Abstract
We expressed the γ-aminobutyric acid (GABA) transporter GAT1 (SLC6A1) in Xenopus laevis oocytes and performed GABA uptake experiments under voltage clamp at different membrane potentials as well as in the presence of the specific GAT1 inhibitors SKF-89976A and NO-711. In the absence of the inhibitors, GAT1 mediated the inward translocation of 2 net positive charges across the plasma membrane for every GABA molecule transported into the cell. This 2:1 charge flux / GABA flux ratio was the same over a wide range of membrane potentials from −110 mV to +10 mV. Moreover, when GABA-evoked (500 μM) currents were measured at −50 and −90 mV, neither SKF-89976A (5 and 25 μM) nor NO-711 (2 μM) altered the 2:1 charge flux / GABA flux ratio. The results are not consistent with previous hypotheses that (i) GABA evokes an uncoupled channel-mediated current in GAT1, and (ii) GAT1 inhibitors block the putative uncoupled current gated by GABA. Rather, the results suggest tight coupling of GAT1-mediated charge flux and GABA flux.
Keywords: Neurotransmitter:sodium symporter, SLC6, Sodium- and chloride-coupled transport, Neurotransmitter transporter, GABA, human GAT1, SLC6A1, Channel mode, Uptake under voltage clamp
INTRODUCTION
γ-Aminobutyric acid (GABA) transporters (GATs) are electrogenic Na+- and Cl−-coupled transporters that are responsible for maintaining low resting levels of GABA in the central nervous system, as well as for modulating synaptic and extra-synaptic GABAergic neurotransmission (Borden, 1996; Nelson, 1998; Dalby, 2003; Richerson and Wu, 2003; Conti et al., 2004). The GABA transporters (GATs) belong to the large neurotransmitter/Na+ symporter family (NSS; 2.A.22 according to the transporter classification system; SLC6 according to the Human Genome Organization classification) (Nelson, 1998; Busch and Saier, 2002; Chen et al., 2004; Saier et al., 2006). Solute transport in these transporters is driven by the electrochemical potential gradient of Na+ and Cl− (Kanner and Schuldiner, 1987; Wu et al., 2007) and, in fact, Na+ and Cl− are cotranslocated with GABA during each transport cycle (Radian and Kanner, 1983; Keynan and Kanner, 1988; Loo et al., 2000). While dependence on Na+ is absolute, the dependence on Cl− is not absolute and its degree varies with the isoform examined (Clark et al., 1992; Keynan et al., 1992; Mager et al., 1993, 1996; Clark and Amara, 1994; Matskevitch et al., 1999; Loo et al., 2000; Sacher et al., 2002; Whitlow et al., 2003; Karakossian et al., 2005; Gonzales et al., 2007). Chloride dependence also varies with the membrane potential, as transport appears to be less Cl−-dependent at hyperpolarized membrane potentials (Mager et al., 1993; Loo et al., 2000).
Results from many laboratories have suggested the ion/substrate transport stoichiometry of GAT1 to be 2 Na+ : 1 Cl− : 1 GABA per transport cycle (Radian and Kanner, 1983; Keynan and Kanner, 1988; Lu and Hilgemann, 1999a; Loo et al., 2000; Wu et al., 2007). Although this ion/GABA coupling stoichiometry suggests that one net positive charge is translocated across the plasma membrane per GABA molecule, numerous studies have shown that, in fact, two net positive charges are transported for every GABA molecule translocated across the plasma membrane (Mager et al., 1996; Loo et al., 2000; Sacher et al., 2002; Whitlow et al., 2003; Karakossian et al., 2005; Gonzales et al., 2007). Thus, there appears to be a discrepancy between the predicted (1 net charge) and measured (2 net charges) number of net positive charges translocated per GABA molecule per transport cycle. Interestingly, the 2:1 charge flux / GABA flux ratio is not altered in the absence of external Cl−, leading Loo et al. (2000) to hypothesize that a Cl−-Cl− exchange mechanism may play a role during the transport cycle. This hypothesis has found both support and opposition (Bicho and Grewer, 2005; Zomot et al., 2007).
It has been suggested that charge translocation in excess of that predicted by the 2 Na+ : 1 Cl− : 1 GABA stoichiometry may be due to uncoupled ion conduction through GAT1 (Cammack et al., 1994; Risso et al., 1996; Krause and Schwarz, 2005). In a series of experiments which examined the effect of the selective blocker of GAT1, SKF-89976A, Krause and Schwarz (2005) proposed that the apparent excess charge may be attributed to a GABA-gated channel mode of GAT1. These investigators referred to this current as an “uncoupled transmitter-gated current” and proposed that charge permeation via this mode is not coupled to GABA translocation across the plasma membrane. They also suggested that Na+ was the main carrier of this uncoupled current. Krause and Schwarz (2005) suggested that SKF-89976A differentially inhibits the cotransport and putative channel modes of the transporter with half-inhibition achieved at 9 μM and 0.03 μM SKF-89976A, respectively. However, that study did not perform two key measurements necessary to support these hypotheses. (i) The current-voltage (I-V) relationship and, hence, reversal potential of the putative “uncoupled transmitter-gated current” was not reported. This measurement is essential for attributing the putative uncoupled current to Na+ conduction. (ii) The charge flux / GABA flux ratio was not determined in the presence of SKF-89976A in individual cells under voltage clamp. According to the proposed hypothesis, the measured charge flux / GABA flux ratio, which is 2 in the absence of SKF-89976A, should change to 1 net positive charge for every translocated GABA molecule when measured in the presence of a sufficiently high concentration of inhibitor to block the putative channel mode.
In the present study, we have tested the hypothesis put forth by Krause and Schwarz (2005) by directly measuring the number of positive charges that enter the cell per GABA molecule over a wide range of membrane potentials (−110 mV to +10 mV), as well as in the presence of the GAT1 inhibitors SKF-89976A and NO-711. While the present study does not reconcile the measured 2:1 charge:GABA transport ratio with the known 2 Na+ : 1 Cl− : 1 GABA transport stoichiometry, our results do suggest that GAT1-mediated charge flux and GABA flux are tightly coupled processes regardless of the imposed membrane potential or presence of GAT1 inhibitors. Our results are not consistent with a GABA-induced channel mode of conduction in GAT1.
EXPERIMENTAL PROCEDURES
Expression in Xenopus Oocytes
Stage V–VI Xenopus laevis oocytes were injected with 50 ng of cRNA for human GAT1 (SLC6A1) (Nelson et al., 1990; Chen et al., 2004). After cRNA injection, oocytes were maintained in Barth's medium (in mM: 88 NaCl, 1 KCl, 0.33 Ca(NO3)2, 0.41 CaCl2, 0.82 MgSO4, 2.4 NaHCO3, 10 HEPES, pH 7.4, and 50 μg/mL gentamicin, 100 μg/mL streptomycin, and 100 units/mL penicillin) at 18 °C for up to 14 days until used in experiments. All experiments were performed at 21 ± 1 °C.
Experimental Solutions and Reagents
Unless otherwise indicated, experiments were performed in a NaCl buffer containing (in mM): 100 NaCl, 2 KCl, 1 CaCl2, 1 MgCl2, 10 HEPES, pH 7.4. Na+-free buffer was prepared by equimolar replacement of NaCl with choline-Cl. GABA, 1-(4,4-Diphenyl-3-butenyl)-3-piperidinecarboxylic acid (SKF-89976A), and/or 1-[2-[[(diphenylmethylene)imino]oxy]ethyl]-1,2,5,6-tetrahydro-3-pyridinecarboxylic acid (NO-711) were added to the NaCl buffer as indicated. [3H]-GABA was obtained from GE Healthcare (Piscataway, NJ). All other reagents were purchased from Fisher Scientific (Pittsburgh, PA) or Sigma (St. Louis, MO).
Electrophysiological Measurements and Data Analysis
The two-microelectrode voltage clamp technique was used for the recording of whole-cell transporter-mediated currents. Oocytes were voltage clamped at the indicated membrane potential (Vm) by using the Warner Oocyte Clamp (OC-725C; Warner Instrument Corporation; Hamden, CT). In the experimental recording chamber, oocytes were initially stabilized in the NaCl buffer, and the composition of the bath was changed as indicated. In all experiments, the reference electrodes were connected to the experimental oocyte chamber via agar bridges (3% agar in 3 M KCl). For continuous holding current measurements, currents were low-pass filtered at 1 Hz (LPF 8; Warner Instrument Corporation), and sampled at 10 Hz (pCLAMP 8.1, Axon Instruments; Union City, CA). For steady-state current-voltage (I-V) relations, the pulse protocol (pCLAMP 8.1, Axon Instruments; Union City, CA) consisted of 400-ms voltage pulses from a holding potential of −50 mV to a series of test voltages (Vm) from +100 to −140 mV in 20-mV steps. Currents were low-pass filtered at 500 Hz, and sampled at 2 kHz. At each voltage, the GABA-evoked current was obtained as the difference in steady-state current at the end of the 400-ms pulse in the absence and presence of GABA and/or inhibitor. As the GAT1-mediated GABA-evoked current is Na+- and Cl−-coupled (Loo et al., 2000), it is referred to as (Gonzales et al., 2007).
Both SKF-89976A and NO-711 are competitive inhibitors of GAT1 and, thus, the data for the inhibition experiments were fitted to Equation 1 (Krause and Schwarz, 2005; Segel, 1975):
| (1) |
where Imax is the maximum current evoked by a saturating concentration of GABA in the absence of the blocker (B, here SKF-89976A or NO-711), I is the evoked current in the presence of the indicated concentrations of GABA and blocker (B), is the GABA concentration at which I is half of Imax (25 μM at −50 mV; Gonzales et al., 2007), and is the blocker concentration at which I is 50% of Imax (apparent half-inhibition constant).
To determine the relationship between the GABA-evoked current and GABA flux, uptake experiments were performed under voltage clamp in individual control and GAT1-expressing cells (Eskandari et al., 1997; Forster et al., 1999; Loo et al., 2000; Sacher et al., 2002; Whitlow et al., 2003; Karakossian et al., 2005; Gonzales et al., 2007). The membrane potential was held at the indicated value (+10, −10, −30, −50, −70, −90, or −110 mV), and the holding current was continuously monitored. Oocytes were initially incubated in the NaCl buffer until baseline was established. GABA (500 μM) and [3H]-GABA (28 nM) were added to the perfusion solution for 5–10 minutes. At the end of the incubation period, GABA and the isotope were removed from the perfusion solution until the holding current returned to the baseline. The oocytes were removed from the experimental chamber, washed in ice-cold choline-Cl buffer, and solubilized in 10% sodium dodecyl sulfate (SDS). Oocyte [3H]-GABA content was determined in a liquid scintillation counter (Beckman LS 6500; Fullerton, CA). Net inward charge was obtained from the time integral of the GABA-evoked inward current and correlated with GABA influx in the same cell. GABA uptake under voltage clamp was also performed in the presence of GAT1 inhibitors SKF-89976A (5 μM and 25 μM) and NO-711 (2 μM). In these experiments, after the initial stabilization in the NaCl buffer, the oocyte was exposed to the inhibitor at the indicated concentration for at least 5 minutes prior to the addition of GABA (500 μM), and the inhibitor was also included at the same concentration in all subsequent solutions perfusing the chamber. In all experiments, GAT1-mediated GABA uptake was obtained by subtracting endogenous GABA uptake in control cells from the same batch that were subjected to the same experimental condition as GAT1-expressing cells. Endogenous GABA uptake was ≤ 5% of total GABA uptake (endogenous + GAT1-mediated uptake).
Where sample sizes are indicated (n), they refer to the number of oocytes in which the experiments were repeated. Reported errors represent the standard error of the mean obtained from data from several oocytes.
RESULTS
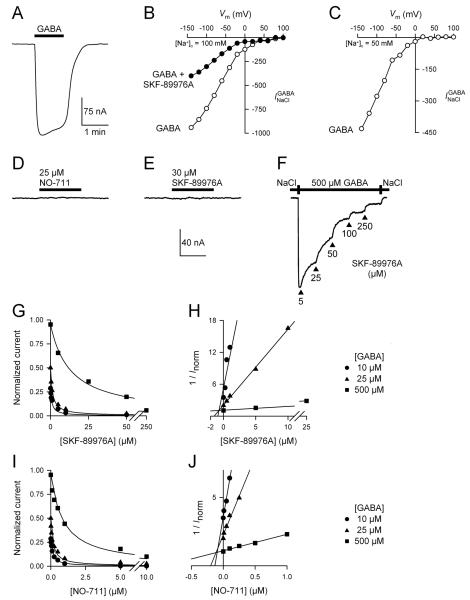
The GABA-evoked current of GAT1 () is voltage-dependent (Fig. 1, A–C). is directly proportional to Na+, Cl−, and GABA influx and, thus, is a good assay of GAT1 transport function (Loo et al., 2000; see also Figs. 2–4). In the voltage range tested (−140 to +100 mV) and under the zero-trans conditions of our experiments, (500 μM GABA) increased with hyperpolarization and only began showing evidence of saturation at the most negative membrane potential of −140 mV (Fig. 1B). At an external Na+ concentration of 100 mM (Fig. 1B) or 50 mM (Fig. 1C), decreased with membrane depolarization and did not reverse under these conditions even at membrane potentials more positive than the predicted Na+ equilibrium potential (VNa) (Fig. 1, B and C). Assuming that the cytoplasmic Na+ concentration is 6–10 mM (Nakhoul et al., 2001), VNa is predicted to be 58–71 mV at 100 mM external Na+, and 41–54 mV at 50 mM external Na+. In the presence of 25 μM SKF-89976A, the GABA-evoked (500 μM) current was reduced by ∼65% (Fig. 1B). Inhibition of by SKF-89976A did not reveal an outward current beyond the Na+ equilibrium potential (Fig. 1B). Similar results were obtained with NO-711 (used at 2 μM; not shown).
Fig. 1. Pharmacological inhibition of GAT1-mediated GABA-evoked current () does not reveal a channel component conductive to Na+ ions.
(A–C) A representative GAT1-mediated GABA-evoked current trace is shown (−50 mV), and the corresponding current-voltage relationships are shown for voltages ranging from −140 mV to +100 mV. [GABA] = 500 μM. When measured at an extracellular Na+ concentration of 100 mM (B) or 50 mM (C), did not show any evidence of reversal. Therefore, under the zero-trans conditions of our experiments, does not have an outward component even at membrane potentials more positive than the predicted Na+ equilibrium potential. When tested at 25 μM, SKF-89976A inhibited the inward current evoked by 500 μM GABA by ∼65% (B). (D–F) Application of NO-711 alone (25 μM) (D) or SKF-89976A alone (30 μM) (E) to a cell expressing GAT1 did not reveal a constitutive leak mode, however, both agents inhibited in a concentration dependent manner. A representative trace is shown for SKF-89976A at 500 μM GABA and −50 mV (F). The current scale bar is the same for panels D–F. The time scale bar is 4 minutes for panels D and E, and 15 minutes for panel F. (G) SKF-89976A inhibition of was carried out at 10 μM, 25 μM, and 500 μM GABA. Vm = −50 mV. The data were adequately fitted with Eq. 1 for competitive inhibition at a single site. (H) Replotting the data in a Dixon plot yielded a Ki value of 700 nM for SKF-89976A inhibition of GAT1-mediated . (I) NO-711 inhibition of was carried out at 10 μM, 25 μM, and 500 μM GABA. Vm = −50 mV. The data were adequately fitted with Eq. 1 for competitive inhibition at a single site. (J) Replotting the data in a Dixon plot yielded a Ki value of 95 nM for NO-711 inhibition of GAT1-mediated . In panels H and J, each data point was normalized to the maximum GABA-evoked current (Imax) obtained in the same cell. Imax was obtained at a saturating GABA concentration (5 mM) and in the absence of inhibitor.
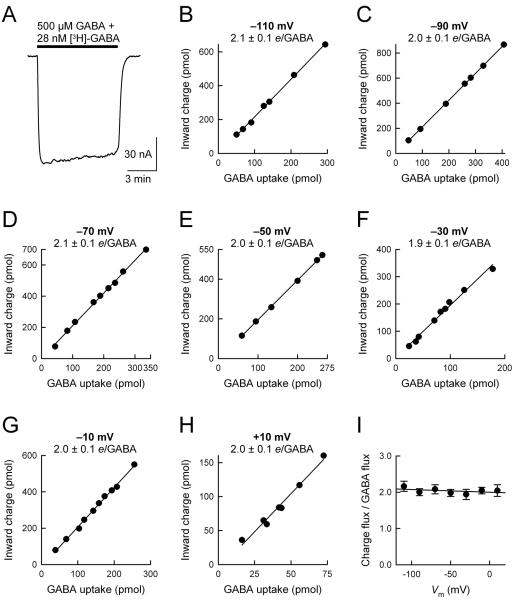
Fig. 2. The ratio of GAT1-mediated charge flux to GABA flux is not altered by changes in the membrane potential.
(A) GABA uptake experiments were performed under voltage clamp at membrane potentials ranging from −110 mV to +10 mV. (B–H) Net inward charge flux is plotted as a function of GABA uptake in individual GAT1-expressing cells. Cells expressing GAT1 were held at the indicated membrane potential and exposed to 500 μM GABA and 28 nM [3H]-GABA for 5–10 minutes. After washout of GABA and isotope, the cells were solubilized in 10% SDS and cellular GABA content was determined by using a liquid scintillation counter. In the same cell, the net inward charge flux was obtained from time integral of the current trace. To determine GAT1-mediated GABA uptake, endogenous uptake of GABA was determined in control cells from the same batch and subtracted from total GABA uptake in GAT1-expressing cells. Endogenous GABA uptake was not voltage dependent but varied in cells obtained from different donor frogs (0.28–0.66 pmol/min/oocyte). In GAT1-expressing cells, uptake rates ranged from 5 to 401 pmol/min/oocyte. In all experiments, endogenous GABA uptake was ≤ 5% of total GABA uptake. The ratio of charge flux to GABA flux (i.e., net positive charges per GABA, e/GABA) was 2.1 ± 0.1 at −110 mV (n = 7), 2.0 ± 0.1 at −90 mV (n = 7), 2.1 ± 0.1 at −70 mV (n = 9), 2.0 ± 0.1 at −50 mV (n = 6), 1.9 ± 0.1 at −30 mV (n = 9), 2.0 ± 0.1 at −10 mV (n = 10), and 2.0 ± 0.1 at +10 mV (n = 7). In each panel, the smooth line is a linear regression through the data points. (I) The ratio of charge flux to GABA flux is plotted as a function of the imposed membrane potential. At all membrane potentials examined (−110 mV to +10 mV), the ratio of charge flux to GABA flux was 2 positive charges/GABA. The smooth line is a linear regression through the data points, and its slope is not significantly different from zero (p = 0.34).
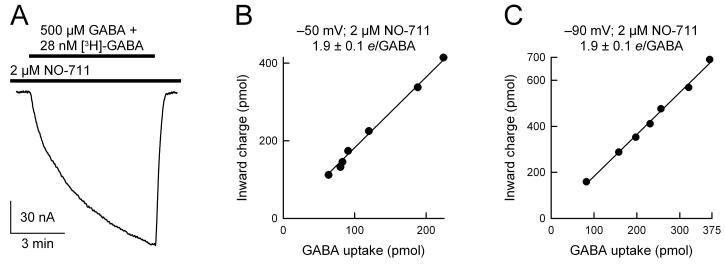
Fig. 4. The ratio of GAT1-mediated charge flux to GABA flux is not altered by NO-711.
GABA uptake was performed under voltage clamp in the presence of 2 μM NO-711 at −50 mV (B) and −90 mV (C). GABA was 500 μM and [3H]-GABA was 28 nM. In the presence of 2 μM NO-711, the ratio of charge flux to GABA flux (e/GABA) was 1.9 ± 0.1 at −50 mV (n = 7) and 1.9 ± 0.1 at −90 mV (n = 8). In each panel, the smooth line is a linear regression through the data points.
In the absence of GABA, application of GAT1 blockers NO-711 (25 μM) or SKF-89976A (30 μM) alone to GAT1-expressing cells did not reveal a constitutive GAT1-mediated leak current (Fig. 1, D and E). These inhibitor levels were sufficiently high because at these concentrations and in the absence of GABA, SKF-89976A and NO-711 lead to complete inhibition of transporter conformational changes (not shown) (Mager et al., 1993; Lu and Hilgemann, 1999b; Li et al., 2000; Forlani et al., 2001; Meinild et al., 2009). SKF-89976A and NO-711 led to complete inhibition of in a concentration dependent manner (Fig. 1, F, G, and I). To better understand the mechanism of inhibition by SKF-89976A and NO-711, as well as to determine the appropriate inhibitor concentration to be used in the uptake under voltage clamp experiments (see Figs. 3 and 4), inhibition kinetics experiments were carried out at different GABA concentrations (Vm = −50 mV) (Fig. 1, G and I). At each GABA concentration (10, 25, and 500 μM), SKF-89976A and NO-711 inhibition of could be adequately described as competitive inhibition at a single site (Equation 1) (Fig. 1, G and I). For SKF-89976A, the Ki value obtained from all trials was 640 ± 50 nM (n = 12), and that obtained from the Dixon plot was 700 nM (Fig. 1H). For NO-711, the Ki value obtained from all trials was 78 ± 12 nM (n = 9), and that obtained from the Dixon plot was 95 nM (Fig. 1J).
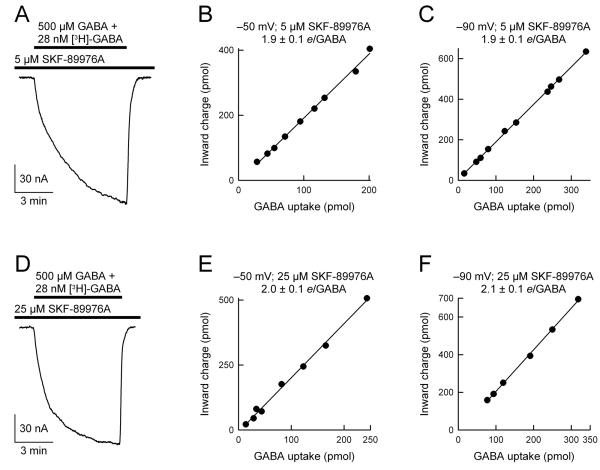
Fig 3. The ratio of GAT1-mediated charge flux to GABA flux is not altered by SKF-89976A.
GABA uptake was performed under voltage clamp in the presence of 5 μM SKF-89976A (A-C) or 25 μM SKF-89976A (D-F) at −50 mV (B and E) and −90 mV (C and F). GABA was 500 μM and [3H]-GABA was 28 nM. In the presence of 5 μM SKF-89976A, the ratio of charge flux to GABA flux (e/GABA) was 1.9 ± 0.1 at −50 mV (n = 9) and 1.9 ± 0.1 at −90 mV (n = 10). In the presence of 25 μM SKF-89976A, the ratio of charge flux to GABA flux was 2.0 ± 0.1 at −50 mV (n = 8) and 2.1 ± 0.1 at −90 mV (n = 7). In each panel, the smooth line is a linear regression through the data points.
Despite numerous attempts in > 50 GAT1-expressing cells, we were not able to record a GABA-evoked outward current (even at Vm values more positive than VNa) (see Fig. 1, B and C). Further, inhibition of by SKF-89976A or NO-711 always blocked an inward current (irrespective of Vm) and not an outward current (see Fig. 1A). These results do not support the proposal that GABA evokes an uncoupled Na+ conductive mode in GAT1 (Krause and Schwarz, 2005).
The voltage-dependence of the GABA-evoked current (Fig. 1, B and C) prompted us to examine the relationship between the GABA-evoked current () and GABA uptake over a wide range of membrane potentials. If a GABA-evoked channel mode of GAT1 exists (Cammack et al., 1994; Risso et al., 1996; Krause and Schwarz, 2005), it is likely that the conductance of the channel mode would be different than that associated with Na+/Cl−/GABA cotransport, such that alterations in the driving force would differentially affect the current conducted via each of the two modes of function. Therefore, it is predicted that the relative contribution of the channel mode to the total GABA-evoked current would be different at different membrane potentials. Moreover, this difference should be reflected in the measured GAT1-mediated charge flux for every GABA molecule translocated across the plasma membrane. For the related dopamine (DAT, SLC6A3) and serotonin (SERT, SLC6A4) transporters, which have confirmed channel modes of function, such an experimental approach has provided evidence for substrate-evoked currents far in excess of substrate fluxes (Lin et al., 1996; Sonders et al., 1997; Quick, 2003; Carvelli et al., 2004). The experiments of Fig. 2 examined this possibility for GAT1.
In the experiments of Fig. 2, [3H]-GABA uptake was measured under voltage clamp at the indicated membrane potential (−110 mV to +10 mV) in individual GAT1-expressing cells. This experimental protocol yielded two measured parameters for each cell: (i) net GABA-evoked inward charge translocation during the recording period (charge flux), and (ii) GABA uptake in the same cell (GABA flux) (Fig. 2A). The ratio of charge flux to GABA flux was 2.1 ± 0.1 (−110 mV; n = 7), 2.0 ± 0.1 (−90 mV; n = 8), 2.1 ± 0.1 (−70 mV; n = 9), 2.0 ± 0.1 (−50 mV; n = 6), 1.9 ± 0.1 (−30 mV; n = 9), 2.0 ± 0.1 (−10 mV; n = 10), and 2.0 ± 0.1 (+10 mV; n = 7) (Fig. 2, B–H). Thus, in the membrane potential range −110 mV to +10 mV, the ratio of charge influx to GABA influx was 2 positive charges per GABA (Fig. 2I). These results suggest a fixed charge:GABA translocation stoichiometry, and argue against a GABA-evoked channel mode of GAT1 (Cammack et al., 1994; Risso et al., 1996; Krause and Schwarz, 2005).
To further examine the ratio of charge influx to GABA influx, we performed similar uptake under voltage clamp experiments in the presence of GAT1 competitive inhibitors SKF-89976A and NO-711 (Figs. 3 and 4). In these experiments, two concentrations of SKF-89976A were chosen (5 μM and 25 μM), and were based on the results of the data shown in Fig. 1. Based on the data of Fig. 1, at 500 μM GABA, 5 μM and 25 μM SKF-89976A block the GABA-evoked current by ∼30% and ∼65%, respectively. In the study of Krause and Schwarz (2005), it was reported that SKF-89976A blocks two modes of GAT1; an uncoupled channel mode (Ki = 0.03 μM) and a coupled Na+/Cl−/GABA cotransport mode (Ki = 9 μM). Thus, when used at 5 μM, SKF-89976A is expected to inhibit the putative GABA-gated channel mode by >99%. Based on the data of Krause and Schwarz (2005), we hypothesized that in the presence of SKF-89976A, the ratio of charge influx to GABA influx should decrease, as the putative GABA-evoked channel component would be blocked nearly completely. Contrary to this prediction, we observed that regardless of the SKF-89976A concentration (5 μM or 25 μM), the ratio of charge influx to GABA influx remained the same: 2 positive charges/GABA (Fig. 3). Moreover, the 2:1 charge flux to GABA flux ratio obtained in the presence of SKF-89976A was independent of the imposed membrane potential (−50 mV or −90 mV) (Fig. 3). These results argue against the existence of a GABA-evoked channel mode of GAT1 that is sensitive to SKF-89976A, as suggested previously (Risso et al., 1996; Krause and Schwarz, 2005).
Finally, we examined the ratio of charge influx to GABA influx in the presence of another high-affinity, competitive blocker of GAT1, NO-711 (Fig. 4). For these experiments, a concentration of 2 μM was chosen based on the data obtained in Fig. 1. At this concentration and in the presence of 500 μM GABA, NO-711 inhibits the GABA-evoked current by ∼60%. Similar to the results obtained with SKF-89976A blockade of GAT1 (Fig. 3), NO-711 did not alter the ratio of charge influx to GABA influx (Fig. 4). Further, this result was independent of the membrane potential (Fig. 4, B and C).
DISCUSSION
The purpose of the present study was to test a specific hypothesis put forth by Krause and Schwarz (2005) that GABA binding to the GABA transporter GAT1 evokes a GABA-gated uncoupled Na+ current that is mediated by a channel mode of GAT1. Overall, our findings did not support this hypothesis because (i) we did not detect an outward current evoked by GABA at membrane potentials more positive than the predicted Na+ equilibrium potential (VNa); (ii) inhibition of the GABA-evoked current by SKF-89976A or NO-711 did not reveal an outward current at any Vm; (iii) the ratio of GAT1-mediated charge flux to GABA flux was 2 positive charges per GABA and was independent of the membrane potential; and (iv) the 2:1 charge flux / GABA flux ratio was not altered in the presence of GAT1 competitive inhibitors SKF-89976A and NO-711.
Fixed Charge/GABA Translocation Stoichiometry of GAT1
Krause and Schwarz (2005) proposed that the GAT1-mediated GABA-evoked current has two components: (i) a transport-associated component, which is directly coupled to and results from electrogenic cotranslocation of 2 Na+, 1 Cl−, and 1 GABA across the plasma membrane, and (ii) an “uncoupled transmitter-gated current” that is carried by Na+ ions via a channel mode of GAT1. Implicit in this proposal is the fact that, under the zero-trans conditions of our experiments, a current-voltage (I-V) relationship of the GABA-evoked current should reveal an outward current at membrane potential values more positive than the Na+ equilibrium potential (VNa). As Krause and Schwarz (2005) did not extend the I-V relationship to voltages beyond zero mV, in this study, we measured the GABA-evoked current mediated by GAT1 at a wide voltage range from +100 mV down to −140 mV. Our results did not reveal an outward current at any voltage tested (Fig. 1B), and this result was consistent even when the external Na+ concentration was reduced to 50 mM so as to shift VNa to less positive values (Fig. 1C). Thus, our data are not consistent with the existence of a GABA-gated uncoupled current of GAT1 that is carried by Na+ ions.
Another proposal put forth by Krause and Schwarz (2005) was that the GABA-evoked transport-associated current and the putative uncoupled GABA-gated current of GAT1 exhibit differential sensitivity to the GAT1 inhibitor SKF-89976A with Ki values of 9 μM and 0.03 μM, respectively. Thus, it should be possible to expose GAT1 to a concentration of SKF-89976A that is high enough to completely inhibit the putative channel mode, while leaving the coupled mode of transport intact or only partially inhibited. Under such a treatment, SKF-89976A inhibition of GAT1 GABA-evoked current should reveal an outward current at membrane potential values more positive than VNa. Our examination of the effect of SKF-89976A on the I-V relationship of the GABA-evoked current was not consistent with this possibility (Fig. 1B). Therefore, at least based on the simple criterion of the thermodynamic Na+ equilibrium potential, our results are not consistent with the existence of a GABA-evoked uncoupled current of GAT1 that is carried by Na+ ions.
To further look for the existence of a GABA-evoked channel mode of GAT1, we performed GABA uptake experiments under voltage clamp at voltages ranging from −110 mV to +10 mV in order to correlate GAT1-mediated charge flux and GABA flux in individual cells expressing GAT1. We reasoned that the conductance of a putative channel mode of GAT1 is likely different than the conductance associated with Na+/Cl−/GABA cotransport, such that alterations in the driving force would differentially affect the magnitude of the currents mediated by the two modes of transporter function. Therefore, the relative contribution of the putative channel mode to the total current evoked by GABA should vary with the membrane potential. Consequently, the ratio of GAT1-mediated charge flux to GABA flux should be different at different membrane potentials. If present, this channel mode of GAT1 function may account for the variable ion/GABA stoichiometry suggested by Cammack et al. (1994). The results of our experiments were not consistent with this scenario, as the ratio of GAT-mediated charge flux to GABA flux was 2 net positive charges per GABA molecule at all voltages examined (−110 mV to +10 mV) (Fig. 2), suggesting that, similar to the glycine transporters GlyT1b (SLC6A9) and GlyT2a (SLC6A5) (Roux and Supplisson, 2000), GAT1 GABA-evoked currents are directly proportional to GABA fluxes. Thus, these results do not support the existence of a GABA-evoked uncoupled current of GAT1, and differ from those reported for the related dopamine (DAT, SLC6A3) and serotonin (SERT, SLC6A4) transporters, which have been shown to possess a channel mode of function and in which the ratio of charge flux to substrate flux varies with the membrane potential (Lin et al., 1996; Sonders et al., 1997; Quick, 2003; Carvelli et al., 2004). Previously, we also demonstrated that GAT1-mediated charge flux and GABA flux are tightly coupled at different GABA concentrations (25–500 μM) as well as at different temperatures (21–32 °C) (Gonzales et al., 2007). Altogether, the data suggest tight coupling of GAT1-mediated GABA and charge translocation across the plasma membrane. The data are not consistent with a variable ion/GABA transport stoichiometry (Cammack et al., 1994).
As discussed above, Krause and Schwarz (2005) proposed that a fraction of the electrogenic signal evoked by GABA results from the putative uncoupled mode, and that this mode can be inhibited by SKF-89976A with a Ki of 0.03 μM. Thus, if uptake under voltage clamp experiments are performed in the presence of SKF-89976A, the resulting charge flux to GABA flux should be closer to 1 net positive charge per GABA, as predicted by the 2 Na+ : 1 Cl− : 1 GABA transport stoichiometry. To test this possibility, we performed GABA uptake under voltage clamp in the presence of two different concentrations of SKF-89976A, which ensured complete inhibition of the putative channel mode proposed by Krause and Schwarz (2005), but only partially inhibited the cotransport mode. Our results demonstrate that SKF-89976A inhibition of GAT1 does not alter the ratio of charge flux to GABA flux (Fig. 3). These results do not lend support to the hypothesis that a fraction of the GAT1 electrogenic signal is uncoupled and can be blocked by SKF-89976A. Similar data were obtained with another high-affinity, selective, and competitive GAT1 inhibitor (NO-711) (Fig. 4).
Studies of the effects of SKF-89976A and NO-711 on the presteady-state currents of GAT1 evoked by voltage and/or concentration jumps suggest that, by competing for the GABA binding site, these inhibitors lock the transporter in a conformation that does not allow the transport cycle to be completed (Mager et al., 1996; Lu and Hilgemann, 1999b; Li et al., 2000; Forlani et al., 2001; Hirayama et al., 2001; Krause and Schwarz, 2005; Soragna et al., 2005; Meinild et al., 2009). Thus, we propose that by competing for the GABA binding site, sub-saturating concentrations of GAT1 blockers (SKF-89976A and NO-711) merely reduce the number of active GAT1 molecules that participate in the transport process. The charge/GABA ratio of the GAT1 transport cycle, as judged by the ratio of inward charge flux to GABA flux, is not altered either at low or high SKF-89976A concentrations. Our results are not consistent with the notion that GAT1 inhibitors uncouple charge flux and GABA flux (Eckstein-Ludwig et al., 1999; Krause and Schwarz, 2005).
Uncoupled Modes of Conduction in GABA Transporters
Uncoupled modes of ion conduction have been proposed for a number of electrogenic Na+-coupled transporters including the neurotransmitter transporters (Sonders and Amara, 1996; DeFelice and Goswami, 2007; Andrini et al., 2008; Vandenberg et al., 2008), and are defined as transporter-mediated ion translocation across the plasma membrane that is not thermodynamically coupled to substrate translocation. In general, uncoupled or “leak” currents can be classified as those that are detected in the absence of the substrate, and those that are evoked by substrate binding to the transporter. In both cases, electrophysiological methods have served a pivotal role in identifying and characterizing these uncoupled modes of transporter function.
Evidence for the existence of a GABA-independent uncoupled current in GAT1 appears to depend on the experimental system used. When examined at normal extracellular Na+ and Cl− concentrations and in the absence of GABA, all studies using the Xenopus oocyte expression system have failed to detect an uncoupled or leak current in GAT1 (Mager et al., 1993; Lu and Hilgemann, 1999a; Karakossian et al., 2005; Krause and Schwarz, 2005; Gonzales et al., 2007; current study). However, when the extracellular Na+ is replaced with Li+, the GABA transporters, GAT1 (SLC6A1), GAT3 (SLC6A13), and GAT4 (SLC6A11), exhibit an uncoupled Li+ leak current, which is sensitive to external Na+, and has been proposed to result from a channel mode of transporter function (Mager et al., 1996; Bismuth et al., 1997; MacAulay et al., 2002; Grossman and Nelson, 2003; Kanner, 2003; Karakossian et al., 2005; Zhou et al., 2006; Meinild et al., 2009). Remarkably, when the extracellular Na+ is replaced by a large cation that does not interact with the transporter (e.g., choline or tetraethylammonium), GAT3 (SLC6A13) and GAT4 (SLC6A11) exhibit a Na+-inhibited Cl− leak current which is believed to result from a channel mode of function (Karakossian et al., 2005). The Na+-inhibited Cl− channel mode does not exist in GAT1 (Karakossian et al., 2005).
On the other hand, earlier studies which examined GAT1 expressed in mammalian cell lines detected GABA-independent uncoupled currents that were believed to result from a channel mode of transporter function (Cammack et al., 1994; Cammack and Schwartz, 1996). Using a human embryonic kidney cell line (HEK-293) stably transfected with rat GAT1, Cammack and Schwartz (1996) provided evidence for GAT1-mediated single-channel currents in the absence of GABA. The open-state probability of the channel mode was very low (0.02–0.06), and it was predicted that only a small fraction of the transporters operated in this mode at any given time. This finding suggests that when over-expressed in Xenopus oocytes that can insert up to 1011 transporter copies in the plasma membrane of individual GAT1-expressing cells (Gonzales et al., 2007), a GABA-independent macroscopic leak current should be detectable. As described above, no such currents are detected in oocytes expressing GAT1, and different expression systems may be responsible for the discrepant results obtained by different investigators.
The existence of a GABA-evoked channel mode of GAT1 appears to be more controversial and cannot be attributed to different expression systems. Most studies using the Xenopus oocyte expression system have failed to observe GAT1-mediated GABA-evoked uncoupled currents (Lu and Hilgemann, 1999a; Loo et al., 2000; Gonzales et al., 2007; current study). However, as discussed above and in the Introduction, using the oocyte expression system, Krause and Schwarz (2005) proposed the existence of a GABA-gated, Na+-conductive channel mode of GAT1 that exhibits much greater sensitivity to SKF-89976A than does the cotransport mode of GAT1. The results of the experiments presented in this study are not consistent with their proposal. Rather, our results suggest tight coupling of GAT1-mediated charge flux and GABA flux. We propose that at least in the Xenopus oocyte expression system and under our experimental conditions, GAT1 does not have a GABA-evoked uncoupled mode of function. In contrast, two earlier studies, which examined GAT1 expressed in mammalian cells (HEK-293 and HeLa cells), proposed the existence of GABA-evoked uncoupled currents which arise from a channel mode of function (Cammack et al., 1994; Risso et al., 1996). However, in a more recent study of GAT1 transiently expressed in HEK-293 cells, the steady-state GABA-evoked current was suggested to be consistent with the accepted 2 Na+ : 1 Cl− : 1 GABA stoichiometry (Bicho and Grewer, 2005). Clearly, additional studies are needed to probe this issue further.
Although our data argue against the existence of a channel mode of conduction in GAT1, it is important to emphasize that there is strong evidence for channel modes of function in other neurotransmitter transporters (Lester et al., 1996; Sonders and Amara, 1996; DeFelice and Goswami, 2007; Vandenberg et al., 2008). A substrate-evoked channel mode of function has been shown for the serotonin (SLC6A4; Mager et al., 1994; Lin et al., 1996; Galli et al., 1997), dopamine (SLC6A3; Sonders et al., 1997; Carvelli et al., 2004), and norepinephrine (SLC6A2; Galli et al., 1995, 1996, 1998) transporters, as well as for all five glutamate transporter isoforms (EAAT1–5; SLC1 gene family) (Fairman et al., 1995; Wadiche et al., 1995; Arriza et al., 1997).
It is also important to note that not all uncoupled currents of electrogenic transporters result from a channel mode of function. For example, in the absence of the respective substrate, the Na+/glucose cotransporter (SGLT1; SLC5 gene family) and type II Na+/Pi cotransporter (SLC34 gene family) exhibit leak currents that are carried by Na+ ions in a uniport fashion and are thought to result from transporter conformational changes as characterized by the classical alternating access model (Panayotova-Heiermann et al., 1998; Andrini et al., 2008).
Whether the channel mode of neurotransmitter transporters serves an important physiological role remains to be determined. In the case of the Li+ channel mode of GAT1 and GAT4, and the Cl− channel mode of GAT4, a physiological role is unlikely, as these modes are inhibited entirely at physiological Na+ concentrations of the extracellular fluid (MacAulay et al., 2002; Kanner, 2003; Karakossian et al., 2005). On the other hand, the currents associated with the Cl− channel mode of the dopamine transporter appear to play an important role in setting the firing rate of midbrain dopaminergic neurons (Ingram et al., 2002). Moreover, the glutamate evoked Cl− conductance of the glutamate transporter (EAAT5) expressed in rod bipolar cells leads to hyperpolarization and, hence, a reduction in transmitter release (Veruki et al., 2006). It has also been suggested that leak currents in transport systems may be evolutionarily built-in features that serve to dissipate abrupt changes in ion and/or substrate concentrations in the immediate vicinity of transporters (Nelson et al., 2002).
Although we are not any closer to understanding the discrepancy between the predicted and experimentally measured number of charges translocated per GABA molecule transported into the cell, our current results cast doubt on the possibility that a fraction of the GAT1-mediated GABA-evoked current results from a channel mode of transporter function, as suggested previously (Cammack et al., 1994; Risso et al., 1996; Krause and Schwarz, 2005). Thus, unlike the related monoamine transporters which have channel modes of conduction and exhibit variable and voltage-dependent charge/substrate stoichiometry (DeFelice and Goswami, 2007), GAT1 does not appear to have a channel mode of conduction, and appears to operate with a fixed charge:GABA translocation stoichiometry.
Conclusions
Uptake under voltage clamp experiments performed on GAT1 expressed in Xenopus laevis oocytes demonstrate that for every GABA molecule transported into the cell, GAT1 mediates the net inward translocation of two positive charges across the plasma membrane. While we have not resolved the discrepancy between the predicted (1 net charge) and measured (2 net charges) number of net positive charges translocated per GABA molecule per transport cycle, our data do suggest that GAT-mediated charge and GABA translocation into the cell are tightly coupled processes within a wide range of membrane potential values (−110 mV to +10 mV), as well as in the presence of selective GAT1 inhibitors (SKF-89976A and NO-711). Importantly, the competitive GAT1 inhibitors, SFK-89976A and NO-711, do not uncouple charge flux and GABA flux. Altogether, our data are not consistent with the existence of a channel mode of conduction in GAT1 (in the absence or presence of GABA). Moreover, our data suggest that the ion/GABA transport stoichiometry of GAT1 is fixed.
ACKNOWLEDGEMENTS
We thank Matthew J. Maestas, Jaison J. Omoto, Tiffany A. Steele, and Stephen J. Byrne for technical assistance. This work was supported by a U.S. National Institute of General Medical Sciences grant awarded to S.E. (SC1GM086344).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andrini O, Ghezzi C, Murer H, Forster IC. The leak mode of type II Na+-Pi cotransporters. Channels (Austin) 2008;2:346–357. doi: 10.4161/chan.2.5.6900. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicho A, Grewer C. Rapid substrate-induced charge movements of the GABA transporter GAT1. Biophys. J. 2005;89:211–231. doi: 10.1529/biophysj.105.061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bismuth Y, Kavanaugh MP, Kanner BI. Tyrosine 140 of the γ-aminobutyric acid transporter GAT-1 plays a critical role in neurotransmitter recognition. J. Biol. Chem. 1997;272:16096–16102. doi: 10.1074/jbc.272.26.16096. [DOI] [PubMed] [Google Scholar]
- Borden LA. GABA transporter heterogeneity: Pharmacology and cellular localization. Neurochem. Int. 1996;29:335–356. doi: 10.1016/0197-0186(95)00158-1. [DOI] [PubMed] [Google Scholar]
- Busch W, Saier MH., Jr. The transporter classification (TC) system, 2002. Crit. Rev. Biochem. Mol. Biol. 2002;37:287–337. doi: 10.1080/10409230290771528. [DOI] [PubMed] [Google Scholar]
- Cammack JN, Rakhilin SV, Schwartz EA. A GABA transporter operates asymmetrically and with variable stoichiometry. Neuron. 1994;13:949–960. doi: 10.1016/0896-6273(94)90260-7. [DOI] [PubMed] [Google Scholar]
- Cammack JN, Schwartz EA. Channel behavior in a γ-aminobutyrate transporter. Proc. Natl. Acad. Sci. U.S.A. 1996;93:723–727. doi: 10.1073/pnas.93.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L, McDonald PW, Blakely RD, Defelice LJ. Dopamine transporters depolarize neurons by a channel mechanism. Proc. Natl. Acad. Sci. U.S.A. 2004;101:16046–16051. doi: 10.1073/pnas.0403299101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NH, Reith ME, Quick MW. Synaptic uptake and beyond: the sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflugers Arch. 2004;447:519–531. doi: 10.1007/s00424-003-1064-5. [DOI] [PubMed] [Google Scholar]
- Clark JA, Amara SG. Stable expression of a neuronal γ-aminobutyric acid transporter, GAT-3, in mammalian cells demonstrates unique pharmacological properties and ion dependence. Mol. Pharmacol. 1994;46:550–557. [PubMed] [Google Scholar]
- Clark JA, Deutch AY, Gallipoli PZ, Amara SG. Functional expression and CNS distribution of a β-alanine-sensitive neuronal GABA transporter. Neuron. 1992;9:337–348. doi: 10.1016/0896-6273(92)90172-a. [DOI] [PubMed] [Google Scholar]
- Conti F, Minelli A, Melone M. GABA transporters in the mammalian cerebral cortex: localization, development and pathological implications. Brain Res. Brain Res. Rev. 2004;45:196–212. doi: 10.1016/j.brainresrev.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Dalby NO. Inhibition of γ-aminobutyric acid uptake: anatomy, physiology and effects against epileptic seizures. Eur. J. Pharmacol. 2003;479:127–137. doi: 10.1016/j.ejphar.2003.08.063. [DOI] [PubMed] [Google Scholar]
- DeFelice LJ, Goswami T. Transporters as channels. Annu. Rev. Physiol. 2007;69:87–112. doi: 10.1146/annurev.physiol.69.031905.164816. [DOI] [PubMed] [Google Scholar]
- Eckstein-Ludwig U, Fei J, Schwarz W. Inhibition of uptake, steady-state currents, and transient charge movements generated by the neuronal GABA transporter by various anticonvulsant drugs. Br. J. Pharmacol. 1999;128:92–102. doi: 10.1038/sj.bjp.0702794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandari S, Loo DD, Dai G, Levy O, Wright EM, Carrasco N. Thyroid Na+/I− symporter. Mechanism, stoichiometry, and specificity. J. Biol. Chem. 1997;272:27230–27238. doi: 10.1074/jbc.272.43.27230. [DOI] [PubMed] [Google Scholar]
- Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- Forlani G, Bossi E, Ghirardelli R, Giovannardi S, Binda F, Bonadiman L, Ielmini L, Peres A. Mutation K448E in the external loop 5 of rat GABA transporter rGAT1 induces pH sensitivity and alters substrate interactions. J. Physiol. (Lond.) 2001;536:479–494. doi: 10.1111/j.1469-7793.2001.0479c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster IC, Loo DD, Eskandari S. Stoichiometry and Na+ binding cooperativity of rat and flounder renal type II Na+-Pi cotransporters. Am. J. Physiol. 1999;276:F644–F649. doi: 10.1152/ajprenal.1999.276.4.F644. [DOI] [PubMed] [Google Scholar]
- Galli A, Blakely RD, DeFelice LJ. Norepinephrine transporters have channel modes of conduction. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8671–8676. doi: 10.1073/pnas.93.16.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli A, Blakely RD, DeFelice LJ. Patch-clamp and amperometric recordings from norepinephrine transporters: Channel activity and voltage-dependent uptake. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13260–13265. doi: 10.1073/pnas.95.22.13260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli A, DeFelice LJ, Duke BJ, Moore KR, Blakely RD. Sodium-dependent norepinephrine-induced currents in norepinephrine-transporter-transfected HEK-293 cells blocked by cocaine and antidepressants. J. Exp. Biol. 1995;198:2197–2212. doi: 10.1242/jeb.198.10.2197. [DOI] [PubMed] [Google Scholar]
- Galli A, Petersen CI, deBlaquiere M, Blakely RD, DeFelice LJ. Drosophila serotonin transporters have voltage-dependent uptake coupled to a serotonin-gated ion channel. J. Neurosci. 1997;17:3401–3411. doi: 10.1523/JNEUROSCI.17-10-03401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales AL, Lee W, Spencer SR, Oropeza RA, Chapman JV, Ku JY, Eskandari S. Turnover rate of the γ-aminobutyric acid transporter GAT1. J. Membr. Biol. 2007;220:33–51. doi: 10.1007/s00232-007-9073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman TR, Nelson N. Effect of sodium lithium and proton concentrations on the electrophysiological properties of the four mouse GABA transporters expressed in Xenopus oocytes. Neurochem. Int. 2003;43:431–443. doi: 10.1016/s0197-0186(03)00032-9. [DOI] [PubMed] [Google Scholar]
- Hirayama BA, Díez-Sampedro A, Wright EM. Common mechanisms of inhibition for the Na+/glucose (hSGLT1) and Na+/Cl−/GABA (hGAT1) cotransporters. Br. J. Pharmacol. 2001;134:484–495. doi: 10.1038/sj.bjp.0704274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram SL, Prasad BM, Amara SG. Dopamine transporter-mediated conductances increase excitability of midbrain dopamine neurons. Nat. Neurosci. 2002;5:971–978. doi: 10.1038/nn920. [DOI] [PubMed] [Google Scholar]
- Kanner BI. Transmembrane domain I of the γ-aminobutyric acid transporter GAT-1 plays a crucial role in the transition between cation leak and transport modes. J. Biol. Chem. 2003;278:3705–3712. doi: 10.1074/jbc.M210525200. [DOI] [PubMed] [Google Scholar]
- Kanner BI, Schuldiner S. Mechanism of transport and storage of neurotransmitters. CRC Crit. Rev. Biochem. 1987;22:1–38. doi: 10.3109/10409238709082546. [DOI] [PubMed] [Google Scholar]
- Karakossian MH, Spencer SR, Gomez AQ, Padilla OR, Sacher A, Loo DD, Nelson N, Eskandari S. Novel properties of a mouse γ-aminobutyric acid transporter (GAT4) J. Membr. Biol. 2005;203:65–82. doi: 10.1007/s00232-004-0732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynan S, Kanner BI. γ-Aminobutyric acid transport in reconstituted preparations from rat brain: Coupled sodium and chloride fluxes. Biochemistry. 1988;27:12–17. doi: 10.1021/bi00401a003. [DOI] [PubMed] [Google Scholar]
- Keynan S, Suh YJ, Kanner BI, Rudnick G. Expression of a cloned γ-aminobutyric acid transporter in mammalian cells. Biochemistry. 1992;31:1974–1979. doi: 10.1021/bi00122a011. [DOI] [PubMed] [Google Scholar]
- Krause S, Schwarz W. Identification and selective inhibition of the channel mode of the neuronal GABA transporter 1. Mol. Pharmacol. 2005;68:1728–1735. doi: 10.1124/mol.105.013870. [DOI] [PubMed] [Google Scholar]
- Lester HA, Cao Y, Mager S. Listening to neurotransmitter transporters. Neuron. 1996;17:807–810. doi: 10.1016/s0896-6273(00)80213-5. [DOI] [PubMed] [Google Scholar]
- Li M, Farley RA, Lester HA. An intermediate state of the γ-aminobutyric acid transporter GAT1 revealed by simultaneous voltage clamp and fluorescence. J. Gen. Physiol. 2000;115:491–508. doi: 10.1085/jgp.115.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Lester HA, Mager S. Single-channel currents produced by the serotonin transporter and analysis of a mutation affecting ion permeation. Biophys. J. 1996;71:3126–3135. doi: 10.1016/S0006-3495(96)79506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo DD, Eskandari S, Boorer KJ, Sarkar HK, Wright EM. Role of Cl− in electrogenic Na+-coupled cotransporters GAT1 and SGLT1. J. Biol. Chem. 2000;275:37414–37422. doi: 10.1074/jbc.M007241200. [DOI] [PubMed] [Google Scholar]
- Lu CC, Hilgemann DW. GAT1 (GABA:Na+:Cl−) cotransport function. Steady state studies in giant Xenopus oocyte membrane patches. J. Gen. Physiol. 1999a;114:429–444. doi: 10.1085/jgp.114.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CC, Hilgemann DW. GAT1 (GABA:Na+:Cl−) cotransport function. Kinetic studies in giant Xenopus oocyte membrane patches. J. Gen. Physiol. 1999b;114:445–457. doi: 10.1085/jgp.114.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAulay N, Zeuthen T, Gether U. Conformational basis for the Li+-induced leak current in the rat γ-aminobutyric acid (GABA) transporter-1. J. Physiol. (Lond.) 2002;544:447–458. doi: 10.1113/jphysiol.2002.022897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager S, Kleinberger-Doron N, Keshet GI, Davidson N, Kanner BI, Lester HA. Ion binding and permeation at the GABA transporter GAT1. J. Neurosci. 1996;16:5405–5414. doi: 10.1523/JNEUROSCI.16-17-05405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager S, Min C, Henry DJ, Chavkin C, Hoffman BJ, Davidson N, Lester HA. Conducting states of a mammalian serotonin transporter. Neuron. 1994;12:845–859. doi: 10.1016/0896-6273(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Mager S, Naeve J, Quick M, Labarca C, Davidson N, Lester HA. Steady states, charge movements, and rates for a cloned GABA transporter expressed in Xenopus oocytes. Neuron. 1993;10:177–188. doi: 10.1016/0896-6273(93)90309-f. [DOI] [PubMed] [Google Scholar]
- Matskevitch I, Wagner CA, Stegen C, Bröer S, Noll B, Risler T, Kwon HM, Handler JS, Waldegger S, Busch AE, Lang F. Functional characterization of the betaine/γ-aminobutyric acid transporter BGT-1 expressed in Xenopus oocytes. J. Biol. Chem. 1999;274:16709–16716. doi: 10.1074/jbc.274.24.16709. [DOI] [PubMed] [Google Scholar]
- Meinild AK, Loo DD, Skovstrup S, Gether U, Macaulay N. Elucidating conformational changes in the γ-aminobutyric acid (GABA)-transporter-1. J. Biol. Chem. 2009;284:16226–16235. doi: 10.1074/jbc.M109.003137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhoul NL, Hering-Smith KS, Abdulnour-Nakhoul SM, Hamm LL. Ammonium interaction with the epithelial sodium channel. Am. J. Physiol. Renal Physiol. 2001;281:F493–F502. doi: 10.1152/ajprenal.2001.281.3.F493. [DOI] [PubMed] [Google Scholar]
- Nelson H, Mandiyan S, Nelson N. Cloning of the human brain GABA transporter. FEBS Lett. 1990;269:181–184. doi: 10.1016/0014-5793(90)81149-i. [DOI] [PubMed] [Google Scholar]
- Nelson N. The family of Na+/Cl− neurotransmitter transporters. J. Neurochem. 1998;71:1785–1803. doi: 10.1046/j.1471-4159.1998.71051785.x. [DOI] [PubMed] [Google Scholar]
- Nelson N, Sacher A, Nelson H. The significance of molecular slips in transport systems. Nat. Rev. Mol. Cell Biol. 2002;3:876–881. doi: 10.1038/nrm955. [DOI] [PubMed] [Google Scholar]
- Panayotova-Heiermann M, Loo DD, Lam JT, Wright EM. Neutralization of conservative charged transmembrane residues in the Na+/glucose cotransporter SGLT1. Biochemistry. 1998;37:10522–10528. doi: 10.1021/bi9800395. [DOI] [PubMed] [Google Scholar]
- Quick MW. Regulating the conducting states of a mammalian serotonin transporter. Neuron. 2003;40:537–549. doi: 10.1016/s0896-6273(03)00605-6. [DOI] [PubMed] [Google Scholar]
- Radian R, Kanner BI. Stoichiometry of sodium- and chloride-coupled γ-aminobutyric acid transport by synaptic plasma membrane vesicles isolated from rat brain. Biochemistry. 1983;22:1236–1241. doi: 10.1021/bi00274a038. [DOI] [PubMed] [Google Scholar]
- Richerson GB, Wu Y. Dynamic equilibrium of neurotransmitter transporters: Not just for reuptake anymore. J. Neurophysiol. 2003;90:1363–1374. doi: 10.1152/jn.00317.2003. [DOI] [PubMed] [Google Scholar]
- Risso S, DeFelice LJ, Blakely RD. Sodium-dependent GABA-induced currents in GAT1-transfected HeLa cells. J. Physiol. (Lond.) 1996;490(Pt 3):691–702. doi: 10.1113/jphysiol.1996.sp021178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux MJ, Supplisson S. Neuronal and glial glycine transporters have different stoichiometries. Neuron. 2000;25:373–383. doi: 10.1016/s0896-6273(00)80901-0. [DOI] [PubMed] [Google Scholar]
- Sacher A, Nelson N, Ogi JT, Wright EM, Loo DD, Eskandari S. Presteady-state and steady-state kinetics and turnover rate of the mouse γ-aminobutyric acid transporter (mGAT3) J. Membr. Biol. 2002;190:57–73. doi: 10.1007/s00232-002-1024-6. [DOI] [PubMed] [Google Scholar]
- Saier MH, Jr., Tran CV, Barabote RD. TCDB: the Transporter Classification Database for membrane transport protein analyses and information. Nucleic Acids Res. 2006;34:D181–D186. doi: 10.1093/nar/gkj001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segel IH. Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems. John Wiley & Sons, Inc.; New York, NY: 1975. Enzyme Kinetics. [Google Scholar]
- Sonders MS, Amara SG. Channels in transporters. Curr. Opin. Neurobiol. 1996;6:294–302. doi: 10.1016/s0959-4388(96)80111-5. [DOI] [PubMed] [Google Scholar]
- Sonders MS, Zhu SJ, Zahniser NR, Kavanaugh MP, Amara SG. Multiple ionic conductances of the human dopamine transporter: The actions of dopamine and psychostimulants. J. Neurosci. 1997;17:960–974. doi: 10.1523/JNEUROSCI.17-03-00960.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soragna A, Bossi E, Giovannardi S, Pisani R, Peres A. Relations between substrate affinities and charge equilibration rates in the rat GABA cotransporter GAT1. J. Physiol. (Lond.) 2005;562:333–345. doi: 10.1113/jphysiol.2004.076703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg RJ, Huang S, Ryan RM. Slips, leaks and channels in glutamate transporters. Channels (Austin) 2008;2:51–58. doi: 10.4161/chan.2.1.6047. [DOI] [PubMed] [Google Scholar]
- Veruki ML, Mørkve SH, Hartveit E. Activation of a presynaptic glutamate transporter regulates synaptic transmission through electrical signaling. Nat. Neurosci. 2006;9:1388–1396. doi: 10.1038/nn1793. [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Amara SG, Kavanaugh MP. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995;15:721–728. doi: 10.1016/0896-6273(95)90159-0. [DOI] [PubMed] [Google Scholar]
- Whitlow RD, Sacher A, Loo DD, Nelson N, Eskandari S. The anticonvulsant valproate increases the turnover rate of γ-aminobutyric acid transporters. J. Biol. Chem. 2003;278:17716–17726. doi: 10.1074/jbc.M207582200. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang W, Díez-Sampedro A, Richerson GB. Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron. 2007;56:851–865. doi: 10.1016/j.neuron.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zomot E, Kanner BI. Identification of a lithium interaction site in the γ-aminobutyric acid (GABA) transporter GAT-1. J. Biol. Chem. 2006;281:22092–22099. doi: 10.1074/jbc.M602319200. [DOI] [PubMed] [Google Scholar]
- Zomot E, Bendahan A, Quick M, Zhao Y, Javitch JA, Kanner BI. Mechanism of chloride interaction with neurotransmitter:sodium symporters. Nature. 2007;449:726–730. doi: 10.1038/nature06133. [DOI] [PubMed] [Google Scholar]