Abstract
It is widely accepted that addictive drug use is related to abnormal functional organization in the user’s brain. The present study aimed to identify this type of abnormality within the brain networks implicated in addiction by resting-state functional connectivity measured with functional magnetic resonance imaging (fMRI). With fMRI data acquired during resting state from 14 chronic heroin users (12 of whom were being treated with methadone) and 13 non-addicted controls, we investigated the addiction related alteration in functional connectivity between the regions in the circuits implicated in addiction with seed-based correlation analysis. Compared with controls, chronic heroin users showed increased functional connectivity between nucleus accumbens and ventral/rostral anterior cingulate cortex (ACC), and orbital frontal cortex (OFC), between amygdala and OFC; and reduced functional connectivity between prefrontal cortex and OFC, and ACC. These observations of altered resting-state functional connectivity suggested abnormal functional organization in the addicted brain and may provide additional evidence supporting the theory of addiction that emphasizes enhanced salience value of a drug and its related cues but weakened cognitive control in the addictive state.
Introduction
Drug addiction is a major health problem in modern society. It is characterized by the failure to resist one's impulses to obtain and take certain types of addictive drugs despite serious negative consequences (Volkow and Li, 2004). Chronic addictive drug use is often related to abnormal functional organization in the brain, which leads to habitually hypersensitivity to the drug and drug-related cues and ensures their compulsive patterns of drug-seeking behavior (Kalivas and Volkow, 2005).
Resting-state functional connectivity is assessed by the correlation of spontaneous fluctuations of blood oxygen level-dependent (BOLD) signals in different regions of the “resting” brain and is thought to provide a measure of its functional organization (Fox and Raichle, 2007). Studies have outlined a number of resting-state networks corresponding to critical brain functional organizations including movement, vision, audition, language, episodic memory, executive function, and salience detection (Fox and Raichle, 2007). During the acquisition of resting-state functional magnetic resonance imaging (fMRI) data, participants are asked to rest quietly instead of performing tasks, making it potentially more readily applicable than functional activation MRI in clinical settings. A number of groups have begun to study the resting state connectivity in a variety of neuropsychiatric disorders such as Alzheimer’s disease, depression, and schizophrenia (Greicius, 2008; Liu et al., 2007; Wang et al., 2007; Zhou et al., 2008).
According to models of addiction, the main brain regions underlying addiction make up a network of, at a minimum, four interdependent and overlapping circuits (Baler and Volkow, 2006): (i) reward, involving the nucleus accumbens and ventral pallidum (ii) memory and learning, including the amygdala and hippocampus; (iii) cognitive control, located in the prefrontal cortex and dorsal anterior cingulate cortex; and (iv) motivation and/or drive and salience evaluation, located in the orbital frontal cortex. In addition, amygdala and ventral/rostral anterior cingulate cortex (including subgenual area), regions that are associated with craving and emotional regulation, are also likely to affect the reactivity of the above circuits and parts of this network (Bechara, 2005; Bush et al., 2000; Volkow et al., 2005). These regions are modulated by dopamine and interconnected mostly through glutamatergic and GABA-ergic projections. The interactions between these regions are integrated to generate the behavioral output toward a reinforcing stimulus (O'Doherty, 2004), such that addictive drugs intensely activate the reward and motivation circuits, usurp systems underlying reward-related learning and memory, and hijack cognitive control resources (Baler and Volkow, 2006; Bechara, 2005; Everitt and Robbins, 2005; Garavan and Hester, 2007; Goldstein and Volkow, 2002). As a result, under addiction, the saliency value of a drug and its related cues are enhanced, while the inhibitory control is weakened, setting up the stage for an unrestrained cycle which leads to compulsive drug-seeking without regard to its negative consequences.
The neurobiological foundation of these models is mainly based on the results of functional activation studies in human addicts, and the observation that exposure to addictive drugs produces persistent structural and functional changes on cells within this network (Garavan et al., 2000; Goldstein et al., 2007; Kalivas and O'Brien, 2008; Tomasi et al., 2007; Yang et al., 2009). To date, the resting-state functional connectivity within the key regions of drug addiction has not been extensively studied in human addicts. Therefore, in this study, we investigated whether there is any addiction related alteration in resting-state functional connectivity in this network with fMRI data acquired during resting state from chronic heroin users and non-addicted controls.
Methods and Materials
Participants
Twenty seven right-handed male volunteers, including 14 chronic heroin users (HU, heroin using (from the time of their initial heroin use until the time of scanning) for 7.11 ± 2.82 years, range from 2 to 10 years) and 13 non-addicted controls (CN), participated in this study. All HU were recruited from Anhui Detoxification and Rehabilitation Center (Hefei, Anhui province, China), sought medical help on their own initiative and had a DSM-IV diagnosis of heroin dependence or abuse and urine tests positive for heroin before enrolling in the treatment program. According to an interview conducted by a clinical psychologist, all of the patients had never used any other types of illicit drugs, were free of illnesses that required hospitalization or regular monitoring and were deemed to be stable and able to participate in the experiment. Before the fMRI scanning, except 2 of HU who were current heroin users and not under any treatment, the HU were under a methadone treatment and had no illicit drug use during the treatment as confirmed by their care takers. Among the 12 HU under the treatment, 9 were in the detoxification phase (entering the program within one week before the scanning, mean = 2.1 days, SD = 1.8), while the remaining 3 were in a relatively stable maintenance phase (one had been in the program for 2 months and the other two for 6 months). All methadone-treated participants were under daily methadone administrations and their last methadone uses were at least 12 hours before the scanning.
The control (CN) participants were recruited through advertisements and compensated for their time, and none of them reported a history of head injury, psychiatric disorders or substance dependence (other than cigarette smoking). Only male participants were selected as gender effect was not a focus of this study. Both cohorts of HU and CN were current tobacco users. The HU and CN were matched in age (HU, 30.1 ± 5.3 years, range from 22 to 39 years; CN, 29.8 ± 7.2 years, range from 20 to 39 years; t(25) = 0.093, ns) and years of education (HU, 9.71 ± 2.7, range from 5 to 14; CN, 10.8 ± 1.6, range from 8 to 13; t(25) = −1.296, ns). After complete description of the study to the participants, written informed consent was obtained from them for their involvement in this study in accordance with the review board of University of Science & Technology of China.
Imaging
Scanning and image preprocessing
All imaging data were obtained on the 3T Siemens Magnetom Trio scanner (Siemens Medical Solutions, Erlangen, Germany) in the Anhui Provincial Hospital. A circularly polarized head coil was used, with foam padding to restrict head motion. Functional images were acquired with a T2*-weighted echo-planar imaging sequence (TE = 30ms, TR = 2s, FOV = 24cm, Matrix=64×64) with 22 axial slices (slice gap = 0.4 mm, voxel size: 3.75×3.75×4 mm3), covering the parietal lobe, the occipital lobe and a large portion of the frontal lobe and the temporal lobe (the slices were approximately along the AC-PC line and covered about −30 to 60 in the IS direction). Resting-state fMRI data were acquired with one run of six minutes (180 images per slice). Corresponding high-resolution T1-weighted spin-echo (for anatomical overlay) images and three-dimensional gradient-echo (for stereotaxic transformation) images were also collected. Before entering the scanner, all participants were told to close their eyes, remain still and relaxed, and stay awake during the scanning. After the resting-state scanning, several functional activation runs were acquired with cognitive tasks (data to appear elsewhere). All participants could response to these tasks immediately after the resting-state scanning suggesting they may not asleep during the resting state. Before and after the scanning, according to the reports of a clinical psychologist, all the heroin users were ensured to be at a stable state and not intoxicated during the scanning.
The imaging data were mainly processed with Analysis of Functional Neuroimages (AFNI) (Cox, 1996). For each participant, the first four time points were discarded to account for the approach to steady state in the BOLD signal. The raw data were corrected for temporal shifts between slices, corrected for head motion, spatially smoothed with a Gaussian kernel (full width at half maximum = 4 mm) and temporally normalized (for each voxel, the signal of each image was divided by the temporally averaged signal.). We totally scanned 17 HU and 16 CN participants and discarded the data of participants whose head moved more than 1.0 mm in any dimension through the resting-state run. Data of 14 HU and 13 CN participants met the movement criterion and appeared in this study. To further reduce the effect of motion and obtain low frequency fluctuation, we regressed the motion data out of the time series and then preformed band pass temporal filtering (0.01Hz to 0.08 Hz) on the residual signals (Auer, 2008; Birn et al., 2006). These preprocessed time series were used in the subsequent seed-base regions of interest (ROI) correlation analysis.
Generation Seed Regions of Interest
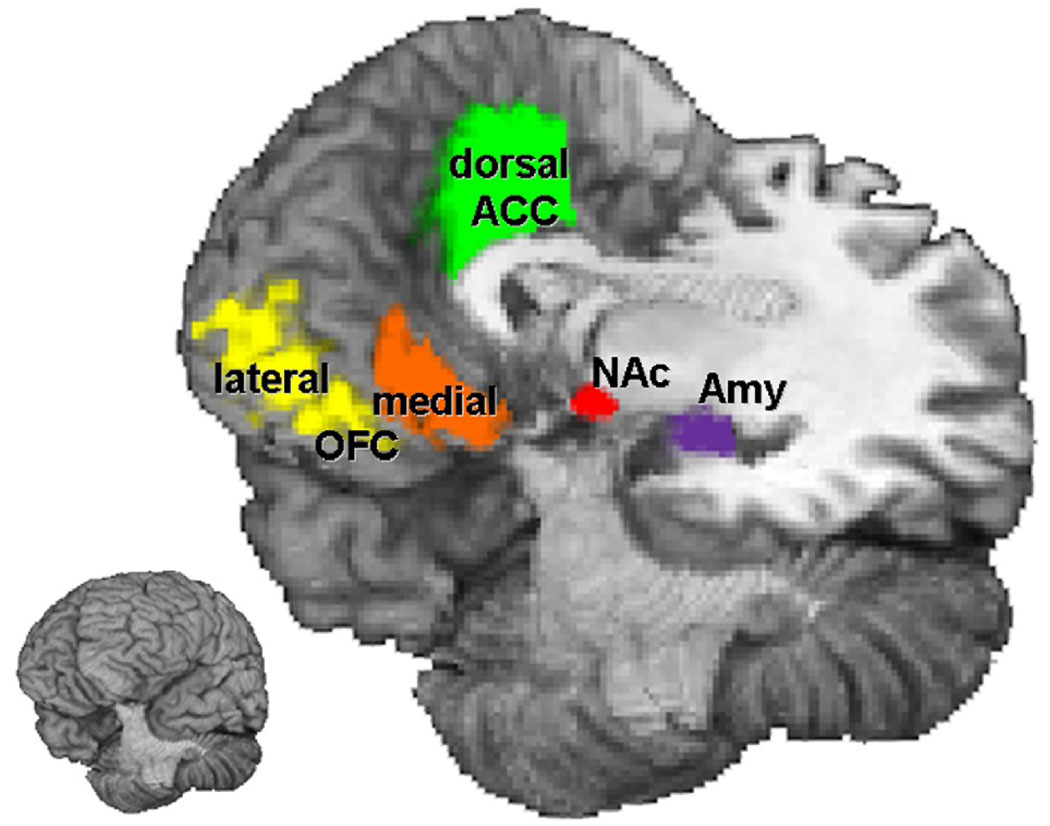
Ten brain regions, five in each hemisphere, were selected as seed regions of interest (seed ROIs) in this study (Figure 1, Table 1). These ROIs were bilateral nucleus accumbens (NAc), amygdala (Amy), dorsal anterior cingulate cortex (Broadman area (BA) 24/32), and orbital frontal cortex (OFC, including the lateral areas, BA 11/47, and the medial areas, BA 11/12). These regions were defined for each participant anatomically as follows. First, a set of masks corresponding to the regions listed above were defined in a standardized coordinate system of Talairach atlas (Talairach et al., 1992) and cortical structures of a standardized brain. Subsequently, these masks were transformed, according to the spatial transformation between the anatomic data and the Talairach space, onto to the image space of each participant and were modified according to individual brain’s cortical structures by referencing to the anatomic boundaries in the high-resolution three-dimensional structural images.
Figure 1. Seed Regions of Interest.
NAc = nucleus accumbens; Amy = amygdale; OFC = orbital frontal cortex; ACC = anterior cingulate cortex.
Table 1.
Seed Regions of Interest (ROI)
| ROI | Hemisphere | Size (mm3) | Coordinates b |
|---|---|---|---|
| NAc | Left | 333 | −10.5, 7.5, −7.9 |
| Right | 335 | 11.6, 7.3, −6.9 | |
| Amy | Left | 1149 | −22.9, −4.8, −14.9 |
| Right | 1147 | 23.6, −4.8, −14.9 | |
| Dorsal ACC | Left | 7887 | −6.0, 15.4, 32.7 |
| 24/32 a | Right | 7995 | 6.0, 15.2, 32.6 |
| Lateral OFC | Left | 13725 | −26.3, 39.3, −5.5 |
| 11/47 | Right | 12972 | 26.3, 39.4, −5.6 |
| Medial OFC | Left | 3636 | −6.0, 36.9, −12.3 |
| 11/12 | Right | 3529 | 6.0, 37.0, −12.3 |
Broadman area.
Coordinates refer to the center of the ROI, in Talairach space (Left to Right, Posterior to Anterior, Inferior to Superior).
Calculation of Functional connectivity
For each participant, correlation map was calculated respectively for each seed ROI by a voxel-wise multiple-regression. Regressors included the template time course extracted by averaging time courses of all the voxels in the seed ROI under consideration, as well as the average time course in white matter and the average time course in cerebrospinal fluid (as nuisance signals). The masks of white matter were determined from each participant’s high-resolution structural image using FAST segmentation program of fMRIb software library (FSL) (www.fmrib.ox.ac.uk). The resulting white matter segmentations were then thresholded to ensure 80% tissue type probability. The cerebrospinal fluid mask was manually drawn according to the anatomic boundaries of the high-resolution three-dimensional structural images of each participant. These nuisance signals were used to account for fluctuations unlikely to be relevant to neuronal activities (Birn et al., 2006; Di Martino et al., 2008; Fox et al., 2005). The resultant t-score maps of the seed ROIs were then converted to z-score maps hereafter referred to as “correlation maps”.
Group Analyses
Group analyses were performed for the correlation maps of each seed ROI. First, these maps were transformed to the Talairach space (re-sampled voxel size: 3×3×3 mm) according to the spatial transformation between the anatomic data and the Talairach space. Second, using AFNI, voxel-wise one-sample t-tests and two-sample t-tests were calculated to compare the seed-based functional connectivity within and between the two groups. For the one-sample t-tests, the correlation maps for each participant in the group (HU or CN, respectively) was entered into the analysis and the z-scores at each voxel were averaged across all participants in the group and compared to zero. Clusters with z-scores significantly larger than zero were determined by combining individual voxel threshold of p < 0.005 with a spatial cluster (cluster size from 29 to 48 voxels among seeds and groups) which yielded a false-positive level of 0.05 over the entire volume according to Monte Carlo simulations conducted with AFNI. For the two-sample t-tests, the correlation maps from both groups were entered into the analysis and the z-scores at each voxel were averaged within each group and then compared between groups. For each seed, the two-sample t-test was restricted to voxels within the mask defined by a logic ‘or’ between the two group-maps of each seed resulting from the one-sample t-tests described above. Clusters with z-scores significantly differed between two groups were determined by combining individual voxel threshold of p < 0.005 with a spatial cluster size (cluster size from 5 to 12 voxels among seeds) which yielded a false-positive level of 0.05 for voxels in these masks according to Monte Carlo simulations conducted with AFNI.
Results
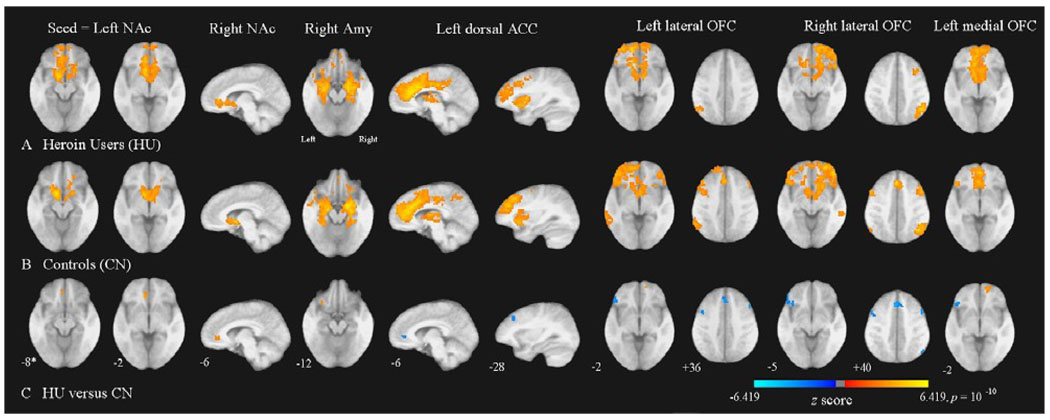
The results are shown in Table 2 and Figure 2. Compared to controls, heroin users showed significantly (p < 0.05, corrected) stronger functional connectivity between NAc and ventral/rostral ACC, between NAc and medial OFC, between amygdala and lateral OFC, between lateral OFC and medial OFC and within medial OFC. On the other hand, compared to controls, heroin users exhibited significantly weaker functional connectivity between lateral OFC and medial, dorsolateral PFC, within lateral OFC, between dorsal ACC and dorsolateral prefrontal cortex (PFC), between dorsal ACC and ventral/rostral ACC and between OFC and some frontal and parietal regions.
Table 2.
Significant Clusters in the Two-Sample t-Tests Comparing Functional Connectivity in Heroin Users (HU) versus Controls (CN).
| Seed ROI | Cluster Anatomical Locations (Brodmann’s Area) |
Cluster Size (mm3) |
Primary Peak Location a |
HU n = 14 Mean b |
CN n = 13 Mean |
|---|---|---|---|---|---|
| HU > CN | |||||
| Left NAc | Left ventral ACC/ medial OFC (24/32) |
459 | −7.5, 34.5, −0.5 | 5.875 | 1.079 |
| Right NAc | Left ventral/rostral ACC (24/32) | 405 | −7.5, 37.5, −0.5 | 3.256 | 0.082 |
| Right Amy | Left lateral OFC (47) | 189 | −28.5, 22.5, −9.5 | 2.854 | 0.081 |
| Left lateral OFC | Right medial OFC (10/12) | 189 | 10.5, 55.5, −0.5 | 5.174 | 1.034 |
| Left medial OFC | Right medial OFC (10/12) | 486 | 13.5, 55.5, −0.5 | 5.127 | 0.540 |
| HU < CN | |||||
| left NAc | Left Precuneus (7) | 189 | −22.5, −79.5, 41.5 |
−0.700 | 1.938 |
| left dorsal ACC | Left ventral/rostral ACC (24/32) | 297 | −10.5, 37.5, 2.5 | 2.039 | 6.217 |
| Left dorsolateral PFC (8/9) | 297 | −28.5, 31.5, 41.5 | 2.068 | 5.588 | |
| Left lateral OFC | Medial PFC (8/9/32) | 2079 | 4.5, 22.5, 41.5 | 0.319 | 3.528 |
| Left IFG (45) | 729 | −43.5, 31.5, −0.5 | 1.077 | 5.007 | |
| Right dorsolateral PFC (9) | 432 | 46.5, 16.5, 32.5 | −0.126 | 2.088 | |
| Left insula (13) | 297 | −37.5, 13.5, 14.5 | 0.146 | 2.857 | |
| Left dorsolateral PFC (8/9) | 270 | −40.5, 7.5, 35.5 | 0.351 | 4.050 | |
| Left dorsolateral PFC (9) | 243 | −49.5, 13.5, 29.5 | 0.551 | 3.456 | |
| Left lateral OFC (10) | 189 | −28.5, 43.5, 8.5 | −0.099 | 3.418 | |
| Right lateral OFC | Left lateral OFC/IFG (47/45) | 2943 | −43.5, 28.5, 2.5 | −0.450 | 2.576 |
| Medial PFC (8/9/32) | 1620 | −1.5, 16.5, 47.5 | 0.102 | 3.481 | |
| Left dorsolateral PFC (8/9) | 1026 | −37.5, 1.5, 38.5 | −0.750 | 2.551 | |
| Right dorsolateral PFC (8/9) | 999 | 46.5, 13.5, 38.5 | 0.848 | 3.597 | |
| Right IPL (39/40) | 405 | 49.5, −61.5, 41.5 | 1.172 | 3.570 | |
| Right anterior PFC (10) | 189 | 37.5, 52.5, 11.5 | 1.949 | 5.066 | |
| Right IPL (39/40) | 189 | 52.5, −58.5, 35.5 | 1.369 | 4.444 | |
| Left medial OFC | Left IFG (45) | 270 | −37.5, 31.5, −0.5 | −0.477 | 2.734 |
Coordinates in Talairach space (Left to Right, Posterior to Anterior, Inferior to Superior)
Within-group averaged z scores (from correlation maps of each participant) of all voxels in clusters. NAc = nucleus accumbens; Amy = amygdale; OFC = orbital frontal cortex; ACC = anterior cingulate cortex; PFC = prefrontal cortex; IFG = inferior frontal gyrus; IPL = inferior parietal lobule.
Figure 2. Altered Functional Connectivity in Heroin Users.
Images of group functional connectivity in heroin users (HU), A, and in non-addictive controls (CN), B. The contrast map in, C, demonstrates most of clusters (see Table 2) where resting-state functional connectivity was altered in HU versus CN. Clusters above thresholds (p < 0.05, corrected) in t-tests were lighten with z-scores converted from t-scores of these tests. The z-score bar is shown below the figures. The left side of the axial images corresponds to the left side of the brain. NAc = nucleus accumbens; Amy = amygdale; ACC = anterior cingulate cortex; OFC = orbital frontal cortex. * Coordinates in Talairach space (Sagittal images, Left to Right, Axial images, Inferior to Superior). For display purposes, a single, averaged, skull striped, stereotaxic transformed image was derived from 27 three-dimensional structural scans taken from all participants of both groups and statistical maps were superimposed on this structural image.
Discussion
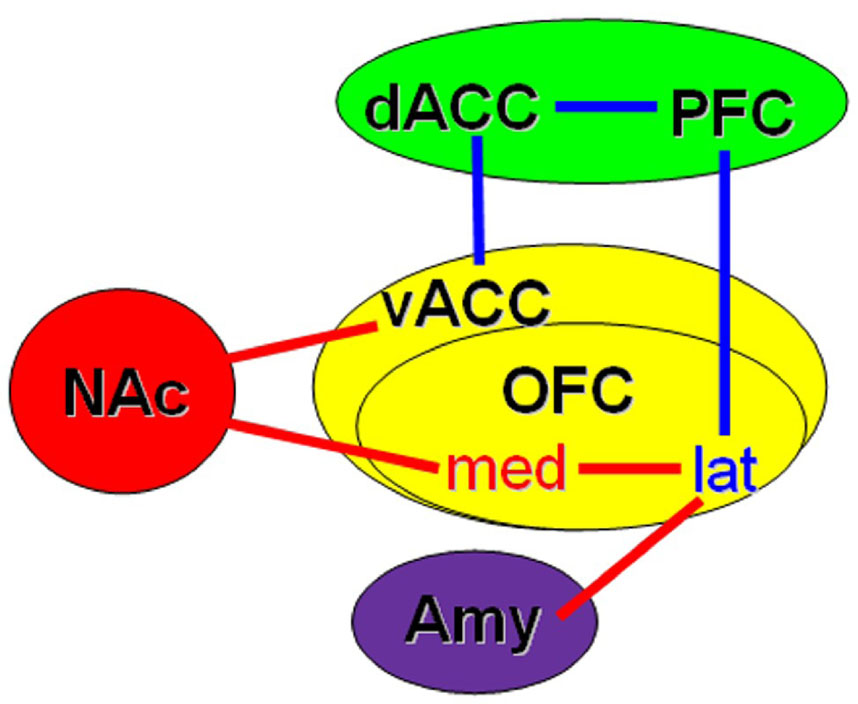
Addiction related alteration in functional connectivity within the key regions implicated in addiction is demonstrated with resting-state fMRI data acquired from chronic heroin users and non-addicted controls. Compared with controls, chronic heroin users showed increased functional connectivity between nucleus accumbens (NAc) and ventral/rostral anterior cingulate cortex (ACC), and orbital frontal cortex (OFC), and between amygdala and OFC; but reduced functional connectivity between PFC and ACC, and OFC (Figure 3).
Figure 3. Schematic diagram showing significant functional connectivity differences between chronic heroin users (HU) and controls.
Red line/word: Enhanced in HU, Blue line/word: Reduced in HU. The colors of backgrounds, as used in a review article (Baler and Volkow, 2006), indicate different roles of the regions in drug addiction: Red = reward; Purple = memory and learning; Green = cognitive control; Yellow = motivation, craving and behavior guidance. NAc = nucleus accumbens; Amy = amygdale; OFC = orbital frontal cortex, med = medial, lat = lateral; PFC = prefrontal cortex; dACC = dorsal anterior cingulate cortex; vACC = ventral/rostral anterior cingulate cortex. Inspired from (Baler and Volkow, 2006).
The BOLD signal has been confirmed to indirectly reflect neural activity, and the low-frequency fluctuations in the resting state, although not conclusively proven, have been attributed to neural spontaneous activity (Fox and Raichle, 2007). Recent studies using diffusion tensor imaging (Greicius et al., 2009) and task-based meta-analyses (Toro et al., 2008) suggest that resting-state functional connectivity reflects structural connectivity and that networks identified in the resting-state mimic those identifiable with a lot of task paradigms. Meanwhile, resting-state functional connectivity was found among some brain regions implicated in addiction in recent studies in normal participants (Di Martino et al., 2008; Margulies et al., 2007; Roy et al., 2009). As dysfunctions in these regions were robustly proven in drug addiction (with human and animal models) (Kalivas and O'Brien, 2008; Volkow et al., 2007), we suggest that the altered functional connectivity found in HU in the present study may be a neurobiological indicator of addiction related abnormal functional organization in neural networks related to reward, craving and motivation processing which leads to addiction related compulsive drug-seeking behaviors.
Nucleus accumbens plays a central role in reward processing (Baler and Volkow, 2006; Knutson and Wimmer, 2007). It is widely accepted that the initial reinforcing effects of most drugs of abuse rely heavily upon the induction of large and rapid increases in the level of dopamine in the NAc, which can render the drugs as highly salient, drive motivation and produce compulsive behaviors (Nestler, 2005; Volkow et al., 2007). Ventral/rostral ACC is the affective subdivision of ACC and primarily involved in assessing the salience of emotional and motivational information and regulating emotional responses (Allman et al., 2001; Bush et al., 2000). This subdivision has extensive connections with other limbic areas including the striatum and amygdala (Kalivas and McFarland, 2003). In drug addicts, increased activity in ventral/rostral ACC was found to be associated with their subjective experience of drug craving (Diekhof et al., 2008; Volkow et al., 2005). The OFC is a major area of motivation, drive and salience evaluation, which is impaired in drug addicts and plays an important role in the output of compulsive drug-seeking behaviors (Volkow and Fowler, 2000). In the present study, significant resting-state functional connectivity was found between NAc and ventral/rostral ACC, and medial OFC, consistent with the results of a recent study on the functional connectivity of striatum (Di Martino et al., 2008). Amygdala is thought to primarily contribute to the acquisition, consolidation and expression of learning of the drug-related cues that drive relapse to drug-seeking behaviors (Hyman et al., 2006; Robbins et al., 2008). This area is also important for craving processing, robustly activated under drug-related cues (Diekhof et al., 2008) (even for the unseen ones (Childress et al., 2008; Zhang et al., 2009)), involved in signaling pleasure of immediate prospects which are related to impulsive behaviors (Bechara, 2005) and was found to exhibit robust resting-state functional connectivity with affective brain areas including OFC (Roy et al., 2009). Our finding of higher functional connectivity between NAc and ventral ACC and OFC, between amygdala and OFC in HU may be relevant to previous studies that have demonstrated the involvement of these areas in the pathology of addiction (Bolla et al., 2004; Goldstein and Volkow, 2002), and may underlie addiction related strong craving responses and motivation for drugs in addicts.
PFC, which is believed to be responsible for restraining craving and cognitive control, was found to be impaired in drug abuse or relapse (Goldstein et al., 2004; Yang et al., 2009). Dorsal ACC is in responsible for inhibition controlling and conflict monitoring (Bush et al., 2000). Resting-state functional connectivity was found between these regions in a previous study (Margulies et al., 2007), implicating the dorsal–caudal ACC-based frontoparietal attention networks. In the present study, heroin users showed reduced functional connectivity within the circuit of cognitive control (between dorsal ACC and PFC) and between the circuits of cognitive control and motivation (between PFC and OFC, between dorsal ACC and ventral/rostral ACC). These observations, which are consistent with the results of a recent diffusion tensor imaging study reporting reduced white matter integrity in heroin users in the frontal and cingulate areas (Liu et al., 2008), suggested diminished cognitive control function and weakened cognitive control upon craving and motivation in heroin users. Taken together, the abnormality of functional connectivity in heroin users observed here supports the theories of addiction positing that the drug craving and motivation are enhanced in the addicted state, whereas the strength of cognitive control is weakened (Baler and Volkow, 2006; Bechara, 2005; Everitt and Robbins, 2005; Garavan and Hester, 2007; Goldstein and Volkow, 2002).
The OFC is a functionally heterogeneous region that involved in complex adaptive behaviors: the medial part of OFC appears to be involved in ongoing monitoring of the reward value of reinforcers, while the lateral part is involved in both evaluating the punishment value of reinforcers and behavioral guidance functions which may lead to a change in current behaviour (Elliott et al., 2009; Kringelbach, 2005; Kringelbach and Rolls, 2004; Murray et al., 2007; O'Doherty et al., 2001; O'Doherty, 2007). In the present study, we respectively analyzed the functional connectivity of these two sub-regions of OFC. We found heroin users exhibited enhanced functional connectivity between medial OFC and NAc and within medial OFC, which may correspond to their stronger salience value of drugs. The lateral OFC of heroin users showed stronger functional connectivity to amygdala and to medial OFC but weaker connectivity to PFC and to lateral OFC. In previous studies, the dysfunction of lateral OFC found in drug addicts was thought to be implicated in the core characteristics of drug addiction, such as failure to properly inhibit excessive drug consumption and develop aversive/withdrawal reactions to potentially dangerous situations (Goldstein et al., 2005; Goldstein et al., 2001; Volkow et al., 2003). Our observations may suggest that, in drug addicts, there may be abnormally excessive influences of drug and drug cues-induced craving to the behavioral guidance function, while the ability of behavior changing according to cognitive control and the function of evaluating punishment value of reinforcers (such as negative consequences of drug taking) may be impaired. As drug addiction is characterized by the failure to resist one's impulses to obtain and take addictive drugs despite serious negative consequences (Volkow and Li, 2004), our functional connectivity findings may provide a neurobiological explanation for this phenomenon.
The major points discussed above are mainly based on findings of altered resting connectivity in addicts compared with non-addicted controls. However, as the roles of resting-state functional connectivity in the circuits implicated in addiction, such as those related reward and craving, remains to be elucidated and due to the lack of behavior tasks in the present study, to what extent the present findings are related to the abnormal brain function in drug addicts is still an open question. Thus, future studies focused on the implications of resting-state functional connectivity in brain circuits of reward, craving and cognitive control, especially in those of drug addicts, are needed to provide a more specific and definitive interpretation of the results in the present study.
As most chronic heroin users in our study were under methadone treatment, what effects of methadone would have on the functional connectivity is an interesting question. Known as a drug that prolongs opioid dependence, methadone has similar effects as addictive drugs to some extent (Connock et al., 2007). For example, studies have found that methadone can prime heroin cue response and craving (Curran et al., 1999) and enhance brain response to drug cues (Langleben et al., 2008), which are similar to those in untreated heroin dependence (Daglish et al., 2003). Thus, we speculate that the alteration of functional connectivity related to methadone might be in same direction as that related to heroin addiction. However, as the effect of methadone was not sufficiently controlled in current study, its effect on functional connectivity needs to be clarified in further studies. Nonetheless, as chronic heroin users under methadone treatment are thought to be still at states of addiction (Connock et al., 2007), our findings of alteration in brain functional connectivity in HU may be mainly related to their opioid addiction.
A recent study found the severity of nicotine addiction was negatively correlated with the strength of connectivity between dorsal ACC and striatal circuits (Hong et al., 2009). However, in the present study, in contrast to controls, we found the connectivity between ventral ACC and NAc was stronger in heroin user who reported higher (p < 0.001) Fagerström Test for Nicotine Dependence (FTND) scores, and there was no significant between-group difference on the connectivity between dorsal ACC and NAc. Thus, we proposed that the effect of nicotine addiction severity may not be a main effect of alteration on functional connectivity found in our study. Nonetheless, as our two groups were not well controlled for nicotine addiction severity, this question should be addressed in future studies.
Additional investigations involving larger sample size of and more diverse participants (e.g. ones addictive to other substance and in different genders) are needed for generalizing our results, examining the relationship between behavioral and imaging data, and clarifying the effects of different treatment stages of addiction on functional connectivity. In particular, the present study did not dissociate the effects of methadone treatment on resting-state functional connectivity from those of heroin addiction, so further studies in this regard is needed; such a study may be of potentially clinical significance. Meanwhile, methods that could address potential noise due to low-frequency fluctuations from possible thermal noise, scanner instability, and alias of cardiac and/or respiratory cycling, more advanced analysis, such as independent component analysis (Greicius et al., 2007) and effective connectivity analysis (Fox and Raichle, 2007), and combination with diffusion tensor imaging (Greicius et al., 2009) may be used in further studies.
Taken together, we found enhanced resting-state functional connectivity within the regions related to reward, memory, craving and motivation, but reduced connections within the regions associated with cognitive control and craving and behavioral guidance in addictive drug users with fMRI. These findings suggest an abnormal functional organization in the addictive brain, which may provide additional evidence supporting the theory of addiction (Baler and Volkow, 2006; Bechara, 2005; Everitt and Robbins, 2005; Garavan and Hester, 2007; Goldstein and Volkow, 2002) that emphasizes enhanced salience value of a drug and its related cues but weakened strength of cognitive control in the addictive state.
Acknowledgments
We thank two anonymous reviewers and the editor of Neuroimage for their helpful comments and suggestions. This research is supported by the National Nature Science Foundation of China (30770713, 30870764, and 30670683), Ministry of Science and Technology of China (2006CB500705), Academic Exchange Fund of International Medical MR, (N7014), and the US National Institutes of Health (RO1EB002009 and RO1DA17795). We thank Dr. De-Lin Sun, Xiao-Song He, Zu-Ji Cheng, for their advice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann N Y Acad Sci. 2001;Ann N Y Acad Sci:107–117. [PubMed] [Google Scholar]
- Auer DP. Spontaneous low-frequency blood oxygenation level-dependent fluctuations and functional connectivity analysis of the 'resting' brain. Magn Reson Imaging. 2008;26:1055–1064. doi: 10.1016/j.mri.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Childress AR, Ehrman RN, Wang Z, Li Y, Sciortino N, Hakun J, Jens W, Suh J, Listerud J, Marquez K, Franklin T, Langleben D, Detre J, O'Brien CP. Prelude to passion: limbic activation by "unseen" drug and sexual cues. PLoS ONE. 2008;3:e1506. doi: 10.1371/journal.pone.0001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, Fry-Smith A, Day E, Lintzeris N, Roberts T, Burls A, Taylor RS. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11:1–171. iii–iv. doi: 10.3310/hta11090. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Curran HV, Bolton J, Wanigaratne S, Smyth C. Additional methadone increases craving for heroin: a double-blind, placebo-controlled study of chronic opiate users receiving methadone substitution treatment. Addiction. 1999;94:665–674. doi: 10.1046/j.1360-0443.1999.9456654.x. [DOI] [PubMed] [Google Scholar]
- Daglish MR, Weinstein A, Malizia AL, Wilson S, Melichar JK, Lingford-Hughes A, Myles JS, Grasby P, Nutt DJ. Functional connectivity analysis of the neural circuits of opiate craving: "more" rather than "different"? Neuroimage. 2003;20:1964–1970. doi: 10.1016/j.neuroimage.2003.07.025. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Falkai P, Gruber O. Functional neuroimaging of reward processing and decision-making: a review of aberrant motivational and affective processing in addiction and mood disorders. Brain Res Rev. 2008;59:164–184. doi: 10.1016/j.brainresrev.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Elliott R, Agnew Z, Deakin JF. Hedonic and Informational Functions of the Human Orbitofrontal Cortex. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp092. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychology Review. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: Neuroanatomical specificity for drug users and drug stimuli. American Journal of Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Leskovjan AC, Fowler JS, Wang GJ, Gur RC, Hitzemann R, Volkow ND. Anger and depression in cocaine addiction: association with the orbitofrontal cortex. Psychiatry Res. 2005;138:13–22. doi: 10.1016/j.pscychresns.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, Telang F, Caparelli EC, Chang L, Ernst T, Samaras D, Squires NK, Volkow ND. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am J Psychiatry. 2007;164:43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang GJ, Fowler JS, Volkow ND. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42:1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, Wang GJ, Fowler JS, Rajaram S. Addiction changes orbitofrontal gyrus function: involvement in response inhibition. Neuroreport. 2001;12:2595–2599. doi: 10.1097/00001756-200108080-00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, Thaker GK, Stein EA. Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66:431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Knutson B, Wimmer GE. Splitting the difference: how does the brain code reward episodes? Ann N Y Acad Sci. 2007;1104:54–69. doi: 10.1196/annals.1390.020. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: Linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Langleben DD, Ruparel K, Elman I, Busch-Winokur S, Pratiwadi R, Loughead J, O'Brien CP, Childress AR. Acute effect of methadone maintenance dose on brain FMRI response to heroin-related cues. Am J Psychiatry. 2008;165:390–394. doi: 10.1176/appi.ajp.2007.07010070. [DOI] [PubMed] [Google Scholar]
- Liu H, Li L, Hao Y, Cao D, Xu L, Rohrbaugh R, Xue Z, Hao W, Shan B, Liu Z. Disrupted white matter integrity in heroin dependence: a controlled study utilizing diffusion tensor imaging. Am J Drug Alcohol Abuse. 2008;34:562–575. doi: 10.1080/00952990802295238. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yu C, Liang M, Li J, Tian L, Zhou Y, Qin W, Li K, Jiang T. Whole brain functional connectivity in the early blind. Brain. 2007;130:2085–2096. doi: 10.1093/brain/awm121. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Murray EA, O'Doherty JP, Schoenbaum G. What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. Journal of Neuroscience. 2007;27:8166–8169. doi: 10.1523/JNEUROSCI.1556-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP. Lights, camembert, action! The role of human orbitofrontal cortex in encoding stimuli, rewards, and choices. Ann N Y Acad Sci. 2007;1121:254–272. doi: 10.1196/annals.1401.036. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P, Musolino A, Missir O. Stereotaxic exploration in frontal epilepsy. Adv Neurol. 1992;57:651–688. [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, Volkow ND. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Res. 2007;155:189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cereb Cortex. 2008;18:2553–2559. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Li TK. Science and society - Drug addiction: the neurobiology of behaviour gone awry. Nature Reviews Neuroscience. 2004;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Zhu W, Maynard L, Telang F, Vaska P, Ding YS, Wong C, Swanson JM. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. J Neurosci. 2003;23:11461–11468. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Liang M, Wang L, Tian L, Zhang X, Li K, Jiang T. Altered functional connectivity in early Alzheimer's disease: a resting-state fMRI study. Hum Brain Mapp. 2007;28:967–978. doi: 10.1002/hbm.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Xie J, Shao YC, Xie CM, Fu LP, Li DJ, Fan M, Ma L, Li SJ. Dynamic neural responses to cue-reactivity paradigms in heroin-dependent users: an fMRI study. Hum Brain Mapp. 2009;30:766–775. doi: 10.1002/hbm.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen X, Yu Y, Sun D, Ma N, He S, Hu X, Zhang D. Masked smoking-related images modulate brain activity in smokers. Hum Brain Mapp. 2009;30:896–907. doi: 10.1002/hbm.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Shu N, Liu Y, Song M, Hao Y, Liu H, Yu C, Liu Z, Jiang T. Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophr Res. 2008;100:120–132. doi: 10.1016/j.schres.2007.11.039. [DOI] [PubMed] [Google Scholar]