Abstract
Background
TGF-β family cytokines have diverse actions in the maintenance of cardiac homeostasis. Activin A is a member of this family whose regulation and function in heart is not well understood at a molecular level. Follistatin-like 3 (Fstl3) is an extracellular regulator of Activin A protein, and its function in the heart is also unknown.
Methods and Results
We analyzed the expression of various TGF-β superfamily cytokines and their binding partners in mouse heart. Activin βA and Follistatin-like 3 (Fstl3) were upregulated in models of myocardial injury. Overexpression of Activin A with an adenoviral vector (Ad-actβA) or treatment with recombinant Activin A protein protected cultured myocytes from hypoxia/reoxygenation- induced apoptosis. Systemic overexpression of Activin A in mice, by intravenous injection of Ad-actβA, protected hearts from ischemia/reperfusion injury. Activin A induced the expression of Bcl-2, and ablation of Bcl-2 by siRNA abrogated its protective action in myocytes. The protective effect of Activin A on cultured myocytes was abolished by treatment with Fstl3 or by a pharmacological Activin receptor-Like Kinase (ALK) inhibitor. Cardiac specific Fstl3 knock-out mice showed significantly smaller infarcts after ischemia/reperfusion injury that was accompanied by reduced apoptosis.
Conclusions
Activin A and Fstl3 are induced in heart by myocardial stress. Activin A protects myocytes from death and this activity is antagonized by Fstl3. Thus, the relative expression levels of these factors following injury is a determinant of cell survival in the heart.
Keywords: myocytes, apoptosis, reperfusion, Activin A, Follistatin-like 3
Introduction
The transforming growth factor-beta (TGF-β) family comprises a large number of multifunctional proteins that can be divided into subfamilies including Activins, Bone Morphogenic Proteins (BMPs), Growth and Differentiation Factors (GDFs), and TGF-βs. These secreted proteins have diverse roles in cell proliferation, differentiation, apoptosis, and immune responses1. TGF-β1, the founding member of TGF-β superfamily, is a mediator of cardiac hypertrophy and remodeling2,3. It has also been reported that BMP-24,5, GDF-156,7 and myostatin (GDF-8)8 influence the growth and survival of cardiac myocytes. However, the majority of TGF-β superfamily members have not been examined for their potential cardiac-regulatory functions.
The Follistatin family proteins function as extracellular antagonists of TGF-β superfamily cytokines. Follistatin and Follistatin-like 3 (Fstl3) directly bind to TGF-β superfamily cytokines to inhibit their biological activities1. Recently, Lara-Pezzi et al. reported that Fstl3 transcript expression is upregulated in end-stage failing myocardium and its expression is correlated with molecular markers of disease severity9. They also reported that transcripts encoding Follistatin-like 1 (Fstl1), a distant member of the Follistatin family, is upregulated in heart failure and its expression positively correlated with better functional recovery following implantation of a left ventricular assist device. We have shown that Fstl1 is secreted from cardiac myocytes following injury in animal models, and that it functions to promote cardiac myocyte survival10.
To better understand the regulation of secreted factors from the heart, we performed gene array transcriptome analyses on murine hearts that were subjected to injury and other stimuli11,12. These analyses revealed that members of the Follistatin family of secreted factors were upregulated upon injury or Akt transgene activation10, leading us to hypothesize that there might exist as-yet-unknown networks of autocrine/paracrine factors that control heart function. Here we report that cardiac injuries induce the expression of Activin A and its binding partner Fstl3. Activin A was found to protect cardiac myocytes from stress-induced cell death, whereas Fstl3 abolished the pro-survival effect of Activin A. We propose that Activin A and Fstl3 serve as sensors of cardiac stress and that their relative levels of expression influence cell survival in the injured heart.
Methods
See the online-only Data Supplement for additional details.
Myocyte cultures of neonatal rat ventricular myocytes
Primary culture of neonatal rat ventricular myocytes (NRVMs) were incubated in DMEM supplemented with 7% Fetal Calf Serum (FCS) for 18 to 24 hours after preparation, then with adenoviral vectors at the indicated multiplicity of infection (MOI) for 16 hours in DMEM. The media were then replaced with fresh DMEM without adenovirus and incubated for 12 hours prior to hypoxia-reoxygenation (H/R). In other experiments, serum-deprived NRVMs were incubated with recombinant Activin A protein for 8 hours prior to hypoxia-reoxygenation. A GasPak system (Becton Dickinson) was used to create hypoxic conditions as described previously13. For hypoxia/reoxygenation (H/R) studies, cells were exposed 12 hr hypoxia followed by reoxygenation.
Construction of adenoviral vectors expressing murine Fstl3 and murine Activin βA
Full-length Fstl3 and Activin βA cDNAs were obtained from American Type Culture Collection (ATCC). Enzymatic restriction sites were added by PCR on both N- and C-terminus and the full-length of Fstl3 and Activin βA and the cDNAs were subcloned into an adenovirus shuttle vector. After linearization, the shuttle vectors were co-transformed into competent cells (TOP10; Invitrogen) with the adenoviral backbone plasmid (pAdEasy-1). The recombinant adenoviral DNA with Fstl3 or Activin βA cDNA were extracted from the competent cells and transfected into HEK 293 cells to produce recombinant adenoviral vectors that express Fstl3 (Ad-Fstl3) or Activin βA (Ad-actβA). An adenoviral vector expressing β-galactosidase (Ad-βgal) was used as a control. The adenoviral vectors were purified by the CsCl ultracentrifugation method.
Adenovirus-mediated overexpression of Activin A in mice
Eight to 10 week old male mice were intravenously injected with adenovirus (Ad-actβA or Ad-βgal, 5.0 × 109 plaque forming units/mouse) through the jugular vein. Plasma Activin A was assayed by western blot analysis three days after adenovirus delivery. At this time point mice also underwent myocardial ischemia-reperfusion injury.
Generation of a cardiac specific Fstl-3 knock-out mice
Mice homozygous for an Fstl3 allele with two loxP sites flanking exons 3 through 5 (Fstl3flox/flox) were backcrossed and maintained on the C57BL6/J background. Fstl3flox/flox were crossed with α-myosin heavy chain (α-MHC)-Cre transgenic mice that are maintained on C57BL6/J background. Four different primer pairs were used for genotyping PCR. The loxP site in intron 2 was detected by using Primer1: SJL954 TCTGAGAAGAGGAGGGATTTCAAG and Primer 2: SJL955 ATTTACACCTAGCCACATACTCTG which amplify a ~390-bp fragment for loxP site, while the Fstl3 wild-type allele gives a 330-bp fragment. The loxP site in intron 5 was detected by using Primer 3: SJL956 AACCACATCCCAGATCCAGGTCAC and Primer 4: SJL986 CAGCTATGTAGGCTTTGCATTGCTC, which amplify an approximate 310-bp fragment for loxP site and a 270-bp fragment for wild-type allele. Recombination by Cre leads to an allele that lacks exons 3, 4 and 5 of Fstl3 gene is detected by using primer pair of 1 and 4 that gives a 357-bp fragment. The α-MHC-Cre transgene is detected by using the primer pair of 5’-ATGACAGACAGATCCCTCCTATCTCC and 5’-CTCATCACTCGTTGCATCATCGAC, that amplifies a 300-bp fragment.
Statistical analysis
Data are presented as mean ± SEM. Group differences were analyzed by two-tailed Student’s t test or ANOVA. To compare multiple groups, Mann-Whitney U-test with Bonferroni correction was used. A value of P < 0.05 was considered statistically significant.
The authors had full access to the data and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Activin βA and Fstl3 levels are regulated by stress in the heart
To better understand the roles of the TGF-β superfamily cytokines in heart, we analyzed transcript expression of family members by QRT-PCR using cDNAs from mouse heart (Figure 1A). These analyses focused on Activin βA, its inhibitory binding partners, Follistatin and Fstl3, and Inhibin α. Activin βA showed marked upregulation at 1 and 3 days following LCA ligation in the infarct zone, and returned to baseline at the 6 day time point. These findings are in general agreement with that of Yndestad et al. 14, who previously reported a 15- to 40-fold induction of Activin βA in the ischemia regions of heart following LCA ligation in rats. Fstl3 displayed statistically significant upregulation at days 1, 3 and 6 in the infarct and remote regions following LCA ligation. Follistatin upregulation was observed in the infarct zone at the 3 and 6 day time points. No regulation of Inhibinα, which opposes the action of Activin A, was observed in this model.
Figure 1.
Fstl3 and Activin βA are specifically induced after cardiac injury. Expression analysis of Activin βA, Fstl3, Follistatin, and Inhibinα in murine models of myocardial infarction (MI) (A), transverse aortic constriction (TAC) (B) and ischemia/reperfusion (I/R) (C). In the MI model, samples were taken separately from ischemic zone (infarct area) and non-ischemic zone (remote area) three days after the onset of MI. For the pressure overload model, samples were taken 7 days after TAC surgery. QRT-PCR was performed to determine the mRNA level of each transcript and the data were compared to the GAPDH level and normalized to the mean value of controls. n=4−6. *P<0.05 vs. sham and #P<0.01 vs. sham. (D) Upregulation of Activin A protein following myocardial infarction. The upper panel is a western blot analysis for Activin A performed under non-reducing condition and the lower panel is a blot for α-tubulin using the same samples under reducing conditions. The histogram shows the quantification of the band intensities for Activin A compared to that of tubulin. #P<0.01 vs. sham. (E) Upregulation of Activin A and Fstl3 proteins after H/R treatment in NRVM cultures. Representative images of immunoblots of the culture media, 24 hrs after addition to cells, and the cell pellet lysates.
Activin βA and Fstl3 were upregulated 10- and 3-fold, respectively after pressure overload at 1 week following transverse aortic constriction (Figure 1B), whereas Follistatin transcript level did not change and Inhibin α transcript level declined by a factor of 2 (Figure 1B). In an ischemia/reperfusion (I/R) model, Fstl3 expression was upregulated 4-fold at 12 and 24 hour time points post-perfusion, whereas levels of Activin βA increased 2-fold at the 12 hour time point (Figure 1C). Levels of Follistatin and Inhibin αdid not change in these assays.
Dimers of Activin βA are processed to give rise to the physiologically-active protein Activin A. Activin A levels were measured in hearts 3 days following LCA ligation because the Activin βA transcript was robustly expressed at this time point. A significant increase in Activin A protein could be detected in hearts following infarction (Figure 1D). To document Activin A and Fstl3 expression by cardiac myocytes, NRVMs were cultured under normoxic and hypoxia/reoxygenation conditions (Figure 1E). Both proteins could be detected in lysates of the cell pellets and in the conditioned media. Treatment of cultures by hypoxia/reoxygenation led to a 1.9-fold upregulation of Activin A and a 1.7-fold upregulation of Fstl3 in the culture media (P<0.05, n=6).
Activin A protects cultured myocytes from apoptosis
In non-cardiac cell type Activin A has been reported to promote survival15–17 or apoptosis18,19. Thus far, the effects of Activin A on cardiac myocyte survival has not been reported. To elucidate the functional significance of Activin A in cardiac myocytes, serum-deprived NRVMs were exposed to H/R stress in the presence or absence of recombinant human Activin A protein and analyzed for markers of apoptotic cell death. As shown in Figure 2A, recombinant Activin A protein promoted survival in NRVMs as assessed by an MTS assay. Statistically significant protection against apoptosis was observed when Activin A was incubated with NRVMs at a dose of 25 ng/ml. This level of Activin A is similar to doses that exert anti-apoptotic actions on other cell types20. To corroborate these findings, a nucleosome fragmentation assay of NRVM apoptosis was performed. Treatment with 25 ng/ml Activin A reduced hypoxia/reoxygenation(H/R)-induced apoptosis by 62% (Figure 2B). Furthermore, Caspase 3/7 activity was increased by the H/R stress and treatment with Activin A protein (25 ng/ml) reduced this activity to near baseline levels (Figure 2C).
Figure 2.
Activin A protects cardiac myocytes from H/R induced injury. (A) NRVMs were pretreated with different concentrations of Activin A for 8 hours before the exposure to 12 hours of hypoxia followed by 24 hours of reoxygenation (H/R). Cell viability was determined by the MTS assay. Apoptosis indicated by nucleosome fragmentation assay (B) and caspase-3 and -7 activities (C) were measured in NRVMs pretreated with 25ng/mL of Activin A before exposure to H/R. (D) Thirty minutes before addition of Activin A, NRVMs were pretreated with or without the inhibitor SB431542. Cell viability was measured by MTS assay after H/R treatment. *P<0.05 and #P<0.01.
Activin A signals through Activin receptor-Like Kinases (ALKs)1. Thus NRVMs exposed to hypoxia/reoxygenation stress, were incubated with SB431542, a specific inhibitor of ALK4, 5 and 7, prior to treatment with recombinant Activin A. Cell viability was assessed by MTS assay. As shown in figure 2D, treatment with SB431542 abrogated the protective effect of Activin A, whereas the inhibitor had no effect on basal cell viability. These data suggest that extracellular Activin A protects cardiac myocytes from stress-induced apoptosis through the activities of ALKs.
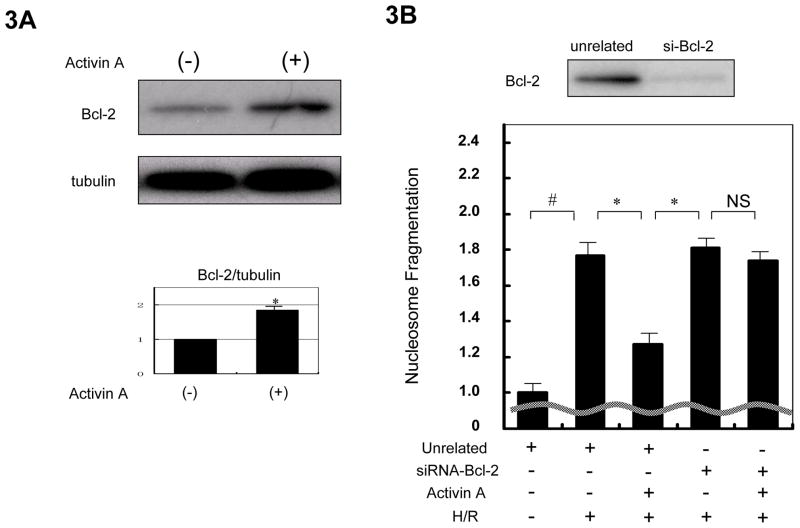
To test whether Bcl-2 is involved in the anti-apoptotic action of Activin A in cardiac myocytes, Bcl-2 protein expression was determined by western blot analysis. Activin A treatment significantly increased Bcl-2 protein levels in NRVMs (Figure 3A). Transduction of NRVMs with siRNA targeting Bcl-2 reduced Bcl-2 protein expression. Knockdown of Bcl-2 with siRNA blocked the inhibitory effect of Activin A on H/R-induced nucleosome fragmentation (Figure 3B). Thus, Activin A cytoprotection is mediated by induction of Bcl-2.
Figure 3.
Cytoprotection by Activin A is mediated by upregulation of the anti-apoptotic protein Bcl-2. (A) Activin A induced Bcl-2 expression in NRVMs. A representative immunoblot is shown. Blots for α-tubulin were performed to indicate the equal loading. The histogram shows quantification of the band intensities to indicate a statistically a significant increase in Bcl-2 protein expression following treatment with Activin A. *P<0.05. (B) Ablation of Bcl-2 expression blocks the cytoprotection conferred by Activin A. The upper panel shows a representative western blot assessing the efficiency of siRNA targeting Bcl-2. The lower panel displays the effect of Bcl-2 knock-down on Activin A-mediated cytoprotection of NRVMs as determined by nucleosome fragmentation assay. Apoptosis was induced by hypoxia/reoxygenation treatment.*P<0.05 and #P<0.01.
Adenovirus-mediated expression of Activin A promotes myocyte survival in vitro and in vivo
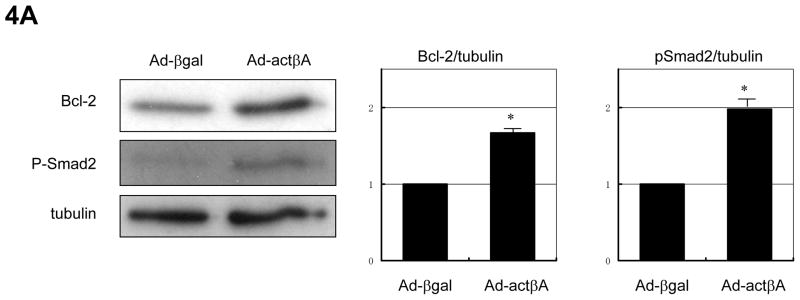
To corroborate and extend the findings obtained with the recombinant human Activin A protein, an adenoviral vector that expresses the mouse Activin βA gene (Ad-actβA) was generated. As shown in figure 4A, transduction with Ad-actβA promoted the expression of Bcl-2 protein and increased the phosphorylation of Smad2 in NRVMs. The magnitude of these effects was similar to that observed with the recombinant Activin A protein (Figure 3A). Transduction of NRVMs with Ad-actβA suppressed apoptosis-induced by H/R as assessed by a nucleosome fragmentation assay (Figure 4B) and an MTS assay of cell viability (Figure 4C).
Figure 4.
Adenovirus-encoded Activin A induces Bcl-2 and protects cardiac myocytes from stress. (A) Representative immunoblot images showing that transfection of adenoviral vector expressing Activin βA (Ad-actβA) at an MOI of 50 resulted in increased expression of Bcl-2 and phosphorylation of Smad2. Membranes were blotted for α-tubulin to indicate equal protein loading. Histogram show quantification of the band intensities. *P<0.05. Transduction of Ad-actβA (MOI=50) reduced apoptosis, assessed by the nucleosome fragmentation assay (B), and preserved cell viability, assessed by the MTS assay, (C) against H/R stress. An adenovirus vector expressing β-galactosidase (Ad-βgal) was used as a control in these experiments at an MOI of 50. *P<0.05 and #P<0.01.
To examine the consequences of Activin A on cardiac myocyte viability in vivo, mice were injected intravenously with ad-actβA or the control vector Ad-βgal. This method of intravenous delivery of adenoviral vectors leads to transduction of the liver, but not heart, and secreted adenovirus- encoded proteins can be detected in the serum10,21. Mice receiving Ad-actβA exhibited detectable Activin A protein expression in serum as assessed by western blot analysis (Figure 5A). In response to myocardial I/R injury, mice treated with Ad-actβA displayed a 53.7% reduction in infarct size. This reduction corresponded to with a decrease in the number of TUNEL-positive, apoptotic cells in the area at risk of the Ad-actβA-treated group (Figure 5B). Collectively, these data show that Activin A protects myocytes from apoptosis in vitro and in vivo and that it minimizes damage from ischemia/reperfusion injury in the heart.
Figure 5.
Adenovirus-mediated overexpression of Activin A protects the heart from ischemia/reperfusion (I/R) injury. (A) Representative western blot analysis, performed under non-reducing conditions, of plasma samples collected three days after injection of Ad-βgal or Ad-actβA. Histogram shows quantification of infarct area induced by I/R three days after adenoviral injection. *P<0.05. (B) Representative images of myocardium stained with TUNEL (green) and sarcomeric actin (red) (upper panels), and merged with DAPI (blue) (lower panels). Histogram shows quantification of TUNEL-positive cells in the myocardium after I/R. *P<0.05.
Fstl3 inhibits Activin A-mediated protection of NRVMs
An adenoviral vector expressing the mouse Fstl3 gene (Ad-Fstl3) was constructed because this factor is also induced by myocardial injury (Figure 1A-C) and it functions as an extracellular binding partner of Activin A. Transduction of NRVMs with Ad-Fstl3 abrogated the ability of Activin A protein to induce Smad2 phosphorylation (Figure 6A). In contrast, adenovirus-mediated overexpression of Fstl1 had no effect on Activin A-induced Smad2 phosphorylation in NRVMs (Figure 6B).
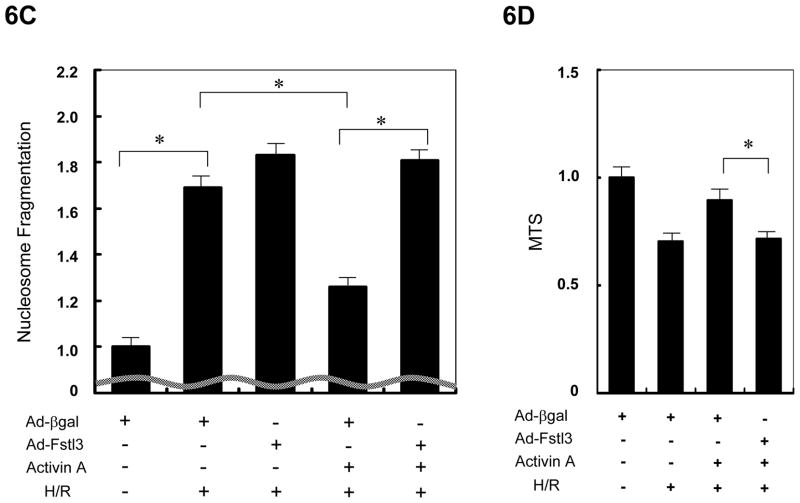
Figure 6.
Fstl3 inhibits Activin A action in NRVMs. (A, B) NRVMs, transduced with Ad-Fstl3 (50MOI) or Ad-Fstl1 (50MOI), were stimulated with 25 ng/mL of recombinant Activin A for indicated periods of time, and phosphorylation of Smad2 was determined by western blot analysis. Immunoblots for α-tubulin were performed as a loading control. (C, D) NRVMs were transduced with Ad-Fstl3 or Ad-βgal at an MOI of 50 (C) or 10 (D) and exposed to H/R treatment in the presence or absence of pretreatment with 25ng/mL of Activin A. Apoptosis was examined by nucleosome fragmentation assay (C) and cell viability was assessed by the MTS assay (D). *P<0.05.
Because Fstl3 is an inhibitor of Activin A, we examined the effects of adeno-mediated induction of Fstl3 on Activin A-mediated protection of NRVMs from stress-induced apoptosis. As shown by nucleosome fragmentation assay, transduction of Ad-Fstl3 abolished the pro-survival actions of Activin A on NRVMs exposed to H/R stress (Figure 6C). The ability of Ad-Fstl3 to block Activin A-mediated NRVM survival was corroborated by the MTS cell viability assay (Figure 6D).
Ablation of Fstl3 in cardiac myocytes protects the heart from ischemia/reperfusion injury in vivo
Cardiac myocyte-specific knockout mice for Fstl3 were generated by crossing Fstl3flox/flox mice with mice expressing Cre recombinase from the αMHC promoter. Cre-mediated recombination of the Fstl3 allele in the hearts of αMHC-Cre x Fstl3flox/flox (CKO) mice was confirmed by PCR (Data Supplement Figure 1). QRT-PCR analysis on the extracts from whole heart revealed a significant, but incomplete, reduction of Fstl3 expression in CKO mice (Cre-f/f) compared to wild-type (W-f/f) mice (Figure 7A). Thus, cardiac myocytes were isolated from adult hearts of both strains of mice and evaluated for Fstl3 expression (Figure 7B). Myocytes isolated from CKO mice were completely void of Fstl3 transcript. Because whole body Fstl3-deficient mice exhibit mild cardiac hypertrophy22, we evaluated heart weight to body weight ratio in the two strains of mice (Figure 7C). Cardiac myocyte-specific Fstl3 knockout mice did not show any difference in heart weight compared to wild-type mice. western immunoblot analysis revealed the upregulation of Bcl-2 protein expression in CKO mice. The upregulation of Bcl-2 expression was also detected by western immunoblot analysis of isolated cardiac myocytes from CKO hearts.
Figure 7.
Fstl3 ablation in heart. (A,B) QRT-PCR was carried out to evaluate Fslt3 mRNA expression in hearts of wild-type (W) or αMHC-Cre (Cre) mice crossed with Fstl3flox/flox mice using cDNA produced from whole heart extracts (A) or isolated cardiac myocytes (B). *P<0.05. (C) Heart weight/body weight ratio (HW/BW) in eight week old male mice. (D, E) Representative images of immunoblots of Bcl-2 expression in whole heart lysates (D) and isolated adult mouse cardiac myocytes (E). Immunoblots for α-tubulin are shown as a loading control.
To examine the functional significance of Fstl3 in myocytes of the heart, CKO and control mice hearts were subjected to I/R injury and infarct size was analyzed by TTC staining. As shown in figure 8A, CKO hearts displayed smaller infarct zones, while the ratio of risk area to left ventricular area did not differ between the two groups (not shown). TUNEL analysis of the area at risk revealed fewer apoptotic cells in the Fstl3 CKO mice (Figure 8B).
Figure 8.
Ablation of Fstl3 protects the heart from ischemia-reperfusion injury. (A) Quantification of infarction size of Fstl3flox/flox crossed with wild-type (W/ff) or αMHC-Cre (Cre/ff) following ischemia/reperfusion injury. *P<0.05. (B) Quantification of TUNEL-positive cells in the myocardium or control (W/ff) and CKO (Cre/ff) mice following ischemia/reperfusion injury. *P<0.05.
Discussion
The heart secretes factors to maintain homeostasis and adapt to stress23–25. Here, we characterize the function of two new members of the cardiac secretome, Fstl3 and Activin A. Fstl3 binds to Activin A and other members of this family and inhibits their ability to activate signaling within target cells1. It has been reported that serum Activin A levels and Fstl3 transcript levels are elevated in heart failure9,14, but the regulatory functions of these factors in heart has not been examined previously. In this study, we show that both Fstl3 and Activin βA mRNA are markedly upregulated in mouse heart in response to multiple types of injury. Functional analyses in vivo and in vitro showed that Activin A is cardio-protective, whereas Fstl3 acts to nullify the protective action of Activin A. These data indicate that the balance of expression between these two molecules can influence how the heart adapts to stress.
Activin A is involved in numerous biological processes including embryonic development26, erythropoiesis27, wound healing28,29, cancer-related cachexia30 and inflammation31. Although it has been demonstrated that Activin A is a pro-survival factor for neuronal cells15–17,20, other studies have demonstrated that Activin A is a pro-apoptotic factor for hematopoietic cells18 and adrenocortical carcinoma cells19. It has also been reported that inhibition of Activin A by Follistatin attenuates apoptosis induced by carbon tetrachloride-injury in liver32. Thus, the mode of Activin A action is highly dependent on tissue and cell type. Here, we present multiple lines of evidence showing that Activin A is cardioprotective. In cultured cardiac myocytes subjected to stress, treatment with recombinant Activin A protein upregulated Bcl-2 protein expression, and reduced caspase activation and cellular apoptosis. Consistent with these results, adenovirus-mediated Activin A overexpression promoted Bcl-2 expression and myocyte viability. Adenovirus-mediated expression of Activin A also reduced infarct size and the frequency of TUNEL-positive cells in hearts that underwent ischemia/reperfusion injury.
The functional significance of Bcl-2 induction by Activin A was assessed by siRNA knock-down experiments in vitro. Treatment with siRNA directed at Bcl-2 effectively ablated Activin A-stimulated expression of this protein by cultured myocytes, and blocked the cytoprotection actions of Activin A. Previous studies have shown that Bcl-2 has roles in promoting cardiac myocyte viability in models of ischemic injury33 and desmin-deficiency-induced cardiomyopathy34. It has also been reported that Activin A induces both Bcl-2 and Bcl-xL in neuroblastoma and pheochromocytoma cells20. However, we did not detect Activin A-stimulated Bcl-xL expression in cardiac myocyte cultures (data not shown).
In this study, it is shown that Fstl3 inhibits the protective actions of Activin A on cardiac myocytes. Pre-treatment with an adenoviral vector expressing Fstl3 abrogates Activin A-mediated suppression of NRVM death under conditions of hypoxia/reoxygenation. Furthermore, cardiac myocyte-specific ablation of Fslt3 reduces infarct size and diminishes the frequency of apoptotic myocytes in the area at risk following ischemia/reperfusion injury.
We previously showed that Fstl1 is upregulated by cardiac injuries in murine models10 and Lara-Pezzi et al. reported that the Fstl1 transcript is upregulated in human heart failure9. In contrast to Fstl3, Fstl1 protects cardiac myocytes from death both in vitro and in vivo10. Also in contrast to Fslt3, it is shown here that Fstl1 does not interfere with Activin A-stimulates Smad2 phosphorylation (Figure 6). In contrast, Fstl1 protection of both cardiac myocytes and endothelial cells is dependent upon the upregulation of Akt signaling10,35. Currently there is no evidence to suggest that Fstl1 functions by binding to TGF-β superfamily members.
It was previously reported that whole-body Fstl3-deficiency results in a low degree of cardiac hypertrophy accompanied by mildly elevated blood pressure in old female mice22. In the current study, we employed cardiac-specific Fstl3-deficient mice, and no change in heart weight to body weight ratio was observed between CKO and wild-type mice. Since elevated blood pressure can lead to cardiac hypertrophy, the cardiac phenotype of the whole-body Fstl3 knock-out mouse may be caused by the indirect actions of whole-body Fstl3-deficiency on the heart.
Other TGF-β family cytokines reported to be produced by the heart under conditions of stress include myostatin/GDF-8 and GDF-156–8,36. Like Activin A, these factors regulate Smad signaling and cause cachexia when administered or overexpressed30,37,38. Both Activin A and GDF-15 have been shown to be increased in patients with heart diseases14,39,40. Collectively, these studies indicate the existence of a broad signaling network involving TGF-β family factors and their extra-cellular inhibitory proteins that controls cardiac adaptation to stress. The expression of these proteins by the damaged heart may also contribute to the systemic wasting response in chronic heart failure.
Conclusions
We show that Activin A and its extracellular inhibitory protein Fstl3 are upregulated in murine heart under conditions of stress. Administration or overexpression of Activin A protects myocytes from stress in vitro and in vivo. In contrast, Fstl3 overexpression inhibits the myocyte-protective activity of Activin A in vitro and cardiac-specific Fstl3-deficient mice display smaller infarcts and less myocyte apoptosis in response to ischemia/reperfusion injury. Thus, we propose that Activin A and Fstl3 function in an opposing manner to regulate myocyte survival, and that the relative expression levels of these factors influence the adaptive response of the heart to injury.
Supplementary Material
Acknowledgments
Sources of Funding
This study was funded by National Institutes of Health grants HL77774, HL86785, AG15052 and HL81587 to K. Walsh. N. Ouchi was supported by American Heart Association grant 0635593T. D.R. Pimentel was supported by National Institutes of Health grant HL71563. Y. Oshima was supported by American Heart Association grant 0625867T. Se-Jin Lee was supported by National Institutes of Health grant U54-AR052646. K.D. Panse was supported by an EMBO fellowship (ASTF 53.00-2009).
Footnotes
Clinical Perspective
The injured heart secretes proteins that influence its function. Here, we characterize two new members of the cardiac “secretome”, Activin A and Fstl3, using genetic gain- and loss-of-function manipulations in mouse models. Activin A and Fstl3 expression was increased in heart following various injuries and in cultured myocytes following hypoxia/reoxygenation. Activin A protected myocytes from cell death and this protective activity was antagonized by Fstl3, which functions as an extracellular inhibitory protein for Activin A. Myocardial ischemia/reperfusion injury was reduced in mice administered Activin A. Genetic ablation of Fstl3 in cardiac myocytes also diminished injury in response to ischemia/reperfusion. We speculate that Activin A and Fstl3 serve as sensors of cardiac stress and that their relative levels of expression influence the adaptive response of the heart to injury.
Disclosures
None
References
- 1.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 2.Schultz Jel J, Witt SA, Glascock BJ, Nieman ML, Reiser PJ, Nix SL, Kimball TR, Doetschman T. TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J Clin Invest. 2002;109:787–96. doi: 10.1172/JCI14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okada H, Takemura G, Kosai K, Li Y, Takahashi T, Esaki M, Yuge K, Miyata S, Maruyama R, Mikami A, Minatoguchi S, Fujiwara T, Fujiwara H. Postinfarction gene therapy against transforming growth factor-beta signal modulates infarct tissue dynamics and attenuates left ventricular remodeling and heart failure. Circulation. 2005;111:2430–7. doi: 10.1161/01.CIR.0000165066.71481.8E. [DOI] [PubMed] [Google Scholar]
- 4.Izumi M, Fujio Y, Kunisada K, Negoro S, Tone E, Funamoto M, Osugi T, Oshima Y, Nakaoka Y, Kishimoto T, Yamauchi-Takihara K, Hirota H. Bone morphogenetic protein-2 inhibits serum deprivation-induced apoptosis of neonatal cardiac myocytes through activation of the Smad1 pathway. J Biol Chem. 2001;276:31133–41. doi: 10.1074/jbc.M101463200. [DOI] [PubMed] [Google Scholar]
- 5.Masaki M, Izumi M, Oshima Y, Nakaoka Y, Kuroda T, Kimura R, Sugiyama S, Terai K, Kitakaze M, Yamauchi-Takihara K, Kawase I, Hirota H. Smad1 protects cardiomyocytes from ischemia-reperfusion injury. Circulation. 2005;111:2752–9. doi: 10.1161/CIRCULATIONAHA.104.490946. [DOI] [PubMed] [Google Scholar]
- 6.Xu J, Kimball TR, Lorenz JN, Brown DA, Bauskin AR, Klevitsky R, Hewett TE, Breit SN, Molkentin JD. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ Res. 2006;98:342–50. doi: 10.1161/01.RES.0000202804.84885.d0. [DOI] [PubMed] [Google Scholar]
- 7.Kempf T, Eden M, Strelau J, Naguib M, Willenbockel C, Tongers J, Heineke J, Kotlarz D, Xu J, Molkentin JD, Niessen HW, Drexler H, Wollert KC. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res. 2006;98:351–60. doi: 10.1161/01.RES.0000202805.73038.48. [DOI] [PubMed] [Google Scholar]
- 8.Morissette MR, Cook SA, Foo S, McKoy G, Ashida N, Novikov M, Scherrer-Crosbie M, Li L, Matsui T, Brooks G, Rosenzweig A. Myostatin regulates cardiomyocyte growth through modulation of Akt signaling. Circ Res. 2006;99:15–24. doi: 10.1161/01.RES.0000231290.45676.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lara-Pezzi E, Felkin LE, Birks EJ, Sarathchandra P, Panse KD, George R, Hall JL, Yacoub MH, Rosenthal N, Barton PJ. Expression of follistatin-related genes is altered in heart failure. Endocrinology. 2008;149:5822–7. doi: 10.1210/en.2008-0151. [DOI] [PubMed] [Google Scholar]
- 10.Oshima Y, Ouchi N, Sato K, Izumiya Y, Pimentel DR, Walsh K. Follistatin-like 1 is an Akt-regulated cardioprotective factor that is secreted by the heart. Circulation. 2008;117:3099–108. doi: 10.1161/CIRCULATIONAHA.108.767673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiekofer S, Shiojima I, Sato K, Galasso G, Oshima Y, Walsh K. Microarray analysis of Akt1 activation in transgenic mouse hearts reveals transcript expression profiles associated with compensatory hypertrophy and failure. Physiol Genomics. 2006;27:156–70. doi: 10.1152/physiolgenomics.00234.2005. [DOI] [PubMed] [Google Scholar]
- 12.Schiekofer S, Belisle K, Galasso G, Schneider JG, Boehm BO, Burster T, Schmitz G, Walsh K. Angiogenic-regulatory network revealed by molecular profiling heart tissue following Akt1 induction in endothelial cells. Angiogenesis. 2008;11:289–99. doi: 10.1007/s10456-008-9112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yndestad A, Ueland T, Oie E, Florholmen G, Halvorsen B, Attramadal H, Simonsen S, Froland SS, Gullestad L, Christensen G, Damas JK, Aukrust P. Elevated levels of activin A in heart failure: potential role in myocardial remodeling. Circulation. 2004;109:1379–85. doi: 10.1161/01.CIR.0000120704.97934.41. [DOI] [PubMed] [Google Scholar]
- 15.Hughes PE, Alexi T, Williams CE, Clark RG, Gluckman PD. Administration of recombinant human Activin-A has powerful neurotrophic effects on select striatal phenotypes in the quinolinic acid lesion model of Huntington’s disease. Neuroscience. 1999;92:197–209. doi: 10.1016/s0306-4522(98)00724-6. [DOI] [PubMed] [Google Scholar]
- 16.Wu DD, Lai M, Hughes PE, Sirimanne E, Gluckman PD, Williams CE. Expression of the activin axis and neuronal rescue effects of recombinant activin A following hypoxic-ischemic brain injury in the infant rat. Brain Res. 1999;835:369–78. doi: 10.1016/s0006-8993(99)01638-8. [DOI] [PubMed] [Google Scholar]
- 17.Schubert D, Kimura H, LaCorbiere M, Vaughan J, Karr D, Fischer WH. Activin is a nerve cell survival molecule. Nature. 1990;344:868–70. doi: 10.1038/344868a0. [DOI] [PubMed] [Google Scholar]
- 18.Valderrama-Carvajal H, Cocolakis E, Lacerte A, Lee EH, Krystal G, Ali S, Lebrun JJ. Activin/TGF-beta induce apoptosis through Smad-dependent expression of the lipid phosphatase SHIP. Nat Cell Biol. 2002;4:963–9. doi: 10.1038/ncb885. [DOI] [PubMed] [Google Scholar]
- 19.Vanttinen T, Liu J, Kuulasmaa T, Kivinen P, Voutilainen R. Expression of activin/inhibin signaling components in the human adrenal gland and the effects of activins and inhibins on adrenocortical steroidogenesis and apoptosis. J Endocrinol. 2003;178:479–89. doi: 10.1677/joe.0.1780479. [DOI] [PubMed] [Google Scholar]
- 20.Kupershmidt L, Amit T, Bar-Am O, Youdim MB, Blumenfeld Z. The neuroprotective effect of Activin A and B: implication for neurodegenerative diseases. J Neurochem. 2007;103:962–71. doi: 10.1111/j.1471-4159.2007.04785.x. [DOI] [PubMed] [Google Scholar]
- 21.Shibata R, Sato K, Kumada M, Izumiya Y, Sonoda M, Kihara S, Ouchi N, Walsh K. Adiponectin accumulates in myocardial tissue that has been damaged by ischemia-reperfusion injury via leakage from the vascular compartment. Cardiovasc Res. 2007;74:471–9. doi: 10.1016/j.cardiores.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Mukherjee A, Sidis Y, Mahan A, Raher MJ, Xia Y, Rosen ED, Bloch KD, Thomas MK, Schneyer AL. FSTL3 deletion reveals roles for TGF-beta family ligands in glucose and fat homeostasis in adults. Proc Natl Acad Sci U S A. 2007;104:1348–53. doi: 10.1073/pnas.0607966104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–18. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frost RJ, Engelhardt S. A secretion trap screen in yeast identifies protease inhibitor 16 as a novel antihypertrophic protein secreted from the heart. Circulation. 2007;116:1768–75. doi: 10.1161/CIRCULATIONAHA.107.696468. [DOI] [PubMed] [Google Scholar]
- 25.Izumiya Y, Shiojima I, Sato K, Sawyer DB, Colucci WS, Walsh K. Vascular endothelial growth factor blockade promotes the transition from compensatory cardiac hypertrophy to failure in response to pressure overload. Hypertension. 2006;47:887–93. doi: 10.1161/01.HYP.0000215207.54689.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matzuk MM, Kumar TR, Vassalli A, Bickenbach JR, Roop DR, Jaenisch R, Bradley A. Functional analysis of activins during mammalian development. Nature. 1995;374:354–6. doi: 10.1038/374354a0. [DOI] [PubMed] [Google Scholar]
- 27.Murata M, Eto Y, Shibai H, Sakai M, Muramatsu M. Erythroid differentiation factor is encoded by the same mRNA as that of the inhibin beta A chain. Proc Natl Acad Sci U S A. 1988;85:2434–8. doi: 10.1073/pnas.85.8.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munz B, Smola H, Engelhardt F, Bleuel K, Brauchle M, Lein I, Evans LW, Huylebroeck D, Balling R, Werner S. Overexpression of activin A in the skin of transgenic mice reveals new activities of activin in epidermal morphogenesis, dermal fibrosis and wound repair. Embo J. 1999;18:5205–15. doi: 10.1093/emboj/18.19.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wankell M, Munz B, Hubner G, Hans W, Wolf E, Goppelt A, Werner S. Impaired wound healing in transgenic mice overexpressing the activin antagonist follistatin in the epidermis. Embo J. 2001;20:5361–72. doi: 10.1093/emboj/20.19.5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matzuk MM, Finegold MJ, Mather JP, Krummen L, Lu H, Bradley A. Development of cancer cachexia-like syndrome and adrenal tumors in inhibin-deficient mice. Proc Natl Acad Sci U S A. 1994;91:8817–21. doi: 10.1073/pnas.91.19.8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones KL, Mansell A, Patella S, Scott BJ, Hedger MP, de Kretser DM, Phillips DJ. Activin A is a critical component of the inflammatory response, and its binding protein, follistatin, reduces mortality in endotoxemia. Proc Natl Acad Sci U S A. 2007;104:16239–44. doi: 10.1073/pnas.0705971104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patella S, Phillips DJ, Tchongue J, de Kretser DM, Sievert W. Follistatin attenuates early liver fibrosis: effects on hepatic stellate cell activation and hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G137–44. doi: 10.1152/ajpgi.00080.2005. [DOI] [PubMed] [Google Scholar]
- 33.Imahashi K, Schneider MD, Steenbergen C, Murphy E. Transgenic expression of Bcl-2 modulates energy metabolism, prevents cytosolic acidification during ischemia, and reduces ischemia/reperfusion injury. Circ Res. 2004;95:734–41. doi: 10.1161/01.RES.0000143898.67182.4c. [DOI] [PubMed] [Google Scholar]
- 34.Weisleder N, Taffet GE, Capetanaki Y. Bcl-2 overexpression corrects mitochondrial defects and ameliorates inherited desmin null cardiomyopathy. Proc Natl Acad Sci U S A. 2004;101:769–74. doi: 10.1073/pnas.0303202101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, Walsh K. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem. 2008;283:32802–11. doi: 10.1074/jbc.M803440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma M, Kambadur R, Matthews KG, Somers WG, Devlin GP, Conaglen JV, Fowke PJ, Bass JJ. Myostatin, a transforming growth factor-beta superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J Cell Physiol. 1999;180:1–9. doi: 10.1002/(SICI)1097-4652(199907)180:1<1::AID-JCP1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 37.Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296:1486–8. doi: 10.1126/science.1069525. [DOI] [PubMed] [Google Scholar]
- 38.Johnen H, Lin S, Kuffner T, Brown DA, Tsai VW, Bauskin AR, Wu L, Pankhurst G, Jiang L, Junankar S, Hunter M, Fairlie WD, Lee NJ, Enriquez RF, Baldock PA, Corey E, Apple FS, Murakami MM, Lin EJ, Wang C, During MJ, Sainsbury A, Herzog H, Breit SN. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat Med. 2007;13:1333–40. doi: 10.1038/nm1677. [DOI] [PubMed] [Google Scholar]
- 39.Kempf T, von Haehling S, Peter T, Allhoff T, Cicoira M, Doehner W, Ponikowski P, Filippatos GS, Rozentryt P, Drexler H, Anker SD, Wollert KC. Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1054–60. doi: 10.1016/j.jacc.2007.04.091. [DOI] [PubMed] [Google Scholar]
- 40.Wollert KC, Kempf T, Peter T, Olofsson S, James S, Johnston N, Lindahl B, Horn-Wichmann R, Brabant G, Simoons ML, Armstrong PW, Califf RM, Drexler H, Wallentin L. Prognostic value of growth-differentiation factor-15 in patients with non-ST-elevation acute coronary syndrome. Circulation. 2007;115:962–71. doi: 10.1161/CIRCULATIONAHA.106.650846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.