal-Wallis one-way ANOVA16 tests (SAS, v9.1, SAS Institute, Cary, NC) were used to detect significant differences (alpha<0.05) in CYP1A1 and CYP1B1 RQs according to smoking (yes/no), age quartile (<50/50-54/55-62/>62 years), ethnicity (white/non-white), alcohol consumption (yes/no), and gender (male/female).
RESULTS
Preferential CYP1A1 induction or similar CYP1A1/CYP1B1 induction in oral/other head and neck SCC lines
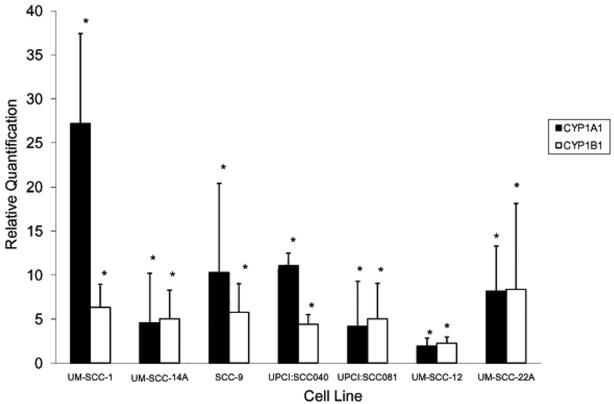
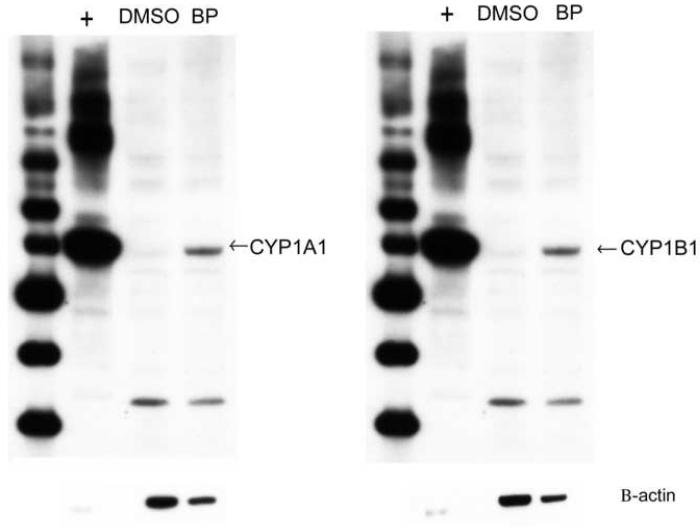
All lines exhibited significant CYP1A1 and CYP1B1 induction with 24h 2μM BP exposure (Figure 1). For the oral SCC lines UM-SCC-1, UPCI:SCC040, and SCC-9, the CYP1A1-to-CYP1B1 fold-change ratios were 4.3, 2.5, and 1.8, respectively, indicating preferential CYP1A1 induction. In the remaining lines, ratios were 0.8-1.0, indicating similar induction of both isoforms. Western blot analysis of untreated and BP-treated UPCI:SCC040 confirmed induction of CYP1A1 and CYP1B1 protein expression (Figure 2).
Figure 1.
CYP1A1 and CYP1B1 mRNA induction in different oral/head and neck SCC cell lines. Cell lines were exposed to 2 μM BP for 24 h. Total RNA was isolated and reverse transcribed to generate cDNA. Real-time qPCR was performed to measure CYP1A1 and CYP1B1 mRNA expression. Each sample was run in triplicate and mean values were used in subsequent calculations. The relative quantification (RQ) (or fold-change in expression between treated and untreated cells) was determined by the 2 -Δ ΔCt method with normalization to β-actin. * indicates target expression is significantly greater in BP-treated versus untreated cells (p < 0.05).
Figure 2.
Western blot analysis demonstrates induction of CYP1A1 and CYP1B1 protein expression by BP in UPCI:SCC040 cells. The cells were exposed to either 2 μM BP or DMSO (dimethylsulfoxide) vehicle (negative control) for 24 h. Microsomes were isolated, and 24 μg microsomal protein was loaded. Recombinant baculovirus-expressed CYP1A1 and CYP1B1 protein (0.025 μg) served as positive controls (+). β-actin loading control is shown below.
BP dose response for CYP1A1 and CYP1B1 induction
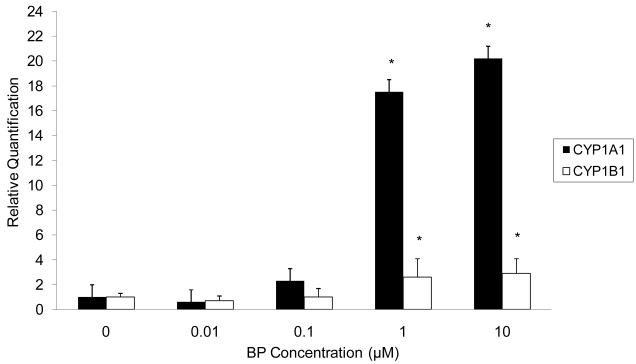
For UM-SCC-14A, significant CYP1A1 and CYP1B1 mRNA induction (p=0.04) was evident at BP concentrations as low as 1μM (Figure 3). Previous investigators have considered the 1μM scale to represent a low and physiologically relevant BP concentration.9
Figure 3.
CYP1A1 and CYP1B1 mRNA expression in UM-SCC-14A in response to different doses of BP. The cells were exposed to .01, .1, 1, or 10 μM BP for 48h. Vehicle-treated cells served as a negative control. Total RNA was isolated and reverse transcribed to generate cDNA. Real-time qPCR was performed to analyze the expression of CYP1A1 and CYP1B1 mRNA. Each sample was run in triplicate and mean values were used in subsequent calculations. The relative quantification (RQ) was determined by the 2 -Δ ΔCt method using normalization to β-actin and comparison to the vehicle-treated control. * indicates that target expression is significantly greater in BP-treated than vehicle-treated cells (p < 0.05).
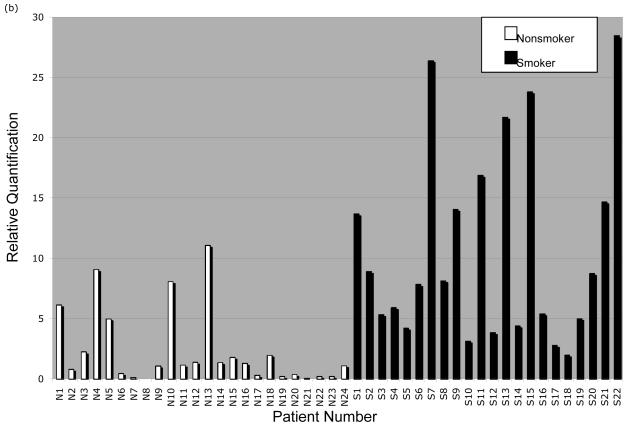
Significantly greater CYP1B1 expression in gingiva from smokers versus nonsmokers
Subject characteristics are summarized in Table 1. Salivary cotinine analysis confirmed each patient’s smoking/nonsmoking status. No patients were taking medications metabolized by CYP1A1/CYP1B1, and none reported occupational chemical exposures known to induce CYP1A1/CYP1B1.
Table 1.
Characteristics of subjects donating normal gingival tissue samples
| Characteristic | Smokers (n=22) | Nonsmokers (n=24) |
|---|---|---|
| Mean age (years) | 51 | 55 |
| Male: female ratio | 1.2:1 | 1.2:1 |
| Ethnicity | 18 Caucasian | 18 Caucasian |
| 4 African American | 6 African American | |
| Mean tobacco exposure (pack-years) | 27.9 | (not applicable) |
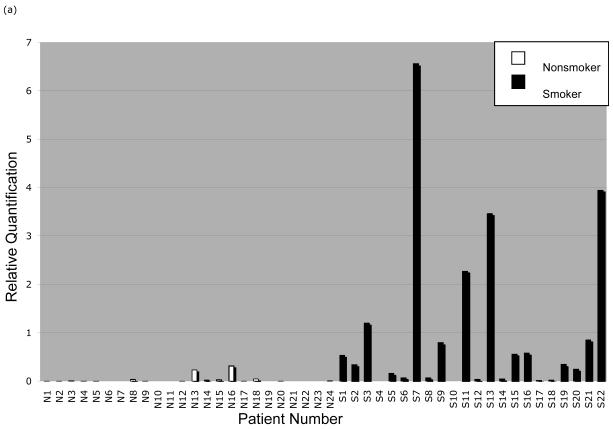
Real-time qPCR analysis (Figure 4A-B) showed significantly greater CYP1A1 and CYP1B1 expression in smokers than nonsmokers (p<0.0001 for CYP1A1 and CYP1B1). Kruskal-Wallis ANOVA tests showed no significant differences in expression by age, gender, ethnicity, or alcohol use. Within the smoking and nonsmoking groups, there was considerable interindividual variation in CYP1A1 (smokers: mean RQ=1.00±1.66, nonsmokers: mean RQ=0.03±0.08) and CYP1B1 expression (smokers: mean RQ=10.70±8.13, nonsmokers: mean RQ=2.31±3.14).
Figure 4.
CYP1A1 and CYP1B1 mRNA quantification in normal gingival tissue samples from nonsmokers and smokers. Gingival tissue samples were obtained from nonsmoking and smoking patients. Total RNA was isolated and reverse transcribed to generate cDNA. Real-time qPCR was performed to analyze the expression of (a) CYP1A1 mRNA and (b) CYP1B1 mRNA. Each patient sample was run in duplicate and mean values were used in subsequent calculations. The relative quantification (RQ) was determined by the 2 -Δ ΔCt method using normalization to the housekeeping gene for TATA-box binding protein (TBP) and comparison to reactions using a constant amount of uninduced UM-SCC-22A cDNA.
DISCUSSION
We demonstrated preferential CYP1A1 induction or similar CYP1A1 and CYP1B1 induction in the oral/other head and neck SCC lines tested. Our results are similar to those of Nagaraj et al. (described above).12 However, our results differ from those of Wen and Walle who reported preferential CYP1B1 induction in SCC-9 cells and bioengineered gingival tissue.9 These authors used semiquantitative analysis based upon branched DNA technology, which may explain their differing results.
In contrast to our cell line analysis, gingival tissue analysis showed more marked CYP1B1 than CYP1A1 induction among smokers. The discordance between in vitro and patient study results may reflect a difference between SCC cells and normal oral mucosa or effects of BP versus whole tobacco smoke. Furthermore, in vitro conditions may not account for in vivo factors that might modulate CYP expression. Lipopolysaccharides and pro-inflammatory cytokines, including tumor necrosis factor(TNF)-α, interleukin(IL)-1β, and transforming growth factor(TGF)-β1, have been reported to downregulate CYP1A1 expression; also, TNF- α and TGF-β1 upregulate CYP1B1 expression.5, 17-19 These observations are from studies of rat liver epithelial and stellate cells and human hepatocytes--it is unclear whether such effects also may apply to human oral tissue. However, this possibility is compelling because of the prevalence of inflammation in the oral cavity; in our study, some gingival samples indeed exhibited inflammation.
The balance between CYP1A1/CYP1B1 expression in a given tissue type is important as it relates to organ-specific susceptibility to BP toxicity and carcinogenesis. This balance is complex, and in vitro models do not always predict in vivo carcinogen sensitivity. Uno et al. showed that Cyp1a1-/- knockout mice exhibit increased hepatic levels of BaP-DNA adducts and greater protection from BP-mediated hepatotoxicity and death compared with Cyp1a1+/+ mice.20 Thus, CYP1A1 paradoxically has a protective role in vivo that would not be predicted by in vitro studies. In contrast, no such paradox has been found in Cyp1b1-/- knockout mice.21-24 CYP1 enzymes are coupled to Phase II detoxification enzymes in vivo. It has been proposed that compared to CYP1B1, CYP1A1 is more tightly coupled to Phase II metabolism and plays a more important role in vivo in detoxification than toxin activation.25
There has been much interest in characterizing CYP1B1 expression in different normal and cancer tissue types. CYP1B1 upregulation has been reported in numerous cancers, including those arising in breast, colon, lung, esophagus, skin, brain, and testis.26 In normal adult tissues, CYP1B1 has been detected only at low levels by Northern blot analysis in kidney, liver, eye, and brain.27-29 Thus, investigators have regarded CYP1B1 as a biomarker of tumor development and potential therapeutic target. In our study, observed CYP1B1 induction in normal oral tissues from smokers suggests CYP1B1 indeed may be an important early biomarker related to risk of tobacco-induced oral SCC development.
Murray et al. cautioned that previous investigations of CYP1B1 expression in normal human tissues have utilized commercially obtained samples.27 Such samples have no corresponding patient histories (e.g., tobacco, chemical, and drug exposure), and typically it is unknown whether such samples represent a single patient or pool of patients. Therefore, our study is significant in that it represents the only investigation of CYP1B1 expression in normal oral tissues obtained from well-characterized smoking and nonsmoking cohorts. Among smokers, we observed marked variation in CYP1A1 and CYP1B1 expression (RQ range 0 to 6-fold for CYP1A1 and 2- to 28-fold for CYP1B1). Interindividual variation in CYP expression is a well-known phenomenon. For instance, Wiley et al. reported significant interindividual variation in CYP1A1 and CYP1B1 expression in bronchial epithelial cells, which might account for interindividual variation in the risk of bronchogenic carcinoma.30 Accordingly, differences in CYP1A1 and CYP1B1 inducibility and activity may account for interindividual variation in tobacco-related oral cancer risk and may relate to CYP1A1 and CYP1B1 polymorphic variants.10, 11, 31, 32
Unlike smokers, most nonsmokers had low or undetectable CYP1B1 expression. Nevertheless, there were some cases (n=7) in which CYP1B1 expression was detected at levels comparable to those in the smoking group. Salivary cotinine assay confirmed reported smoking status for each patient. Thus, we suspect sources of PAH exposure other than tobacco. High PAH levels have been found in grilled or smoked meats.33-35 The ingestion of such foods has been associated with an increased risk of colorectal, gastric, and esophageal cancers, although no such association with oral cancer has been reported.36, 37 Among our subjects, we did not find any occupational exposures or medications that might interact with CYP1A1 or CYP1B1. Nonetheless, various environmental factors (e.g., diesel exhaust, asphalt fumes, wood smoke) are sources of PAH exposure as well.33
We recognize that there were a limited number of patients analyzed in this study, and thus a population-based study with subjects matched by age, gender, and ethnicity would be needed to confirm our findings. Nonetheless, in the current study, the demographic composition of the smoking and nonsmoking groups was similar, and our analysis demonstrates preferential CYPB1 induction in smokers regardless of age, gender, ethnicity, and alcohol use.
In conclusion, this study represents the first well-characterized clinical investigation of tobacco-induced oral CYP1A1 and CYP1B1 expression. We demonstrate preferential induction of CYP1B1 in smokers. In contrast, with BP exposure, oral SCC cell lines show similar induction of both isoforms or preferential CYP1A1 induction. In vitro conditions may not include in vivo factors, such as modulation by inflammatory cytokines. Interindividual variation in CYP1A1 and CYP1B1 expression among smokers may account in part for interindividual variation in tobacco-related oral SCC risk. Moreover, CYP1B1 may be an important early biomarker for risk of tobacco-induced oral SCC development.
ACKNOWLEDGMENTS
This work was supported by the South Carolina Center of Biomedical Research Excellence (COBRE) for Oral Health NIH/NCRR P20RR017696. We would like to thank Dr. Thomas Walle for his encouragement and Dr. Elizabeth Slate for her statistical advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Reibel J. Tobacco and oral diseases. Update on the evidence, with recommendations. Med Princ Pract. 2003;12(Suppl 1):22–32. doi: 10.1159/000069845. [DOI] [PubMed] [Google Scholar]
- 2.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–44. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 3.Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–210. doi: 10.1093/jnci/91.14.1194. [DOI] [PubMed] [Google Scholar]
- 4.Baird WM, Hooven LA, Mahadevan B. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ Mol Mutagen. 2005;45:106–14. doi: 10.1002/em.20095. [DOI] [PubMed] [Google Scholar]
- 5.Umannova L, Machala M, Topinka J, Novakova Z, Milcova A, Kozubik A, et al. Tumor necrosis factor-alpha potentiates genotoxic effects of benzo[a]pyrene in rat liver epithelial cells through upregulation of cytochrome P450 1B1 expression. Mutat Res. 2008;640:162–9. doi: 10.1016/j.mrfmmm.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Xue W, Warshawsky D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: a review. Toxicol Appl Pharmacol. 2005;206:73–93. doi: 10.1016/j.taap.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Thier R, Bruning T, Roos PH, Bolt HM. Cytochrome P450 1B1, a new keystone in gene-environment interactions related to human head and neck cancer? Arch Toxicol. 2002;76:249–56. doi: 10.1007/s00204-002-0349-3. [DOI] [PubMed] [Google Scholar]
- 8.Walle T, Walle UK, Sedmera D, Klausner M. Benzo[a]pyrene-induced oral carcinogenesis and chemoprevention: studies in bioengineered human tissue. Drug Metab Dispos. 2006;34:346–50. doi: 10.1124/dmd.105.007948. [DOI] [PubMed] [Google Scholar]
- 9.Wen X, Walle T. Preferential induction of CYP1B1 by benzo[a]pyrene in human oral epithelial cells: impact on DNA adduct formation and prevention by polyphenols. Carcinogenesis. 2005;26:1774–81. doi: 10.1093/carcin/bgi127. [DOI] [PubMed] [Google Scholar]
- 10.Singh AP, Shah PP, Mathur N, Buters JT, Pant MC, Parmar D. Genetic polymorphisms in cytochrome P4501B1 and susceptibility to head and neck cancer. Mutat Res. 2008;639:11–9. doi: 10.1016/j.mrfmmm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Ko Y, Abel J, Harth V, Brode P, Antony C, Donat S, et al. Association of CYP1B1 codon 432 mutant allele in head and neck squamous cell cancer is reflected by somatic mutations of p53 in tumor tissue. Cancer Res. 2001;61:4398–404. [PubMed] [Google Scholar]
- 12.Nagaraj NS, Beckers S, Mensah JK, Waigel S, Vigneswaran N, Zacharias W. Cigarette smoke condensate induces cytochromes P450 and aldo-keto reductases in oral cancer cells. Toxicol Lett. 2006;165:182–94. doi: 10.1016/j.toxlet.2006.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spivack SD, Hurteau GJ, Jain R, Kumar SV, Aldous KM, Gierthy JF, et al. Gene-environment interaction signatures by quantitative mRNA profiling in exfoliated buccal mucosal cells. Cancer Res. 2004;64:6805–13. doi: 10.1158/0008-5472.CAN-04-1771. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Hollander M, Wolfe DA. Nonparametric Statistical Methods. 2nd edition Wiley-Interscience; New York: 1999. Section 4.1 A Distribution-Free Rank Sum Test (Wilcoxon, Mann and Whitney) pp. 106–24. [Google Scholar]
- 16.Hollander M, Wolfe DA. Nonparametric Statistical Methods. 2nd edition Wiley-Interscience; New York: 1999. Section 6.1 A Distribution-Free Test for General Alternatives (Kruskal-Wallis) pp. 190–99. [Google Scholar]
- 17.Piscaglia F, Knittel T, Kobold D, Barnikol-Watanabe S, Di Rocco P, Ramadori G. Cellular localization of hepatic cytochrome 1B1 expression and its regulation by aromatic hydrocarbons and inflammatory cytokines. Biochem Pharmacol. 1999;58:157–65. doi: 10.1016/s0006-2952(99)00066-0. [DOI] [PubMed] [Google Scholar]
- 18.Ke S, Rabson AB, Germino JF, Gallo MA, Tian Y. Mechanism of suppression of cytochrome P-450 1A1 expression by tumor necrosis factor-alpha and lipopolysaccharide. J Biol Chem. 2001;276:39638–44. doi: 10.1074/jbc.M106286200. [DOI] [PubMed] [Google Scholar]
- 19.Abdel-Razzak Z, Corcos L, Fautrel A, Campion JP, Guillouzo A. Transforming growth factor-beta 1 down-regulates basal and polycyclic aromatic hydrocarbon-induced cytochromes P-450 1A1 and 1A2 in adult human hepatocytes in primary culture. Mol Pharmacol. 1994;46:1100–10. [PubMed] [Google Scholar]
- 20.Uno S, Dalton TP, Shertzer HG, Genter MB, Warshawsky D, Talaska G, et al. Benzo[a]pyrene-induced toxicity: paradoxical protection in Cyp1a1(-/-) knockout mice having increased hepatic BaP-DNA adduct levels. Biochem Biophys Res Commun. 2001;289:1049–56. doi: 10.1006/bbrc.2001.6110. [DOI] [PubMed] [Google Scholar]
- 21.Page TJ, O’Brien S, Holston K, MacWilliams PS, Jefcoate CR, Czuprynski CJ. 7,12-Dimethylbenz[a]anthracene-induced bone marrow toxicity is p53-dependent. Toxicol Sci. 2003;74:85–92. doi: 10.1093/toxsci/kfg115. [DOI] [PubMed] [Google Scholar]
- 22.Buters JT, Mahadevan B, Quintanilla-Martinez L, Gonzalez FJ, Greim H, Baird WM, et al. Cytochrome P450 1B1 determines susceptibility to dibenzo[a,l]pyrene-induced tumor formation. Chem Res Toxicol. 2002;15:1127–35. doi: 10.1021/tx020017q. [DOI] [PubMed] [Google Scholar]
- 23.Buters JT, Sakai S, Richter T, Pineau T, Alexander DL, Savas U, et al. Cytochrome P450 CYP1B1 determines susceptibility to 7, 12-dimethylbenz[a]anthracene-induced lymphomas. Proc Natl Acad Sci U S A. 1999;96:1977–82. doi: 10.1073/pnas.96.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uno S, Dalton TP, Dragin N, Curran CP, Derkenne S, Miller ML, et al. Oral benzo[a]pyrene in Cyp1 knockout mouse lines: CYP1A1 important in detoxification, CYP1B1 metabolism required for immune damage independent of total-body burden and clearance rate. Mol Pharmacol. 2006;69:1103–14. doi: 10.1124/mol.105.021501. [DOI] [PubMed] [Google Scholar]
- 25.Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:23847–50. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- 26.Murray GI, Taylor MC, McFadyen MC, McKay JA, Greenlee WF, Burke MD, et al. Tumor-specific expression of cytochrome P450 CYP1B1. Cancer Res. 1997;57:3026–31. [PubMed] [Google Scholar]
- 27.Murray GI, Melvin WT, Greenlee WF, Burke MD. Regulation, function, and tissue-specific expression of cytochrome P450 CYP1B1. Annu Rev Pharmacol Toxicol. 2001;41:297–316. doi: 10.1146/annurev.pharmtox.41.1.297. [DOI] [PubMed] [Google Scholar]
- 28.Rieder CR, Ramsden DB, Williams AC. Cytochrome P450 1B1 mRNA in the human central nervous system. Mol Pathol. 1998;51:138–42. doi: 10.1136/mp.51.3.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutter TR, Tang YM, Hayes CL, Wo YY, Jabs EW, Li X, et al. Complete cDNA sequence of a human dioxin-inducible mRNA identifies a new gene subfamily of cytochrome P450 that maps to chromosome 2. J Biol Chem. 1994;269:13092–9. [PubMed] [Google Scholar]
- 30.Willey JC, Coy EL, Frampton MW, Torres A, Apostolakos MJ, Hoehn G, et al. Quantitative RT-PCR measurement of cytochromes p450 1A1, 1B1, and 2B7, microsomal epoxide hydrolase, and NADPH oxidoreductase expression in lung cells of smokers and nonsmokers. Am J Respir Cell Mol Biol. 1997;17:114–24. doi: 10.1165/ajrcmb.17.1.2783. [DOI] [PubMed] [Google Scholar]
- 31.Gattás GJ, de Carvalho MB, Siraque MS, Curioni OA, Kohler P, Eluf-Neto J, et al. Genetic polymorphisms of CYP1A1, CYP2E1, GSTM1, and GSTT1 associated with head and neck cancer. Head Neck. 2006;28:819–26. doi: 10.1002/hed.20410. [DOI] [PubMed] [Google Scholar]
- 32.Varela-Lema L, Taioli E, Ruano-Ravina A, Barros-Dios JM, Anantharaman D, Benhamou S, et al. Meta-analysis and pooled analysis of GSTM1 and CYP1A1 polymorphisms and oral and pharyngeal cancers: a HuGE-GSEC review. Genet Med. 2008;10:369–84. doi: 10.1097/GIM.0b013e3181770196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fontana RJ, Lown KS, Paine MF, Fortlage L, Santella RM, Felton JS, et al. Effects of a chargrilled meat diet on expression of CYP3A, CYP1A, and P-glycoprotein levels in healthy volunteers. Gastroenterology. 1999;117:89–98. doi: 10.1016/s0016-5085(99)70554-8. [DOI] [PubMed] [Google Scholar]
- 34.Goldman R, Shields PG. Food mutagens. J Nutr. 2003;133(Suppl 3):965S–73S. doi: 10.1093/jn/133.3.965S. [DOI] [PubMed] [Google Scholar]
- 35.Phillips DH. Polycyclic aromatic hydrocarbons in the diet. Mutat Res. 1999;443:139–47. doi: 10.1016/s1383-5742(99)00016-2. [DOI] [PubMed] [Google Scholar]
- 36.Ward MH, Sinha R, Heineman EF, Rothman N, Markin R, Weisenburger DD, et al. Risk of adenocarcinoma of the stomach and esophagus with meat cooking method and doneness preference. Int J Cancer. 1997;71:14–9. doi: 10.1002/(sici)1097-0215(19970328)71:1<14::aid-ijc4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 37.Kazerouni N, Sinha R, Hsu CH, Greenberg A, Rothman N. Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem Toxicol. 2001;39:423–36. doi: 10.1016/s0278-6915(00)00158-7. [DOI] [PubMed] [Google Scholar]