Abstract
The effects of early life experience on later brain structure and function have been studied extensively in animals, yet the relationship between childhood experience and normal brain development in humans remains largely unknown. Using a unique longitudinal data set including ecologically valid in-home measures of early experience during childhood (at age 4 and 8 years) and high-resolution structural brain imaging during adolescence (mean age 14 years), we examined the effects on later brain morphology of two dimensions of early experience: parental nurturance and environmental stimulation. Parental nurturance at age 4 predicts the volume of the left hippocampus in adolescence, with better nurturance associated with smaller hippocampal volume. In contrast, environmental stimulation did not correlate with hippocampal volume. Moreover, the association between hippocampal volume and parental nurturance disappears at age 8, supporting the existence of a sensitive developmental period for brain maturation. These findings indicate that variation in normal childhood experience is associated with differences in brain morphology, and hippocampal volume is specifically associated with early parental nurturance. Our results provide neuroimaging evidence supporting the important role of warm parental care during early childhood for brain maturation.
Keywords: Childhood experience, Parental nurturance, Environmental stimulation, Hippocampus, Morphology
Introduction
It is widely accepted that individual differences in cognition, emotion and personality result from a combination of genetic and experiential influences on brain development. Although experience may affect human brain structure and function throughout the entire life span (e.g., Maguire et al., 2000; 2003; Draganski et al., 2004), evidence from animal research suggests that early experience may be particularly critical. Rosenzweig et al. (1966; 1978) and Greenough et al. (1975; 1987) carried out some of the earliest systematic investigations exploring the effects of varying environmental conditions on brain development in rodents. This and subsequent work established that the variety and complexity of the cage environment influences many different aspects of brain structure and function, including the number of neurons, glial cells, dendrites and synapses, amounts of myelination, neurotransmitter and growth factor activity, and single cell electrophysiology, all of which can contribute to alterations of animal behavior (for a review, see Van Pragg et al., 2000).
Environmental characteristics responsible for brain changes in animals include a variety of toys which are changed on a regular basis to provide stimulation, novelty and opportunities for perceptual, cognitive, and motor development, as well as social interaction. Environmental variation, such as exploratory behaviour achieved by visiting other cages, is also effective in enhancing later brain structure and function (Tang et al., 2006). Although there was evidence suggesting that environmental enrichment and voluntary exercise may increase hippocampal neurogenesis via distinct mechanisms (Olson et al., 2006), attempts to isolate a single aspect of environmental enrichment that mediates structural and functional brain alterations have failed. For example, the effects of allowing visual access to complex environments without the opportunity for physical exploration (Ferchmin and Bennett, 1975), physical activity in a standard laboratory cage (Bernstein, 1973; Rosenzweig et al., 1978), or social interaction in a standard laboratory cage (Rosenzweig et al., 1978) are either partially or completely ineffective.
Whereas environmental stimulation seems to function as a complex whole in stimulating the development of widespread brain areas, early life stressful experiences may operate somewhat independently (for a review, see Van Pragg et al., 2000; Teicher et al., 2003). For example, the stress of prolonged maternal separation (i.e., hours per day) on young animals has been shown to exert lasting negative effects on the development of hypothalamic-pituitary-adrenal (HPA) responses and hippocampal glucocorticoid receptor levels (Liu et al., 1997; Meaney et al., 1989; Francis et al., 1999). In contrast, brief tactile stimulation, such as handling offspring for several minutes each day, which also separates the animal from its mother, appears beneficial (Liu et al., 2000). The effects of such early experience on later life stress regulation and memory ability may result from their impact on hippocampal development (Meaney et al., 1989; Liu et al., 1997; 2000; Francis et al., 1999; Duffy et al., 2001; Bredy et al, 2003; Olson et al., 2006). The salutary effect of brief separations may result from intensified maternal nurturing behaviour following the separation. The more a mother rat licks her pup following a brief stressor, the better regulated the pup’s later response to stressors and the better its subsequent learning ability (Liu et al., 2000; Bredy et al, 2003). Although the majority of research linking early life experience and later brain development has been conducted with rodents, similar effects of environmental stimulation and stress have also been observed in nonhuman primates (Kozorovitskiy et al., 2005; Parker et al., 2006).
In contrast to animal brain development, much less is known about the effect of childhood experience on the developing human brain. Although behavioral and brain imaging studies of early trauma or deprivation have demonstrated the association between extreme adverse experiences and impaired brain development (Kaler and Freeman, 1994; Chugani et al., 2001; Anderson et al., 2008; for a review, see Teicher et al., 2003; 2006), these findings address the etiology of psychopathology rather than maturational variation during normal brain development. For obvious ethical reasons, it is impossible to carry out prospective experiments in human populations using similar methods in animal studies. Therefore, a longitudinal study that relates childhood experience to later brain structure would provide an important new source of evidence on the effects of various childhood experiences on normal brain development.
The present study employed this research strategy, taking advantage of a unique longitudinal data set that included ecologically valid in-home measures of childhood experience and MRI measures of brain structure in adolescence. The participants consisted of a cohort of male and female children who have been longitudinally followed since their birth (Hurt et al., 1995). At age 4 and 8 years, childhood home environments of these participants were evaluated with the Home Observation for Measurement of the Environment (HOME) inventory (Caldwell et al., 1984). Two aspects of the child’s home life were measured, namely parental nurturance (PN), which reflects the warmth and availability of parental care, and environmental stimulation (ES), which reflects the availability of cognitively stimulating toys and activities, respectively. During adolescence (age 13–16 years), participants completed high-resolution structural brain imaging. The goal of the present study is to investigate the anatomical sequelae of variations in normal childhood experience. In view of the animal literature, we were particularly interested in the hippocampus with specific emphases on the following three questions: First, is variation in normal childhood experience, as opposed to trauma or severe deprivation, associated with measurable differences in brain structures, particularly the hippocampus? Second, are specific aspects of childhood experience associated with the development of specific brain regions? Specifically, is hippocampal morphology in the human brain affected by stress-buffering parental nurturance, as suggested by animal research literature? Third, does age of experience make a difference? Specifically, is early childhood experience more critical and influential than later experience for hippocampal development?
Materials and Methods
Study Participants
A total of 49 African American middle school-aged children (24 female) participated in the study. These participants had been recruited at birth for a study of the effects of gestational cocaine exposure (Hurt et al., 1995). Maternal use of cocaine as well as amphetamines, opiates, barbiturates, benzodiazepines, marijuana, alcohol and tobacco was ascertained by interview and medical record review at time of birth and, for all but the last three substances, maternal and infant urine specimens. Use of drugs other than tobacco, alcohol, marijuana and cocaine was an exclusionary criterion. The children were born at a single inner-city hospital, in which mothers receiving public assistance at or near term, with a mean gestational age of 38.6 weeks (SD = 2.2), a median 5-minute Apgar score of 9 (range from 6 to 10), normal cranial ultrasound and no asphyxiation. Mothers were native English speakers free of major psychiatric illness. None of the children had Fetal Alcohol Syndrome or any chromosomal disorder known to be associated with developmental delay. Since enrollment in the initial study, the children have been evaluated semi-annually for measurements of growth, development, language, cognition, and social-emotional outcomes (Hurt et al., 1996; 1997, Hurt et al., 2001b, a; 2001b, a; 2005). Assent was obtained from all participating children and informed consent was obtained from their parents or guardians. The project was conducted in accordance with the principles expressed in the Declaration of Helsinki and was approved by the Institutional Review Boards of the University of Pennsylvania and Children’s Hospital of Philadelphia.
Measures of Childhood Experiences
Children’s home environments were evaluated at age 4 years (4.1 ± 0.2) and 8 years (8.4 ± 0.5), using the Home Observation for Measurement of the Environment (HOME) Inventory (Caldwell and Bradley, 1984; Bradley, 1994). The HOME is a 1-hour structured interview and observational checklist that includes subscales measuring specific aspects of the child’s home life. Two composite scores, measuring environmental stimulation and parental nurturance, respectively, were created by averaging the z-scores of the relevant subscales listed below. Of all participants with the MRI scan, 41 children had the HOME measurements completed at age 4, 42 had the HOME measurements completed at age 8, and 36 had the HOME measurements completed at both ages.
The Parental Nurturance composite score incorporated subscales that measured the warmth and availability of parental care. For 4 year-olds, these subscales (with two sample items from each) included: Warmth and affection (e.g., “parent holds child close 10–15 minutes per day,” and “parent converses with child at least twice during visit”) and acceptance (e.g., “parent does not scold or derogate child more than once,” and “parent neither slaps nor spanks child during visit”). For 8 year-olds, the subscales included: Emotional and verbal responsivity (e.g., “Child has been praised at least twice during past week for doing something,” and “parent responds to child’s questions during interview”), encouragement of maturity (e.g., “family requires child to carry out certain self care routines,” and “parents set limits for child and generally enforce them”), emotional climate (e.g., “parent has not lost temper with child more than once during previous week,” and “parent uses some term of endearment or some diminutive for child’s name when talking about child at least twice during visit”) and paternal involvement (e.g., “Father [or father substitute] regularly engages in outdoor recreation with child,” and “Child eats at least one meal per day, on most days, with mother and father [or mother and father figure]”).
The Environmental Stimulation composite score incorporated subscales that were analogous to the experiential factors that vary with environmental enrichment in animal studies. For 4 year-olds, these subscales (with two sample items from each) included: Learning stimulation (e.g., “child has toys which teach color,” and “at least 10 books are visible in the apartment”), language stimulation (e.g., “child has toys that help teach the names of animals,” and “mother uses correct grammar and pronunciation,”), academic stimulation (e.g., “child is encouraged to learn colors,” and “child is encouraged to learn to read a few words”), modeling (e.g., “some delay of food gratification is expected,” and “parent introduces visitor to child”), and variety of experience (e.g., “child has real or toy musical instrument,” and “child’s art work is displayed some place in house”). For 8 year-olds, the relevant subscales were: Growth fostering materials and experiences (e.g., “child has free access to at least ten appropriate books,” and ”house has at least two pictures of other type of art work on the walls”), provision for active stimulation (e.g., “family has a television, and it is used judiciously, not left on continuously,” and “family member has taken child, or arranged for child to go to a scientific, historical or art museum within the past year”), family participation in developmentally stimulating experiences (e.g., “Family visits or receives visits from relatives or friends at least once every other week,” and “family member has taken child, or arranged for child to go, on a trip of more than 50 miles from his home”).
Imaging Data Acquisition
During adolescence at 13–16 years of age (mean age 14.2 ± 1.0 years), the participants were recruited back to complete the MRI scan. A Siemens 3.0T Trio whole-body scanner (Siemens AG, Erlangen, Germany) was used to acquire anatomic images, as well as the perfusion (data reported elsewhere, see Rao et al, 2007) and blood oxygen dependent level functional images (data reported elsewhere, see Hurt et al., 2008). High-resolution T1-weighted anatomic images were obtained in a 6 minute scan using 3D-MPRAGE sequence (TR = 1620 ms, TI = 950 ms, TE = 3 ms, flip angle = 15°, 160 contiguous slices of 1.0 mm thickness, in-plane resolution = 1 mm × 1 mm).
Imaging Data Analysis
The symmetric diffeomorphic image registration and unbiased atlas construction approach (Avants and Gee, 2004; Avants et al., 2006; 2007) was used to analyze structural images and quantify brain structural morphology. This approach is based on a mapping between a template or reference brain and each individual participant’s brain. An average shape and appearance template image was computed with symmetric normalization. This approach eliminates template selection bias because it is fully automated and outputs an aggregate image that equally weights each individual in the dataset. A validated, reliable image registration algorithm, which has been shown to perform similarly to an expert user on automated segmentation of the hippocampus, was then used to map the template to each individual participant’s brain. The volume of each individual brain structure, relative to the unbiased template averaged from symmetric normalization of all subjects’ structural images, was calculated and entered to the voxel-wise multiple regression analyses using general linear model (GLM).
Four separate whole brain GLM analyses, each using one of the four home composite scores as the regressor of interest, were conducted to search for brain regions predicted by early childhood parental nurturance, early environmental stimulation, later parental nurturance, and later environmental stimulation, respectively. All analyses included age of subjects at MR scanning, gender, and prenatal drug exposure as the nuisance covariates to account for the variance associated with these variables of no interest. Significance levels were set at P < 0.05, after whole brain false discovery rate (FDR) correction for multiple comparisons. Since we are primarily concerned with the hippocampus, small volume correction (SVC) was applied to hippocampal activation using an a priori defined hippocampus region of interest (ROI) obtained from an automated anatomical labeling ROI library (Tzourio-Mazoyer et al., 2002).
ROI Analysis
To further confirm the unique prediction of PN4 on hippocampal morphology, an independent region of interest analysis was also conducted. This analysis used a priori defined hippocampal structure which was obtained from an automated anatomical labeling ROI library (Tzourio-Mazoyer et al., 2002) and subsequently mapped to the unbiased structural template using symmetric diffeomorphy. For each subject, the relative volume of bilateral hippocampi (relative to the hippocampus in the unbiased template averaged from symmetric normalization of all subjects’ structural images), was calculated and correlated with each individual participant’s home composite scores.
The specific relationship between the composite score of PN4 and the outcome of hippocampal volume was further tested using multiple regression including all variables: the Environmental Stimulation composite scores at age 4 and age 8, the Parental Nurturance composite at age 4 and age 8, the child’s age at time of MR scanning, gender, total brain volume, and prenatal drug exposure status. Correlation analysis was also conducted on the left hippocampal volume and PN4 score for subjects whose mothers reported no use of alcohol during pregnancy in order to rule out potential confounding effects of prenatal alcohol exposure. In order to detect possible functional effects of the volumetric differences measured here, additional correlation analyses were conducted on the hippocampal volume, PN and ES scores, as well as a measure of memory ability. This memory measure was based on two incidental leaning tasks administered when the participants were of middle school age, as reported elsewhere (Farah et al., 2006). The memory score has been found to be significantly correlated with childhood parental nurturance (Farah et al., 2008).
Results
The four composite scores showed significant correlations between PN and ES scores at each age as well as ES scores at different ages (all p < 0.05, Table 1), supporting the stability of in-home measurements. However, no significant correlation was found between PN4 and PN8 scores (p > 0.1), which may be due in part to the different behaviors counted as representative of parental nurturance at the two ages. None of the four composite scores showed significant correlations with total brain volume (Table 1, all p > 0.1).
Table 1.
Correlations between parental nurturance (PN4, PN8) composite scores at the two ages, environmental stimulation (ES4, ES8) composite scores at the two ages, and total brain volume (TBV).
| PN4 | ES4 | PN8 | ES8 | TBV | |
|---|---|---|---|---|---|
| PN4 | -- | R = 0.51 p = 0.001** |
R = 0.07 p = 0.70 |
R = 0.04 p = 0.81 |
R = −0.25 p = 0.15 |
| ES4 | -- | R = 0.34 p = 0.04* |
R = 0.53 p = 0.001** |
R = −0.10 p = 0.52 |
|
| PN8 | -- | R = 0.45 p = 0.003** |
R = 0.04 p = 0.80 |
||
| ES8 | R = −0.08 p = 0.62 |
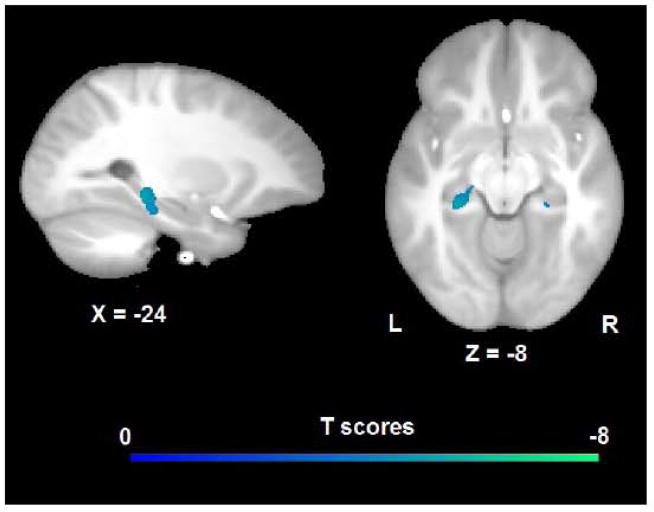
Results from the whole brain analyses demonstrated that the composite score of parental nurturance at the earlier age (PN4) inversely predicted the volume of the adolescent hippocampus, as shown in Fig. 1 and Table 2. This effect was observed primarily in the left hemisphere and in the right hemisphere with less spatial extent. PN4 also inversely predicted the morphology of right middle cingulate cortex and left thalamus (Table 2). In contrast, neither the composite score of parental nurturance at age 8 (PN8), nor the composite scores of environmental stimulation at age 4 (ES4) or 8 (ES8) show any prediction of hippocampal volume, even with a more liberal significance level (uncorrected p < 0.001). However, at this lower threshold, PN8 inversely predicted the volume of lateral orbitofrontal cortex, ES4 inversely predicted the volume of right fusiform cortex, ES8 inversely predicted the volume of left superior parietal cortex and positively predicted the volume of right premotor cortex (Table 3).
Figure 1.
Results from voxel-wise whole brain analysis showed that parental nurturance score at age 4 (PN4) inversely predicted left and right hippocampal volume. Activation maps were overlapped on the unbiased brain template generated from the anatomical images of all 47 participants. Blue color means negative correlation.
Table 2.
Areas showing significant associations with parental nurturance scores at age 4 (PN4) and 8 (PN8). Significance levels were set as whole brain false discovery corrected (FDR) p < 0.05 for age 4 and uncorrected p < 0.001 for age 8.
| Brain Regions | Coordinates* | Z Score | P Value | Cluster size (mm3) | |||
|---|---|---|---|---|---|---|---|
| X | Y | Z | uncorr. | SVC corr. | |||
| PN4, negative prediction | |||||||
| Left hippocampus | −25 | −33 | −6 | 4.44 | < 0.001 | 0.002 | 683 |
| Right hippocampus | 22 | −33 | −8 | 3.81 | < 0.001 | 0.02 | 9 |
| Right cingulate | 8 | −29 | 39 | 4.98 | < 0.001 | − | 1542 |
| Left thalamus | −13 | −22 | 16 | 4.47 | < 0.001 | - | 413 |
| PN4, positive prediction, no area survived FDR corrected p < 0.05 | |||||||
| PN8, negative prediction | |||||||
| Right lateral orbitofrontal | 32 | 38 | −9 | 3.91 | < 0.001 | - | 1146 |
| Left lateral orbitofrontal | −25 | 28 | −12 | 3.43 | < 0.001 | − | 166 |
| PN8, positive prediction, no area survived uncorrected p < 0.001 | |||||||
Note the coordinates given in all tables are based on our brain template from symmetric diffeomorphy and only approximately refer to the Talairach and Tournoux template.
uncorr: uncorrected p value; SVC corr: small volume corrected p value.
Table 3.
Areas showing significant associations with environmental stimulation scores at age 4 (ES4) and 8 (ES8). Significance level was set as uncorrected p < 0.001.
| Brain Regions | Coordinates* | Z Score | P Value | Cluster size (mm3) | |||
|---|---|---|---|---|---|---|---|
| X | Y | Z | uncorr. | SVC corr. | |||
| ES4, negative prediction | |||||||
| Right fusiform | 37 | −9 | −34 | 3.87 | < 0.001 | - | 148 |
| ES4, positive prediction, no area survived uncorrected p < 0.001 | |||||||
| ES8, negative prediction | |||||||
| Left superior parietal | −6 | −57 | 63 | 3.59 | < 0.001 | - | 372 |
| ES8, positive prediction | |||||||
| Right premotor | 39 | −1 | 39 | 3.39 | < 0.001 | - | 127 |
Note the coordinates given in all tables are based on our brain template from symmetric diffeomorphy and only approximately refer to the Talairach and Tournoux template.
uncorr: uncorrected p value; SVC corr: small volume corrected p value.
To address the question of whether early parental nurturance (PN4) was substantially more predictive of left hippocampal volume than the other three measures of childhood experience, we conducted a voxel-vise whole brain analysis using multiple regression with all four measures of childhood experience as potential predictors. The association of PN4 and left hippocampal volume remained significant (Table 4) after including all childhood experience scores in the multiple regression analysis.
Table 4.
Areas showing significant inverse associations with parental nurturance at age 4 (PN4) from the multiple regression analysis of 36 subjects, including all childhood experience scores and age, gender, prenatal cocaine exposure as the covariates. Significance level was set as uncorrected p < 0.001.
| Brain Regions | Coordinates* | Z Score | P Value | Cluster size (mm3) | |||
|---|---|---|---|---|---|---|---|
| X | Y | Z | uncorr. | SVC corr. | |||
| PN4, negative prediction | |||||||
| Left hippocampus | −19 | −24 | −8 | 3.53 | < 0.001 | 0.04 | 782 |
| Left thalamus | −13 | −22 | 14 | 3.56 | < 0.001 | - | 342 |
| PN4, positive prediction | |||||||
| Left precuneus | −12 | −57 | 36 | 3.90 | < 0.001 | - | 1011 |
| Left cingulate | −6 | −36 | 44 | 3.37 | < 0.001 | - | 211 |
| Left angular/supramarginal | −42 | −45 | 30 | 3.34 | < 0.001 | - | 162 |
Note the coordinates given in all tables are based on our brain template from symmetric diffeomorphy and only approximately refer to the Talairach and Tournoux template.
uncorr: uncorrected p value; SVC corr: small volume corrected p value.
Results from the independent region of interest (ROI) analysis further confirmed the unique role of early childhood parental nurturance on hippocampal volume. A significant inverse association was found between PN4 score and left hippocampal volume (r = −0.48, p = 0.001, Fig. 2), while no such significant relationship emerged for PN8 and ES scores respectively (PN8, r = −0.01, p = 0.94; ES4, r = −0.25, p = 0.1; ES8, r = −0.004, p = 0.98, Fig. 2). The strength of the inverse prediction for PN4 is significantly stronger than that for PN8 and ES8 (both p < 0.02). Removing the outliers in the data did not change the significance of these results. By including all childhood experience scores at age 4 and age 8, as well as the age of subjects at the time of scanning, gender, total brain volume, and prenatal drug exposure in a multiple regression model, only PN4 was significantly associated with left hippocampal volume (Table 5). PN4 explained about 1/4 (25%) of the total variance in the structural volume of the adolescent left hippocampus. However, prenatal cocaine exposure did not influence the hippocampal volume, which is consistent with the results from voxel-wise comparisons between exposed and non-exposed subjects (Avants et al., 2007).
Figure 2.
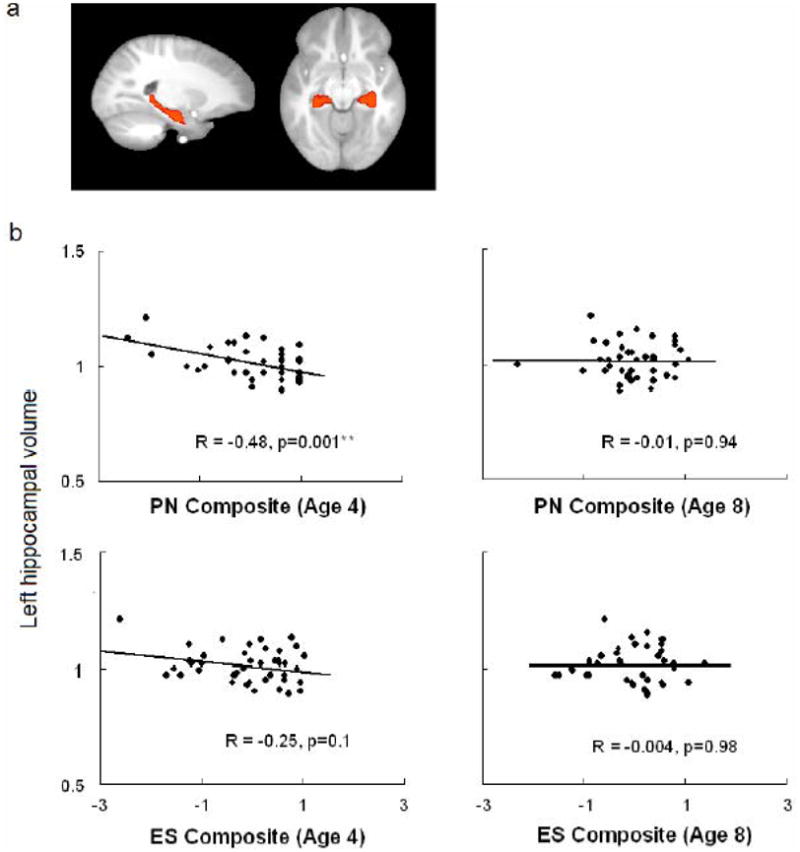
Results from independent region of interest (ROI) analysis showed that only parental nurturance score at age 4 (PN4), but not parental nurturance score at age 8 (PN8), nor environmental stimulation scores (ES4 and ES8), inversely predicted the left hippocampal morphology. a) A priori defined hippocampus mapped to and overlapped on the unbiased brain template, b) Correlations between left hippocampal volume and childhood experience scores.
Table 5.
Multiple regression analysis on the region of interest data showed unique relationship between parental nurturance at age 4 and left hippocampal volume
| PN4 | ES4 | PN8 | ES8 | Age | Gender | TBV | Drug exposure | |
|---|---|---|---|---|---|---|---|---|
| Beta | −0.51 | 0.09 | −0.004 | 0.04 | 0.06 | −0.11 | 0.23 | 0.10 |
| p | 0.018* | 0.75 | 0.98 | 0.87 | 0.76 | 0.59 | 0.28 | 0.59 |
p < 0.05.
Given the negative effect of prenatal alcohol exposure on hippocampal structure and function (Sakata-Haga et al., 2003; Relila et al., 2006), we carried out an additional correlation analysis for the 31 subjects whose mothers reported no use of alcohol during pregnancy. This analysis confirmed the significant negative association between PN4 and left hippocampal volume (r = −0.47, p = 0.009). Finally, in light of the previously mentioned finding of a selective relation between parental nurturance and memory development in a larger sample of children (Farah et al., 2008), we also carried out a correlation relating our sample’s PN and ES scores, hippocampal volumes, and memory function. This analysis showed no significant correlation between hippocampal volume and memory ability (all p > 0.1), however, it did show a significant correlation between PN8 and memory scores (r = 0.36, p = 0.02).
Discussion
The significant effect of childhood parental nurturance on hippocampal morphology is consistent with animal literature suggesting that early parenting experience affects later hippocampal development (Meaney et al., 1989; Francis et al., 1999; Liu et al., 1997; 2000; Bredy et al, 2003; Olson et al., 2006). However, the direction of the relationship, namely more nurturance associated with smaller hippocampi is, at least ostensibly, contrary to findings of enhanced neuronal survival in animals. However, this negative correlation may be less surprising in the context of pediatric literature. Although human studies of Post Traumatic Stress Disorder (PTSD) have generally documented smaller hippocampal volumes in adults experiencing pathologically high levels of stress (Bremner et al., 1997; Stein, 1997), pediatric PTSD studies show either equivalent or larger hippocampal volumes in children with PTSD compared with control subjects (De Bellis et al, 1999; Carrion et al., 2001; Tupler and DeBellis, 2006). These findings are consistent with the negative relationship between hippocampal volume and the quality of parental care found in the present healthy sample.
Additional context for understanding the inverse relationship between early parental nurturance and hippocampal volume is provided by a recent MRI study (Gogtay et al., 2006) on hippocampal development in normal human brain. This longitudinal study demonstrated a complex pattern of expansion and contraction of different subregions of the hippocampus, many of which follow inverse U-shaped developmental curves. Specifically, in the left middle hippocampus, the region where we found that early childhood parental nurturance negative correlated with adolescent hippocampal volume, the developmental curve (see region C in the left middle hippocampus at Figure 3 in Gogtay et al., 2006) clearly showed that volume in this subregion increased from early childhood at age 4 to a peak during adolescence at about age 12, and subsequently decreased from adolescence to adulthood. The age range of adolescent participants in the present study fell in the decreasing part of curve. Moreover, meta-analysis of hippocampal morphology (Van Petten, 2004) in developing brains has shown little change of absolute hippocampal volume between the ages of 4 and 18 in the context of continuous expansion of whole brain volume. In light of this evidence, the negative association between early childhood parental nurturance on adolescent hippocampal volume is at least consistent with accelerated hippocampal maturation for adolescents with a high level of parental nurturance or conversely a delay in hippocampal maturation for children with lower levels of parental nurturance.
Animal studies on environmental enrichment and maternal care both support the notion of hippocampal changes associated with early experience. However, the lack of relationship between environmental stimulation and hippocampal volume is consistent with prior animal studies showing that hippocampal neurogenesis is not necessary related to the behavioral effects of environmental enrichment (Meshi et al., 2006). It is also possible that such effects are simply weaker than the effects of nurturance, or depend on aspects of environmental stimulation not well measured by the HOME or not sufficiently variable in our sample. Nevertheless, the unique predictive value of parental nurturance at age 4 in the present study suggests a more important role for warm parental care over cognitive stimulation for normal hippocampal development, especially during early childhood years. Particularly, our findings that the hippocampal volume is modulated by parental nurturance at age 4 but not age 8 are concordant with a recent study showing that the hippocampus was maximally sensitive to the effects of childhood sexual abuse occurring at age 3–5 years, but not at 6–8 or 9–10 years (Anderson et al., 2008). These results provide converging evidence supporting the hypothesis that brain structures susceptible to stress may have unique periods during which they are maximally sensitive to early stress during brain development (Teicher et al. 2006).
A limitation of the present study was that the children in our study sample were all from African American families of low socioeconomic status (SES). SES is associated with many aspects of childhood experiences. Lower SES is associated with less environmental stimulation in the forms of toys, books, and recreational opportunities, as well as less maternal care from the family as a whole and less supportive parenting practice (Bradley et al., 2001). Individuals with lower SES may be exposed to more stressful events in their lives (Dohrenwend, 1973), have more health problems (Anderson and Armstead, 1995), and differ in some aspects of neurocognitive development and function (Hackman & Farah, 2009). Therefore, we cannot be certain whether the results obtained with this sample would generalize to children of different ethnicity or socioeconomic background. However, with over 17% of American children living below the poverty line according to the 2004 census, low SES is not abnormal or atypical in our society. Our results demonstrate that even within the low level of SES, the quality of parental nurturance at early childhood can alter hippocampal volume in adolescence. This finding is consistent with a recent report (Buss et al., 2007) showing that maternal care modulates the relationship between prenatal risk and hippocampal volume in adults, with birth weight predicting hippocampal volume in adulthood only in female subjects with low levels of maternal nurturance. Future studies will be needed to further examine whether the same effects of parental nurturance on hippocampal volume can be observed in children from families of middle to higher SES.
Without manipulating or controlling for genetic relatedness, for example by studying adoptees, we cannot be certain that the relationships reported here between childhood experience and later brain morphology exactly reflect the effects of experience on brain development. Alternative patterns of causality could also be present. For example, children who have smaller hippocampi might also tend to receive higher levels of nurturance from their parents, or parents who innately provide greater nurturance might also tend to have genes that predispose their children to smaller hippocampi. Further research will be needed to provide more strict control to further clarify these alternatives. However, at present, although neither of the alternative scenarios can be ruled out, neither has any independent support. In contrast, animal research has shown that early experience actually exerts an effect on hippocampal development (Meaney et al., 1989; Liu et al., 1997; 2000; Francis et al., 1999; Duffy et al., 2001; Bredy et al, 2003; Olson et al., 2006), which supports the present conclusion.
In conclusion, three aspects of the present results deserve emphasis. First, as expected from the animal literature but never before demonstrated in humans, variations in childhood experience of healthy human bears a significant relationship to brain structure. Second, the effect of childhood experience may be highly selective, with parental nurturance but not environmental stimulation being related to hippocampal morphology. Third, the timing of this relationship between childhood experience and hippocampal structure is consistent with the existence of a sensitive developmental period, with only the earlier measure of parental nurturance at age 4 predicting adolescent hippocampal volume. The present findings thus provide an important bridge between the study of normal neurocognitive development and early experience in humans, which has only been validated in animal models until this time.
Acknowledgments
This research was supported by US NSF Grant BCS-0517935, NIH Grants P30 NS045839, R01 HD043078, HD055689, DA015149, DA014129 and Sun Yat-Sen University 985 project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson NB, Armstead CA. Toward understanding the association of socioeconomic status and health: a new challenge for the biopsychosocial approach. Psychosomatic Med. 1995;57:213–225. doi: 10.1097/00006842-199505000-00003. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20:292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Gee JC. Geodesic estimation for large deformation anatomical shape averaging and interpolation. NeuroImage. 2004;23(Suppl 1):S139–150. doi: 10.1016/j.neuroimage.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Avants BB, Hurt H, Giannetta J, Epstein CL, Shera DM, Rao H, Wang J, Gee JC. Effects of heavy in utero cocaine exposure on adolescent caudate morphology. Pediatr Neurol. 2007;37:275–279. doi: 10.1016/j.pediatrneurol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Avants BB, Schoenemann PT, Gee JC. Lagrangian frame diffeomorphic image registration: Morphometric comparison of human and chimpanzee cortex. Med Image Anal. 2006;10:397–412. doi: 10.1016/j.media.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Bernstein L. A study of some enriching variables in a free-environment for rats. J Psychosom Res. 1973;17:85–88. doi: 10.1016/0022-3999(73)90008-1. [DOI] [PubMed] [Google Scholar]
- Bradley RH. The HOME Inventory: review and reflections. In: Reese HW, editor. Advances in child development and behavior. Vol. 25. San Diego, CA: Academic Press; 1994. pp. 241–288. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Convyn RF, Burchinal M, McAdoo HP, Coll CG. The home environments of children in the United States part II: relations with behavioral development through age thirteen. Child Dev. 2001;72:1868–1886. doi: 10.1111/1467-8624.t01-1-00383. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse--a preliminary report. Biol Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy TW, Grant RJ, Champagne DL, Meaney MJ. Maternal care influences neuronal survival in the hippocampus of the rat. Eur J Neurosci. 2003;18:2903–2909. doi: 10.1111/j.1460-9568.2003.02965.x. [DOI] [PubMed] [Google Scholar]
- Buss C, Lord C, Wadiwalla M, Hellhammer DH, Lupien SJ, Meaney MJ, Pruessner JC. Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. J Neurosci. 2007;27:2592–2595. doi: 10.1523/JNEUROSCI.3252-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH. Home Observation for Measurement of the Environment (HOME) Little Rock, AR: University of Arkansas at Little Rock; 1984. [Google Scholar]
- Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, Reiss AL. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 2001;50:943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. NeuroImage. 2001;14:1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, Ryan ND. Developmental traumatology, part II Brain development. Biol Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- Dohrenwend BP. Social status and stressful life events. J Pers Soc Psychol. 1973;28:225–235. doi: 10.1037/h0035718. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Duffy SN, Craddock KJ, Abel T, Nguyen PV. Environmental enrichment modifies the PKA-dependence of hippocampal LTP and improves hippocampus-dependent memory. Learn Mem. 2001;8:26–34. doi: 10.1101/lm.36301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ, Shera DM, Savage JH, Betancourt L, Giannetta JM, Brodsky NL, Malmud EK, Hurt H. Childhood poverty: specific associations with neurocognitive development. Brain Res. 2006;1110:166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Betancourt L, Shera DM, Savage JH, Giannetta JM, Brodsky NL, Malmud EK, Hurt H. Environmental stimulation, parental nurturance and cognitive development in humans. Dev Sci. 2008;11:793–801. doi: 10.1111/j.1467-7687.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- Ferchmin PA, Bennett EL. Direct contact with enriched environment is required to alter cerebral weights in rats. J Comp Physiol Psychol. 1975;88:360–367. doi: 10.1037/h0076175. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Nugent TF, 3rd, Herman DH, Ordonez A, Greenstein D, Hayashi KM, Clasen L, Toga AW, Giedd JN, Rapoport JL, Thompson PM. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Greenough WT. Experiential modification of the developing brain. Am Sci. 1975;63:37–46. [PubMed] [Google Scholar]
- Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Dev. 1987;58:539–559. [PubMed] [Google Scholar]
- Hackman D, Farah MJ. Socioeconomic status and brain development. Trends Cogn Sci. 2009 doi: 10.1016/j.tics.2008.11.003. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt H, Brodsky NL, Betancourt L, Braitman LE, Malmud E, Giannetta J. Cocaine-exposed children: follow-up through 30 months. J Dev Behav Pediatr. 1995;16:29–35. [PubMed] [Google Scholar]
- Hurt H, Brodsky NL, Betancourt L, Braitman LE, Belsky J, Giannetta J. Play behavior in toddlers with in utero cocaine exposure: a prospective, masked, controlled study. J Dev Behav Pediatr. 1996;17:373–379. doi: 10.1097/00004703-199612000-00001. [DOI] [PubMed] [Google Scholar]
- Hurt H, Brodsky NL, Roth H, Malmud E, Giannetta JM. School performance of children with gestational cocaine exposure. Neurotoxicol Teratol. 2005;27:203–211. doi: 10.1016/j.ntt.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Hurt H, Giannetta J, Brodsky NL, Malmud E, Pelham T. Are there neurologic correlates of in utero cocaine exposure at age 6 years? J Pediatr. 2001b;138:911–913. doi: 10.1067/mpd.2001.113709. [DOI] [PubMed] [Google Scholar]
- Hurt H, Giannetta JM, Korczykowski M, Hoang A, Tang KZ, Betancourt L, Brodsky NL, Shera DM, Farah MJ, Detre JA. Functional magnetic resonance imaging and working memory in adolescents with gestational cocaine exposure. J Pediatr. 2008;152:371–377. doi: 10.1016/j.jpeds.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Hurt H, Malmud E, Betancourt L, Braitman LE, Brodsky NL, Giannetta J. Children with in utero cocaine exposure do not differ from control subjects on intelligence testing. Arch Pediatr Adolesc Med. 1997;151:1237–1241. doi: 10.1001/archpedi.1997.02170490063011. [DOI] [PubMed] [Google Scholar]
- Hurt H, Malmud E, Betancourt L, Brodsky NL, Giannetta JM. A prospective comparison of developmental outcome of children with in utero cocaine exposure and controls using the Battelle Developmental Inventory. J Dev Behav Pediatr. 2001a;22:27–34. doi: 10.1097/00004703-200102000-00005. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Kaler SR, Freeman BJ. Analysis of environmental deprivation: cognitive and social development in Romanian orphans. J Child Psychol Psychiatry. 1994;35:769–781. doi: 10.1111/j.1469-7610.1994.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Gross CG, Kopil C, Battaglia L, McBreen M, Stranahan AM, Gould E. Experience induces structural and biochemical changes in the adult primate brain. Proc Natl Acad Sci USA. 2005;102:17478–17482. doi: 10.1073/pnas.0508817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Spiers HJ, Good CD, Hartley T, Frackowiak RS, Burgess N. Navigation expertise and the human hippocampus: a structural brain imaging analysis. Hippocampus. 2003;13:250–259. doi: 10.1002/hipo.10087. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Viau V, Sharma S, Sarrieau A. Neonatal handling alters adrenocortical negative feedback sensitivity and hippocampal type II glucocorticoid receptor binding in the rat. Neuroendocrinology. 1989;50:597–604. doi: 10.1159/000125287. [DOI] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–731. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc Natl Acad Sci USA. 2006;103:3000–3005. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H, Wang J, Korczykowski M, Giannetta J, Shera D, Avants B, Gee J, Detre JA, Hurt H. Altered resting cerebral blood flow in adolescents with in-utero cocaine exposure revealed by perfusion functional MRI. Pediatrics. 2007;120:e1245–1254. doi: 10.1542/peds.2006-2596. [DOI] [PubMed] [Google Scholar]
- Redila VA, Olson AK, Swann SE, Mohades G, Webber AJ, Weinberg J, Christie BR. Hippocampal cell proliferation is reduced following prenatal ethanol exposure but can be rescued with voluntary exercise. Hippocampus. 2006;16:305–311. doi: 10.1002/hipo.20164. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR. Environmental complexity, cerebral change, and behavior. Am Psychol. 1966;21:321–332. doi: 10.1037/h0023555. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL, Hebert M, Morimoto H. Social grouping cannot account for cerebral effects of enriched environments. Brain Res. 1978;153:563–76. doi: 10.1016/0006-8993(78)90340-2. [DOI] [PubMed] [Google Scholar]
- Sakata-Haga H, Sawada K, Ohta K, Cui C, Hisano S, Fukui Y. Adverse effects of maternal ethanol consumption on development of dorsal hippocampus in rat offspring. Acta Neuropathol. 2003;105:30–36. doi: 10.1007/s00401-002-0606-9. [DOI] [PubMed] [Google Scholar]
- Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- Tang AC, Akers KG, Reeb BC, Romeo RD, McEwen BS. Programming social, cognitive, and neuroendocrine development by early exposure to novelty. Proc Natl Acad Sci USA. 2006;103:15716–15721. doi: 10.1073/pnas.0607374103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Tomoda A, Andersen SL. Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Ann N Y Acad Sci. 2006;1071:313–323. doi: 10.1196/annals.1364.024. [DOI] [PubMed] [Google Scholar]
- Tupler LA, De Bellis MD. Segmented hippocampal volume in children and adolescents with posttraumatic stress disorder. Biol Psychiatry. 2006;59:523–529. doi: 10.1016/j.biopsych.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]