Abstract
The notion that the brain is organized into two complementary networks, one that is task-positive and supports externally-oriented processing, and the other that is task-negative and supports internally-oriented processing, has recently attracted increasing attention. The goal of the present study was to investigate involvement of the task-positive and task-negative networks in overlapping activity between episodic memory encoding and retrieval. To this end, we performed a functional MRI study that included both encoding and retrieval tasks. We hypothesized that during the study phase, encoding success activity (remembered > forgotten) involves mainly the task-positive network, whereas encoding failure activity (forgotten > remembered) involves mainly the task-negative network. We also hypothesized that during the test phase, retrieval success activity (old > new) involves mainly the task-negative network, whereas novelty detection activity (new > old) involves mainly the task-positive network. Based on these hypotheses, we made 3 predictions regarding study-test overlap. First, there would be relatively high level of overlap between encoding success and novelty detection activity involving the task-positive network. Second, there would be relatively high level of overlap between encoding failure and retrieval success activity involving the task-negative network. Third, there would be relatively low level of overlap between encoding success and retrieval success activity as well as between encoding failure and novelty detection activity. The results fully confirmed our 3 predictions. Taken together, the present findings clarify roles of the task-positive and task-negative networks in encoding and retrieval and the function of overlapping brain activity between encoding and retrieval.
Keywords: default mode, functional MRI, human memory, memory encoding, memory retrieval
Introduction
Episodic memory consists of multiple subprocesses, and a fundamental distinction can be drawn between encoding and retrieval processes. Most prior functional neuroimaging studies of episodic memory have focused either on encoding or retrieval processes, whereas relatively few have directly compared the two processes within the same study. An influential view for relating encoding and retrieval activations has been the “reinstatement” hypothesis, which postulates that successful retrieval of episodic information involves reactivation of several of the brain regions that were activated during encoding of that information (e.g., Nyberg et al., 2000; Wheeler et al., 2000). Consistent with this hypothesis, several studies (e.g., Persson and Nyberg, 2000; Johnson and Rugg, 2007) have found significant activation overlaps between encoding and retrieval. However, in practically all studies that have examined this issue, the extent of overlap was highly limited, involving only a small fraction of sensory/perceptual regions activated during encoding. Thus, the reinstatement hypothesis, while helpful for interpreting memory activity in parts of sensory/perceptual regions, is of limited value for understanding memory activity in other brain regions.
Here, we propose a more global, system-wide model to relate brain activity elicited during study (encoding) and test (retrieval) phases, and we test 3 predictions derived from the model. A fundamental characteristic of the model is that it considers not only encoding and retrieval activations but also encoding and retrieval deactivations. This feature allows the model to link the contribution of brain regions to memory processes to their roles within distributed networks that consistently activate or deactivate during cognitive tasks. It has become well established in recent years that during attention-demanding cognitive tasks, a set of widespread regions routinely show activity increases, whereas a different set of wide-spread regions routinely show activity decreases (Binder et al., 1999; Fransson, 2005; Fox et al., 2005; Golland et al., 2008). These two sets of regions have been termed the task-positive and the task-negative network, respectively (Fox et al., 2005). The task-positive network includes lateral prefrontal cortex (PFC), dorsal parietal cortex, sensory-motor cortices, subcortical areas, and the cerebellum (Cabeza and Nyberg, 2000; Naghavi and Nyberg, 2005; Shulman, Corbetta et al., 1997), whereas the task-negative network, also known as the default-mode network (Raichle et al., 2001), consists of anterior and medial PFC, the precuneus, and the angular gyrus (Shulman, Fiez et al., 1997; Gusnard and Raichle, 2001). There is increasing evidence that the task-positive network is activated for processing externally presented information, including stimulus processing, task-execution, and monitoring external environments (e.g., Cabeza and Nyberg, 2000; Naghavi and Nyberg, 2005; Golland et al., 2008), whereas the task-negative network is activated (or less deactivated) for processing internally generated information, including self-referential processing, task-unrelated thoughts, and theory of mind (e.g., Gusnard et al., 2001; Fransson, 2006; McKiernan et al., 2006; Mason et al., 2007; Buckner et al., 2008). In short, externally-oriented processing is associated with increased activity in the task-positive network and decreased activity in the task-negative network, whereas internally-oriented processing is associated with increased activity in the task-negative network and decreased activity in the task-positive network. This opposing (i.e., anticorrelated) relationship between the networks can be also observed in spontaneous low-frequency fluctuations of blood oxygenation level-dependent signal (Fransson, 2005; Fox et al., 2005; Golland et al., 2008). On the basis of hypothesized associations of the task-positive and task-negative networks with encoding and retrieval activity, we made 3 specific predictions regarding study-test overlap (see below).
To directly compare brain activity during study and test phases, we performed an fMRI study that included both encoding and retrieval tasks. In each encoding trial of the present study, subjects studied a “mini word-list” comprising 4 instances (e.g., horse, chicken, sheep, goat) of a semantic category (e.g., farm animal). At test, they performed an old/new recognition test with confidence ratings that included studied words as well as unrelated, new words. Previous studies (Otten and Rugg, 2001; Wagner and Davachi, 2001; Daselaar et al., 2004) have shown that some activity at study phase is positively correlated with subsequent remembering (i.e., remembered > forgotten), whereas other activity is negatively correlated (i.e., forgotten > remembered), and attention must be given to both effects to fully account for subsequent memory effects. Thus, using the subsequent memory procedure we coded the two forms of activity in the study phase: (i) activity positively correlated with subsequent hit rates, or encoding success activity; (ii) activity negatively correlated with subsequent hit rates, or encoding failure activity. Activity elicited during test phase includes not only processes associated with successful retrieval (i.e., old > new), but also processes associated with detection of novel information (i.e., new > old; Tulving et al., 1996; Buckner et al., 2001). Both behavioral and neuroimaging evidence indicates that novelty is an important determinant of memory processing (Tulving and Kroll, 1995; Ranganath and Rainer, 2003). Thus, we also coded two forms of activity in the test phase: (i) greater activity for high-confidence hits than for high-confidence correct rejections, or retrieval success activity; (ii) greater activity for high-confidence correct rejections than for high-confidence hits, or novelty detection activity. We used high-confidence responses in the contrasts because we were interested in recollection rather than familiarity.
Our model linking activity during study and test phases to the task-positive and task-negative networks consist of four hypotheses, which correspond to the four cells of Table 1. We assume that during the study phase, encoding stimulus information into long-term memory is supported by externally-oriented attention to the study item, but interfered by internally-oriented processing, such as stimulus-independent thoughts (McKiernan et al., 2006; Mason et al., 2007; Uncapher and Wagner, 2009). In other words, activity in the task-positive network facilitates encoding by directing attention appropriately to the word list, whereas activity in the task-negative network interferes with encoding by taking processing resources away from the word list. Thus, we hypothesize that encoding success activity involves mainly the task-positive network, whereas encoding failure activity involves mainly the task-negative network. Additionally, we assume that during the test phase, vivid remembering of specific contextual details, or recollection, redirects attention to internal mnemonic associations, whereas detection of novel information is supported by externally-oriented attention. In other words, activity in the task-positive network facilitates novelty detection by directing attention externally to the word cue, whereas activity in the task-negative network is associated with internal re-experience of episodes associated with the word cue. Thus, we hypothesize that retrieval success activity involves mainly the task-negative network, whereas novelty detection activity involves mainly the task-positive network. The hypothetical associations of encoding success and novelty detection activity with the task-positive network and encoding failure and retrieval success activity with the task-negative network are meant to be relative rather than absolute. This is particularly true for retrieval success activity, because access to the internal mnemonic associations is dependent upon externally-oriented attention toward the memory cue. Thus, retrieval success activity should include at least some subcomponents of the task-positive network, possibly centered in sensory/perceptual region.
Table 1.
Model linking encoding and retrieval activity to widespread networks consistently activated or deactivated during cognitive performance
| Task-positive Network External Orientation (lateral PFC, dorsal parietal ctx., sensorimotor ctx., etc.) | Task-negative Network Internal Orientation (anterior and medial PFC, precuneus, angular gyrus, etc.) | |
|---|---|---|
| Study Phase | Encoding success activity | Encoding failure activity |
| Test Phase | Novelty detection activity | Retrieval success activity |
On the basis of our model (i.e., the 2 × 2 matrix in Table 1), we made the following 3 predictions regarding study-test overlap. First, we predicted relatively high level of overlap between encoding success and novelty detection activity involving the task-positive network. Second, we predicted relatively high level of overlap between encoding failure and retrieval success activity involving the task-negative network. Third, we predicted relatively low level of overlap between encoding success and retrieval success activity, as well as between encoding failure and novelty detection activity. In other words, we predict that overlaps between study-phase and test-phase activity will occur along the columns of the matrix (the task-positive and task-negative networks) rather than along the diagonals of the matrix.
Materials and Methods
Participants
The basis of the present report was a re-analysis of the data reported in prior studies, focusing on the encoding phase (Kim and Cabeza, 2007a) and on the retrieval phase (Kim and Cabeza 2007b, 2009), respectively. The goals of these studies are different from the present one, and the critical contrasts investigated are also different. Sixteen young adults participated in the experiment. They were healthy, right-handed, native English speakers, with no history of neurological or psychiatric episodes. All subjects gave informed consent to a protocol approved by the Duke University Institutional Review Board. Four subjects were excluded due to a sparse number (<10) of high-confidence correct rejection responses, which was one of the critical trial types in fMRI analyses (see below). Thus, the reported results are based on the data from the remaining 12 subjects (6 female; age range 18–31).
Stimulus materials
The stimulus materials were 72 categorical 6-word lists selected from category norms (Battig and Montague, 1969; Yoon et al., 2004). Each list consisted of the 6 most typical instances (e.g., cow, pig, horse, chicken, sheep, goat) of a natural/artificial category (e.g., farm animal), with minor exceptions. In each list, the last 4 typical instances were used as encoding stimuli (Old words) in the study phase, whereas the first and the second typical instances were used as ‘critical lures’ (Lure words) in the test phase. Additionally, semantically unrelated words, matched in letter number, frequency, and concreteness to the category words, were used as distractors (New words) in the test phase.
Behavioral procedures
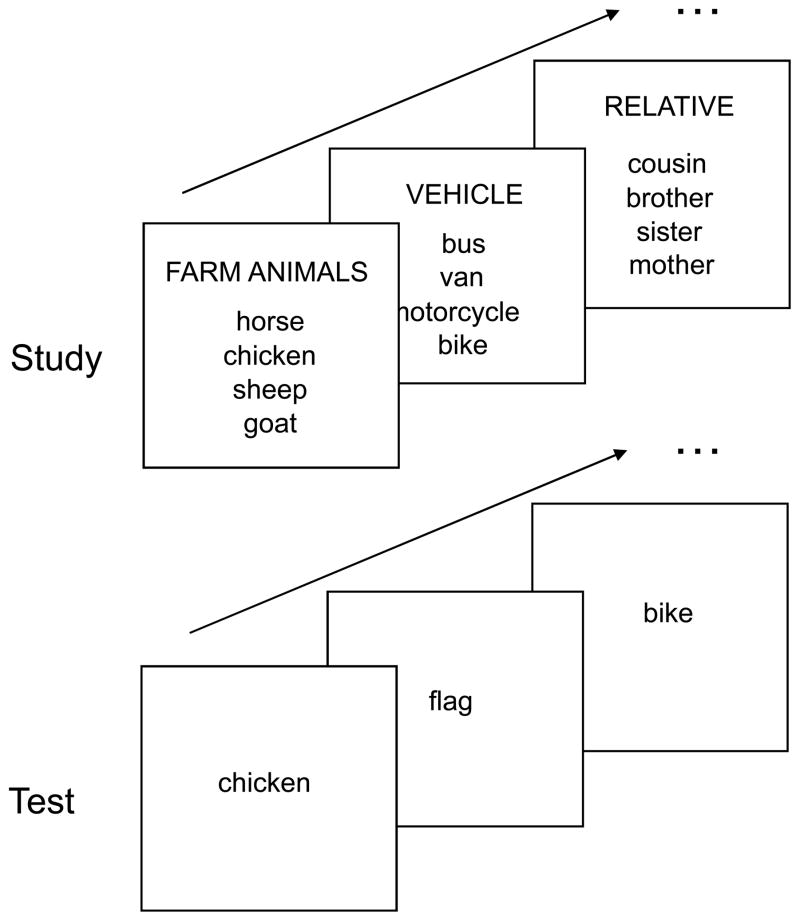
The behavioral paradigm is illustrated by Fig. 1. The study phase was a single scan consisting of 82 trials/lists. Each encoding screen simultaneously showed a category name at the top and its 4 instances below the category name in column format. Each list was presented for 4 sec. The subjects’ task was to decide whether all 4 or only 3 instances belonged to the category. They responded by pressing one of the two keys in a response box using their right hand. In 72 ‘critical’ trials, all 4 words were members of the category, whereas in 10 ‘catch’ trials, only 3 of the 4 words belonged to the category. The words were displayed in colors to promote the encoding of sensory/perceptual information (Cabeza et al., 2001). In a given trial, all 4 words were displayed in the same color, but 5 different colors were alternatively used across trials. For each encoding trial, we calculated a subsequent hit measure by counting how many studied words were later remembered (range: 0–4). Only high-confidence hit responses were considered as “remembered”, because we were interested in recollection rather than familiarity. Based on the subsequent hit measure, we conducted a parametric study of fMRI signals at the study phase (see below).
Fig. 1.
Behavioral paradigm. The encoding task was a category judgment task. The retrieval task was an old-new recognition test with confidence ratings that included studied words (Old words) and nonstudied, unrelated words (New words).
The test phase, which started approximately 10 min after completion of the study phase, consisted of 6 scans. There were a total of 288 Old-word, 144 New-word, and 144 Lure-word trials across all scans. The Lure-word trials generated very high false alarm rates, and were the critical items in a previous study focused on false memory processes (Kim and Cabeza, 2007b). Since these trials are not relevant for the goal of the present study, they were not considered in the analyses of the present study, except in analyses to determine whether overall activity is task-positive, task-negative, or neutral (see below). Trials were presented in a predetermined, pseudo-random order. In each trial, a word was shown for 2 sec, followed by a fixation cross for 1 sec. All words in the test phase were displayed in white color against black background. Subjects responded by pressing one of four keys using their right-hand according to whether the word was judged to be ‘sure old’, ‘unsure old’, ‘unsure new’, or ‘sure new’. A fixation period, ranging from 1.5 sec to 4.5 sec, was interspersed across both study and test trials to ‘jitter’ the onset times of trials and allow event-related fMRI analyses.
fMRI procedures
MRI scanning was conducted using a 4-T GE magnet. Scanner noise was reduced with earplugs, and head motion was reduced with foam pads and headbands. Stimuli were presented with liquid-crystal display goggles. Anatomical scanning started with a T2-weighted sagittal localizer series. The anterior (AC) and posterior commissures (PC) were identified in the midsagittal slice, and 34 contiguous oblique slices were prescribed parallel to the AC-PC plane. High-resolution T1-weighted structural images were collected with a 500-msec repetition time (TR), a 14-msec echo time (TE), a 24-cm field of view (FOV), a 2562 matrix, 68 slices, and a slice thickness of 1.9 mm. Functional images were acquired using an inverse spiral sequence with a 1500-msec TR, a 6-msec TE, a 24-cm FOV, a 642 matrix, and a 60° flip angle. Thirty-four contiguous slices were acquired with the same slice prescription as the anatomical images. Slice thickness was 3.75 mm, resulting in cubic 3.75 mm3 isotropic voxels.
Image processing and analyses were performed using SPM2 software (www.fil.ion.ucl.ac.uk/spm/). After discarding the first 6 volumes, the functional images were slice-timing corrected and motion-corrected, and then spatially normalized to the Montreal Neurological Institute (MNI) templates implemented in SPM2. The coordinates were later converted to Talairach and Tournoux’s (1988) space. Subsequently, the functional images were spatially smoothed using an 8 mm isotropic Gaussian kernel, and resliced to a resolution of 3.75 mm3 isotropic voxels. Trial-related fMRI activity was first modeled by convolving a vector of the onset times of the stimuli with a canonical hemodynamic response function (HRF). The general linear model (GLM), as implemented in SPM2, was used to model the effects of interest and other confounding effects (e.g., head movement and magnetic field drift). Statistical Parametric Maps (SPM) pertaining to the effects of interest were identified and subsequently integrated across subjects using a random-effects model.
Preliminary fMRI analyses
An assumption intrinsic to the main hypotheses of the present study is that regions showing task-positive and task-negative activity are similar across the study and test phases. To test this assumption, we performed a conjunction analysis involving task-positive and task-negative activity in the two phases. In these analyses, all encoding trials (ENC) and all retrieval trials (RET) were treated as one trial type, respectively. To identify overlapping task-positive activity across the two phases, we performed 3 steps: (i) created a T-map for the contrast ENC > 0 (i.e., baseline activity); (ii) created a T-map for the contrast RET > 0; and iii) identified voxels that were significant in both T-maps, each with a statistical threshold of P < .05 (T = 1.80), uncorrected. While probabilities are not completely independent, this results in an approximate conjoint probability of .0025 (= .05 × .05). The extent threshold was set at 15 contiguous voxels. To identify overlapping task-negative activity across the two phases, we performed similar 3 steps, using the same height and extent thresholds. The purpose of these preliminary analyses was only to show that task-positive and task-negative activity in the study versus test phases involves similar regions. Thus, the results were not used as a “mask” in any main fMRI analyses (see below).
Main fMRI analyses
Encoding success, encoding failure, retrieval success, and novelty detection activity were identified, respectively, in the following manner. To identify encoding success activity, the height of the modeled HRF of encoding trials was parametrically modulated by the subsequent hit measure using a linear increase function [i.e., −2, −1, 0, 1, 2], and to identify encoding failure activity, using a linear decrease function. Thus, encoding success activity reflected encoding trial activity positively correlated with later hit rate for words from those trials, and encoding failure activity reflected encoding trial activity negatively correlated with later hit rate for words from those trials. At the test phase, retrieval success activity was identified by the contrast high-confidence hit > high-confidence correct rejection, and novelty detection activity by the reverse contrast, i.e., high-confidence correct rejection > high-confidence hit. The resulting T-map images for encoding success, encoding failure, retrieval success, and novelty detection activity were examined each with a statistical threshold set at P < .005, uncorrected, and an extent threshold of 15 contiguous voxels, which yields a false positive probability of 0.00001 per voxel according to Monte Carlo simulations of spatially correlated data (Forman et al., 1995). To identify overlap across encoding success and novelty detection activity, we determined voxels that were significant in both T-maps identifying encoding success and novelty detection activity, each with a statistical threshold of P < .033 (T = 2.04), uncorrected. While probabilities are not completely independent, this results in an approximate conjoint probability of .001 (= .033 × .033). The extent threshold was set at 15 contiguous voxels. To identify overlap across encoding failure and retrieval success activity, encoding success and retrieval success activity, and encoding failure and novelty detection activity, respectively, we performed similar conjunction analyses, using the same height and extent thresholds.
In addition to SPM-based group contrasts, for each significant cluster identified in the group contrasts, a follow-up region-of-interest (ROI) analysis was performed to determine whether its overall activity is task-positive, task-negative, or neutral. For each cluster, the parameter estimate for ENC or RET (or both) was extracted for each significant voxel and averaged across voxels within the cluster and participants. A cluster indentified in the study-phase contrasts was classified as task-positive if ENC is significantly greater than zero (P < .05, two-tailed), task-negative if ENC is significantly less than zero (P < .05), and neutral if otherwise. A cluster indentified in the test-phase contrasts was classified as task-positive, task-negative, or neutral using a similar approach based on the contrast RET versus zero. For a cluster identified in conjunction analyses involving both study and test phases, its study- and test-phase activity respectively was classified as task-positive, task-negative, or neutral.
As noted above, in addition to Old and New words, the retrieval test included “Lure words” that were semantically related to studied lists. A caveat for the current study is whether the presence of false items in the recognition test affected the processing of old and new items. We believe this is not a significant problem because the inclusion of false items in a recognition test may affect mainly the placement of the decision criterion (e.g., make decisions more conservative) rather than the nature of retrieval processing per se. Though the nature of retrieval processing may also be affected, it is unlikely that this factor significantly alters the hypothesized association of retrieval processing with the task-positive network.
Results
Behavioral Performance
Category judgment at the study phase was highly accurate (mean, 95% correct). Behavioral results at the study phase, sorted by the number of subsequent high-confidence hits (5 conditions), are summarized in Table 2. The mean proportion of trials was significantly different for the 5 conditions (F4, 44 = 6.31, P < .001), reflecting relatively low proportion of trials associated with extremely high or low number (i.e., 4 or 0) of subsequent high-confidence hits (see Table 2). The mean RT was not significantly different for the 5 conditions (F4, 44 = .27, P > .80). There was no significant correlation between RT and the number of subsequent high-confidence hits across trials, computed within each subject and then averaged across subjects (r = .039, t11 = .76, P > .40). Behavioral results at the test phase, sorted by response type (sure old, unsure old, unsure new, sure new) and item status (old, new), are summarized in Table 3. Corrected recognition scores (hits – false alarms) were .44 for high-confidence responses and .09 for low-confidence responses. These scores were significantly above chance levels of performance in both high-confidence (t11 = 16.53, P < .001) and low-confidence responses (t11 = 2.33, P < .05). The mean RT was significantly longer for high-confidence correct rejection than for high-confidence hit (t11 = 6.51, P < .001).
Table 2.
Behavioral results at the study phase: mean proportion of trials and reaction time (SD)
| Number of subsequent high-confidence hits | Proportion of trials | Reaction time (ms) |
|---|---|---|
| 4 | .11 (.07) | 2701 (319) |
| 3 | .24 (.10) | 2705 (316) |
| 2 | .29 (.08) | 2692 (312) |
| 1 | .23 (.11) | 2631 (303) |
| 0 | .13 (.11) | 2739 (561) |
Table 3.
Behavioral results at the test phase: mean proportion of trials and reaction time (SD)
| Proportion of trials |
Reaction time (ms) |
|||
|---|---|---|---|---|
| Old | New | Old | New | |
| Sure old | .49 (.11) | .05 (.04) | 1240 (140) | 1502 (388) |
| Unsure old | .23 (.09) | .14 (.11) | 1490 (164) | 1534 (257) |
| Unsure new | .18 (.09) | .50 (.21) | 1542 (189) | 1500 (179) |
| Sure new | .08 (.07) | .28 (.20) | 1527 (264) | 1467 (209) |
Task-positive and task-negative activity
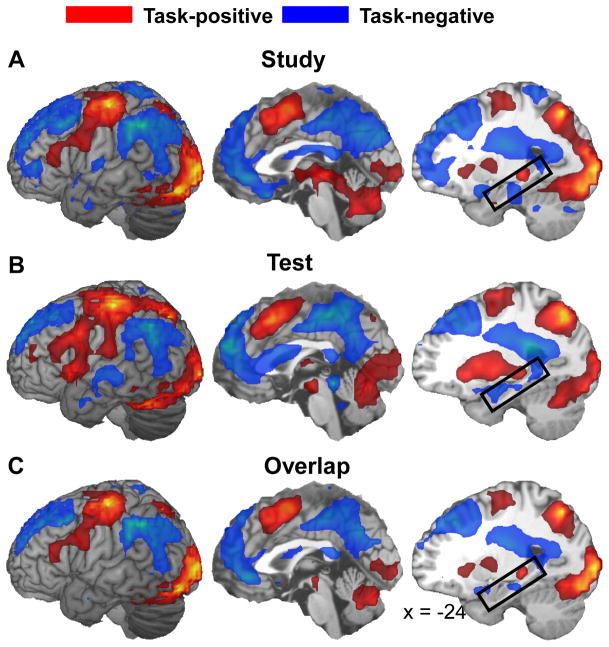
Fig. 2A illustrates study-phase task-positive and task-negative activity, Fig. 2B test-phase task-positive and task-negative activity, and Fig. 2C overlap between the two. Consistent with the distinction of task-positive and task-negative networks, despite different stimulus display and task requirements during encoding and retrieval, there was extensive overlap between encoding and retrieval activations (task-positive network) and between encoding and retrieval deactivations (task-negative network). During both encoding and retrieval, activations (task-positive network) included ventrolateral PFC, premotor/motor cortex, supplementary motor area (SMA)/preSMA, dorsal posterior parietal cortex (PPC), visual sensory/perceptual regions, basal ganglia, thalamus and cerebellum. The cluster centered in the thalamus (Talairach coordinate xyz = −19, −29, 1) included a left posterior hippocampal region (xyz = −23, −31, −4; see the boxed regions in Fig. 2. During both encoding and retrieval, deactivations (task-negative network) included anterior PFC, dorsolateral PFC, anterior cingulate/medial PFC, posterior cingulate/precuneus, ventral PPC, and bilateral hippocampal formation. These task-positive and task-negative regions are comparable to that observed in a number of relevant prior studies (e.g., Fox et al., 2005). The extensive overlap between encoding and retrieval activations (task-positive network) is also consistent with a recent report by Wiesmann and Ishai (2008), which showed a similar extensive overlap based on a nonverbal memory task.
Fig. 2.
Brain regions showing task-positive (red) and task-negative (blue) activity in the study phase (A) and the test phase (B), and overlap between the two (C). The boxed regions in the third column mark the hippocampal formation.
Encoding success, encoding failure, retrieval success, and novelty detection activity
Table 4 lists regions showing significant encoding success, encoding failure, retrieval success, and novelty detection activity, respectively. The regions were in general agreement with those reported in a number of prior studies of each activity (e.g., Tulving et al., 1996; Wagner et al., 1998; Henson et al., 1999; Otten and Rugg, 2001; Spaniol et al., 2009). We found significant encoding success activity within the left hippocampus, but no medial temporal region associated with retrieval success, encoding failure or novelty detection activity. As noted in the Behavioral results, the mean RT was significantly longer for high-confidence correct rejection versus high-confidence hit. However, ‘time on task’ is unlikely to adequately account for the present novelty detection effects, because no region showing the effects was associated with a significant correlation between RT and parameter estimate across several retrieval conditions listed in Table 3, computed within each subject and then averaged across subjects (P > .20 for each correlation).
Table 4.
Brain regions showing encoding success (A), encoding failure (B), retrieval success (C), and novelty detection activity (D)
| Talairach |
||||||||
|---|---|---|---|---|---|---|---|---|
| Voxels | TP/TN | Region | H | BA | x | y | z | t |
| A. Encoding success activity | ||||||||
| 176 | TP | Ventrolateral PFC | L | 44/45 | −46 | 20 | 24 | 5.21 |
| 28 | TP | Premotor cortex | L | 6 | −38 | −1 | 56 | 5.32 |
| 50 | TP | Medial premotor cortex | L | 6 | −4 | 11 | 56 | 5.26 |
| 7 | Ne | Hippocampus | L | - | −30 | −11 | −6 | 4.48 |
| 19 | TP | Occipital cortex | R | 19 | 49 | −77 | 14 | 5.42 |
| 472 | TP | Occipital cortex | B | 18/19 | −19 | −88 | −2 | 7.78 |
| Cerebellum | B | - | 8 | −82 | −21 | 5.57 | ||
| 80 | TP | Caudate | B | - | −15 | −4 | 8 | 6.6 |
| B. Encoding failure activity | ||||||||
| 209 | TN | Anterior medial PFC | B | 10 | 4 | 52 | 1 | 9.34 |
| Anterior cingulate cortex | B | 32 | 8 | 41 | 5 | 5.54 | ||
| 77 | TN | Dorsolateral PFC | L | 9 | −23 | 42 | 33 | 5.22 |
| 207 | TN | Dorsolateral PFC | R | 9 | 27 | 39 | 33 | 6.77 |
| 24 | TN | Dorsolateral PFC | R | 8 | 19 | 17 | 52 | 4.89 |
| 827 | TN | Precuneus | B | 7 | −8 | −76 | 42 | 10.5 |
| Posterior cingulate cortex | B | 23/31 | 11 | −35 | 37 | 14.2 | ||
| 249 | TN | Ventral PPC | R | 39/40 | 64 | −43 | 27 | 7.39 |
| C. Retrieval success activity | ||||||||
| 188 | TN | Precuneus | B | 7 | 4 | −62 | 31 | 5.33 |
| Posterior cingulate cortex | B | 31 | −15 | −65 | 24 | 4.47 | ||
| 30 | TN | Ventral PPC | R | 39 | 46 | −72 | 28 | 4.16 |
| 57 | TN | Ventral PPC | L | 39 | −49 | −58 | 31 | 4.74 |
| 59 | TP | Occipito-parietal cortex | R | 19/7 | 30 | −67 | 52 | 6.15 |
| 78 | TP | Ventral occipital cortex | L | 18/19 | −11 | −74 | −3 | 6.04 |
| D. Novelty detection activity | ||||||||
| 623 | TP | Premotor cortex | B | 6 | −30 | −12 | 57 | 7.20 |
| Pre/Postcentral cortex | L | 1/3/4 | −34 | −24 | 50 | 7.00 | ||
| 38 | TP | Insula | L | 13 | −42 | −3 | 10 | 3.92 |
| 268 | TP | Occipital cortex | R | 18 | 13 | −78 | −3 | 3.95 |
| Cerebellum | R | - | 8 | −67 | −9 | 8.05 | ||
| 19 | TP | Cerebellum | L | - | −34 | −53 | −26 | 4.36 |
| 16 | TP | Thalamus | L | - | −15 | −22 | 8 | 4.11 |
TP, task-positive; TN, task-negative; Ne, neutral; H, hemisphere; L, left; R, right; B, bilateral; BA, Brodmann area; PFC, prefrontal cortex; PPC, posterior parietal cortex.
For each significant cluster listed in Table 4, we performed a follow-up ROI analysis to determine whether its overall activity at the study or test phase is task-positive, task-negative, or neutral. The results are shown in the second column of Table 4. Confirming our hypotheses, encoding success activity and novelty detection activity respectively were associated mainly with the task-positive network, whereas encoding failure activity and retrieval success activity respectively were associated mainly with the task-negative network. There were only 3 “atypical” clusters out of 23 listed in Table 4. First, the hippocampal region associated with encoding success activity showed no significant difference from baseline. Second, the left ventral occipital and occipito-parietal regions associated with retrieval success activity showed task-positive activity.
Overlapping activity
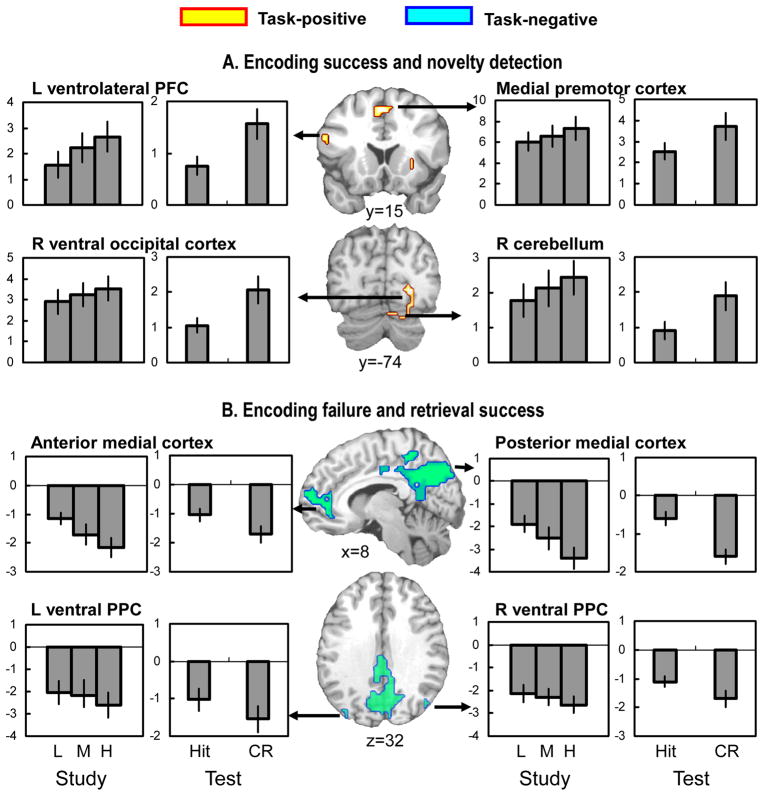
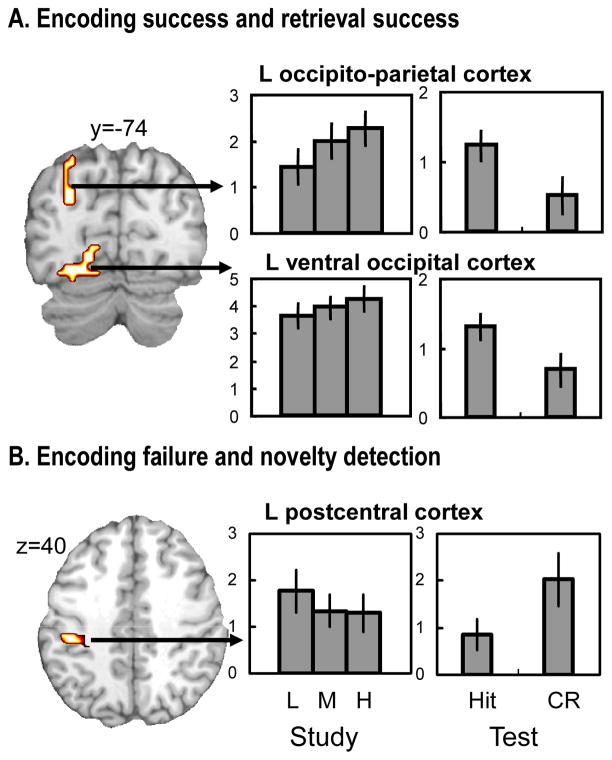
Table 5 shows overlaps along the columns and diagonals of the matrix in Table 1. As previously noted, we predicted overlaps along the columns but not along the diagonals of the matrix. Fig. 3 shows overlapping activity between encoding success and novelty detection and between encoding failure and retrieval success (i.e., the columns), whereas Fig. 4 shows overlapping activity between encoding success and retrieval success and between encoding failure and novelty detection (i.e., the diagonals). Results of follow-up ROI analyses to confirm overall activation (task-positive) and deactivation (task-negative) are listed in the second and third column of Table 5. First, confirming the first prediction regarding study-test overlap, there was high level of overlap between encoding success and novelty detection activity involving the task-positive network (Table 5A, Fig. 3A). The overlapping regions included left ventrolateral PFC, left premotor cortex, medial premotor cortex, right ventral occipital cortex, and cerebellum. Second, confirming the second prediction, there was high level of overlap between encoding failure and retrieval success activity involving the task-negative network (Table 5B, Fig. 3B). The overlapping regions included anterior cingulate/medial PFC, posterior cingulate/precuneus, and right ventral PPC, and at a slightly lowered extent threshold (10 voxels), left ventral PPC. Third, confirming the third prediction, there was relatively low level of overlap between encoding success and retrieval success activity (Table 5C, Fig. 4A) as well as between encoding failure and novelty detection activity (Table 5D, Fig. 4B). The overlapping region across encoding success and retrieval success activity was confined to left sensory/perceptual area involving left ventral occipital and left occipito-parietal regions. These regions were within the task-positive network. The overlapping region across encoding failure and novelty detection activity was found in a single region involving postcentral cortex. This region was also within the task-positive network.
Table 5.
Brain regions showing overlapping activity across the study- and test-phases
| TP/TN |
Talairach |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Voxels | Study | Test | Region | H | BA | x | y | z | t |
| A. Both encoding success and novelty detection activity | |||||||||
| 101 | TP | TP | Ventrolateral PFC | L | 44 | −52 | 16 | 24 | 4.47 |
| Premotor cortex | L | 6 | −46 | 5 | 27 | 6.39 | |||
| 15 | TP | TP | Insula | R | 47 | 27 | 22 | −4 | 4.21 |
| 16 | TP | TP | Premotor cortex | L | 6 | −38 | −1 | 56 | 5.32 |
| 29 | TP | TP | Medial premotor cortex | B | 6 | 0 | 10 | 52 | 3.57 |
| 90 | TP | TP | Ventral occipital cortex | R | 18 | 23 | −74 | 0 | 6.92 |
| Cerebellum | R | - | 14 | −74 | −19 | 3.38 | |||
| B. Both encoding failure and retrieval success activity | |||||||||
| 93 | TN | TN | Anterior medial PFC | R | 10 | 11 | 59 | 4 | 3.83 |
| Anterior cingulate cortex | R | 32 | 8 | 41 | −5 | 2.75 | |||
| 636 | TN | TN | Precuneus | B | 7 | 4 | −62 | 31 | 5.33 |
| Posterior cingulate cortex | B | 23/31 | −15 | −65 | 24 | 4.47 | |||
| 23 | TN | TN | Ventral PPC | R | 39 | 46 | −72 | 28 | 4.16 |
| C. Both encoding success and retrieval success activity | |||||||||
| 126 | TP | TP | Ventral occipital cortex | L | 18/19 | −15 | −92 | −2 | 6.3 |
| 46 | TP | TP | Occipito-parietal cortex | L | 19/7 | −30 | −72 | 31 | 4.78 |
| D. Both encoding failure and novelty detection activity | |||||||||
| 26 | TP | TP | Postcentral cortex | L | 2 | −49 | −27 | 40 | 4.36 |
TP, task-positive; TN, task-negative; Ne, neutral; H, hemisphere; L, left; R, right; B, bilateral; BA, Brodmann area; PFC, prefrontal cortex; PPC, posterior parietal cortex.
Fig. 3.
(A) Brain regions showing both encoding success and novelty detection activity, and (B) brain regions showing both encoding failure and retrieval success activity. The bar graphs display mean parameter estimates (in arbitrary units) across all significant voxels within each cluster. The error bars indicate ± 1 standard error. For illustration of parametric effects in the study phase, encoding trials were classified into low (L; x = 0, 1), medium (M; x = 2), and high (H; x = 3 or 4) subsequent hits. The bars in the study-phase graphs represent the parameter estimates of these 3 trial types. PFC, prefrontal cortex; PPC, posterior parietal cortex; CR, correct rejection.
Fig. 4.
(A) Brain regions showing both encoding success and retrieval success activity, and (B) brain regions showing both encoding failure and novelty detection activity. See Fig. 3 legend for explanation of the bar graphs.
Discussion
Several recent studies support the idea that there are two complementary brain networks, a task-positive network dedicated to externally-oriented processing and a task-negative network dedicated to internally-oriented processing (e.g., Binder et al., 1999; Fox et al., 2005; Fransson, 2005; Golland et al., 2008). However, functional neuroimaging studies of episodic memory have yet to fully explore implication of this notion. The present study investigated what roles the task-positive and task-negative networks play in overlapping activity across episodic memory encoding and retrieval. There were 3 main findings. First, there was relatively extensive overlap between encoding success and novelty detection activity involving the task-positive network. Second, there was relatively extensive overlap between encoding failure and retrieval success activity involving the task-negative network. Third, there was relatively limited overlap between encoding success and retrieval success activity and between encoding failure and novelty detection activity. These 3 findings are discussed in separate sections below.
Roles of the task-positive network
Consistent with the first column of the matrix in Table 1, encoding success activity was associated mainly with the task-positive network, novelty detection activity was associated mainly with the task-positive network, and overlaps between the two activities occurred within the task-positive network. It is worth noting that association of encoding success activity and novelty detection activity each with the task-positive network does not necessarily predict overlap between the two, because they could a priori involve different subcomponents of the task-positive network. Thus, the finding of actual overlap underscores regional similarity between encoding success and novelty detection activity within the task-positive network. Since both encoding success and novelty detection activity are heavily dependent upon attention to external stimulus, association of the two activities with the task-positive network fits well with the notion that the network is principally involved in externally-oriented processing. Though a few previous studies (e.g., Kirchhoff et al., 2000; Stark and Okado, 2003) have reported co-localization of novelty and subsequent memory effects within specific regions of interest, the present results are important in showing the overlap in more wide-spread areas of the brain involving the task-positive network. Incidentally, the present finding that encoding success and novelty detection activity share widespread areas of the brain is consistent with the “novelty-encoding” hypothesis (Tulving et al., 1996), which posits that novelty assessment is an early stage of long-term memory encoding, and hence, predicts regional overlap between novelty assessment and encoding activity.
The task-positive regions showing overlap between encoding success and novelty detection activity involved left ventrolateral PFC, premotor cortex, occipital cortex, and cerebellum. Both encoding success and novelty detection activity consist of multiple subprocesses including sensory/perceptual processing, attention, response selection, and other executive processes. Most likely, each overlapping region mediates differential subcomponents of encoding success and novelty detection activity, and more generally, externally-oriented processing. For example, activation of occipital cortex may reflect sensory/perceptual processing of stimulus information, and activation of ventrolateral PFC semantic processing (e.g., Gabrieli et al., 1998). Activation of premotor area and cerebellum may reflect response selection and motor-related alertness and readiness to changes in external environments (e.g., Shulman, Corbetta et al., 1997; Fransson, 2006).
Roles of the task-negative network
Consistent with the second column of the matrix in Table 1, encoding failure activity was associated mainly with the task-negative network, retrieval success activity was associated mainly with the task-negative network, and overlaps between the two activities occurred within the task-negative network. Though association of encoding failure activity and retrieval success activity each with the task-negative network was noted in some prior studies (e.g., Daselaar et al., 2004; Buckner and Carroll, 2007; Addis et al., 2007), the present report is the first to show widespread overlap within the task-negative network. Again, the finding of actual overlap between encoding failure and retrieval success activity within the task-negative network is important, because it does not necessarily follow from separate associations of each activity with the task-negative network. Encoding failure activity likely involves stimulus-independent thoughts or mind wandering (McKiernan et al., 2006; Mason et al., 2007), and retrieval success activity, attention to internal mnemonic representations and subsequent remembering state. Thus, association of encoding failure and retrieval success activity with the task-negative network fits well with the notion that the network is principally involved in internally-oriented processing. Association of encoding failure activity with the task-negative network is also consistent with other recent evidence (Weissman et al., 2006; Li et al., 2007) that activation of the task-negative network during attention-demanding cognitive tasks is associated with performance errors. Given that access to internal mnemonic associations is central to association of retrieval success activity with the task-negative network, vivid remembering, but not necessarily non-vivid remembering, may involve the task-negative network. Consistent with this hypothesis, additional analyses involving low-confidence hits showed that low-confidence recognition is associated mainly with the task-positive network (see Table S1 in supplementary material available online). This association may reflect goal-directed, controlled search processes when remembering is difficult.
The task-negative regions showing overlap between encoding failure and retrieval success activity involved most major components of the task-negative network, including anterior cingulate/medial PFC, posterior cingulate/precuneus, and bilateral ventral PPC. These regions are most likely to be involved in different aspects of internally-oriented processing. Activation of anterior medial cortex may be associated possibly with self-referential processing (e.g., Gusnard et al., 2001), activation of lateral ventral parietal cortex possibly with bottom-up attention to the retrieval output (e.g., Cabeza et al., 2008), and activation of posterior medial cortex possibly with integration of mnemonic and other internally-oriented processing (e.g., Buckner et al., 2008). In a recent meta-analysis (Daselaar et al., 2009), we found that across five different fMRI studies, activity in ventral parietal and posterior midline regions was associated with unsuccessful encoding (encoding failure activity) but with successful retrieval (hits > misses). The present results generalize this finding to other components of the task-negative network and to other retrieval contrasts (hits > correct rejections).
A recent meta-analysis (Binder et al., in press) of 120 functional neuroimaging studies focusing on semantic processing suggested a large overlap between conceptual processing and task-negative networks and between perceptual processing and task-positive networks. Commenting on these overlaps, the authors noted that “conceptual processes operate on ‘internal’ sources of information, such as semantic and episodic memory, which can be retrieved and manipulated at any time, independent of ongoing external events. In contrast, perceptual processes operate on ‘external’ information derived from immediate, ongoing sensory and motor processes that coordinate interactions with the external environment”. This conceptual-perceptual perspective provides a potentially important rationale for why the task-negative network involves mainly heteromodal (or amodal) association areas as well as why the task-positive network involves mainly unimodal sensory-perceptual and motor areas (see Fig. 2).
Roles of opposing modes of processing
As expected from the association of encoding success activity mainly with the task-positive network and retrieval success activity mainly with the task-negative network, we found relatively small overlap between the two activities (one of the diagonals in the matrix of Table 1). This overlap involved the task-positive network. Consistent with prior reports (e.g., Persson and Nyberg, 2000; Johnson and Rugg, 2007), the overlapping region was limited to sensory/perceptual areas, involving left ventral occipital and occipito-parietal regions. As the reinstatement hypothesis suggests, activation of this region during the test phase may represent reactivation of encoding-related sensory/perceptual activity (Nyberg et al., 2000; Wheeler et al., 2000). A question of interest is whether this reactivation is specific to high-confidence recognition, or common to high- and low-confidence recognition (Wheeler et al., 2000; Johnson and Rugg, 2007). To address this question, we performed additional ROI analyses involving high-confidence as well as low-confidence hit responses. The results showed that activation of the sensory/perceptual area was common to high- and low-confidence hits (see Fig. S1 in supplementary material available online). Thus, reactivation of the sensory/perceptual area may support processes common to high-and low-confidence recognition, and by implication, recollection and familiarity. Supposing that all recollected items are also familiar (e.g., Joordens and Merikle, 1993; Knowlton, 1998), such common processes may be familiarity-related.
Also as expected, we found low level of overlap between encoding failure and novelty detection activity (i.e., the other diagonal of the matrix in Table 1). The overlap was detected in a single region involving left postcentral cortex. This region was within the task-positive network, making its association with encoding failure activity more surprising than its association with novelty detection activity. While some caution in interpreting this finding seems necessary given that location of this region was beyond our predictions, it is noteworthy that a similar region (xyz = −34, −24, 54) was also associated with task-positive, encoding failure activity in a previous study (Daselaar et al. 2004). Activation of this somatosensory region may represent misallocation of processing resources that is detrimental to encoding.
Conclusions
The present study represents one of the more comprehensive attempts to relate the notion of the task-positive and task-negative networks to episodic memory function. There were 3 main findings. First, there was a relatively high level of overlap between encoding success and novelty detection activity within the task-positive network, reflecting common involvement of externally-oriented processing. Second, there was a relatively high level of overlap between encoding failure and retrieval success activity within the task-negative network, reflecting common involvement of internally-oriented processing. Third, there was a low level of overlap between encoding success and retrieval success activity, and between encoding failure and novelty detection activity, respectively, reflecting involvement of mainly opposing modes of processing in the two activities. Collectively, these findings clarify roles of the task-positive and task-negative networks in encoding and retrieval, and support a theoretical account of overlapping encoding-retrieval networks that goes beyond the reinstatement hypothesis. More generally, the findings indicate that the notion of two complementary networks provides a helpful framework for interpreting functional neuroimaging results.
Supplementary Material
Acknowledgments
This work was supported by a Daegu University research grant in 2008 to H.K., and National Institutes of Health Grant AG19731 and AG23770 to R.C. We thank Amber Baptiste for participant recruitment, Rakesh Arya for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, Wong AT, Schacter DL. Remembering the past and imaging the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battig WF, Montague WE. Category norms for verbal items in 56 categories: A replication and extension of the Connecticut norms. J Exp Psychol. 1969;80:1–46. [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system ? a critical review and meta-analysis of 129 functional neuroimaging studies. Cereb Cortex. doi: 10.1093/cercor/bhp055. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Rao SM, Cox RW. Conceptual processing during the conscious resting state: a functional MRI study. J Cogn Neurosci. 1999;11:80–93. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Wheeler ME, Sheridan MA. Encoding processes during retrieval tasks. J Cogn Neurosci. 2001;13:406–415. doi: 10.1162/08989290151137430. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Rao SM, Wagner AD, Mayer AR, Schacter DL. Can medial temporal lobe regions distinguish true from false? An event-related functional MRI study of veridical and illusory recognition memory. Proc Natl Acad Sci USA. 2001;98:4805–4810. doi: 10.1073/pnas.081082698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. NeuroImage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Dennis NA, Hayes SM, Kim H, Cabeza R. Posterior midline and ventral parietal activity is associated with retrieval success and encoding failure. Front Hum Neurosci. 2009;3:13. doi: 10.3389/neuro.09.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Essen DCV, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci USA. 1998;95:906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golland Y, Golland P, Bentin S, Malach R. Data-driven clustering reveals a fundamental subdivision of the human cortex into two global systems. Neuropsychologia. 2008;46:540–553. doi: 10.1016/j.neuropsychologia.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Rugg MD. Recollection and the reinstatement of encoding-related cortical activity. Cereb Cortex. 2007;17:2507–2515. doi: 10.1093/cercor/bhl156. [DOI] [PubMed] [Google Scholar]
- Joordens S, Merikle PM. Independence or redundancy? two models of conscious and unconscious influences. J Exp Psychol Gen. 1993;122:462–467. [Google Scholar]
- Kim H, Cabeza R. Differential contributions of prefrontal, medial temporal, and sensory-perceptual regions to true and false memory formation. Cereb Cortex. 2007a;17:2143–2150. doi: 10.1093/cercor/bhl122. [DOI] [PubMed] [Google Scholar]
- Kim H, Cabeza R. Trusting our memories: dissociating the neural correlates of confidence in veridical versus illusory memories. J Neurosci. 2007b;27:12190–12197. doi: 10.1523/JNEUROSCI.3408-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Cabeza R. Common and specific brain regions in high- versus low-confidence recognition memory. Brain Res. 2009;1282:103–113. doi: 10.1016/j.brainres.2009.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J Neurosci. 2000;20:6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton BJ. The relationship between remembering and knowing: a cognitive neuroscience perspective. Acta Psychol. 1998;98:253–265. doi: 10.1016/s0001-6918(97)00045-0. [DOI] [PubMed] [Google Scholar]
- Li CS, Yan P, Bergquist KL, Sinha R. Greater activation of the “default” brain regions predicts stop signal errors. NeuroImage. 2007;38:640–648. doi: 10.1016/j.neuroimage.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, D’Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: an fMRI investigation. NeuroImage. 2006;29:1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration? Conscious Cogn. 2005;14:390–425. doi: 10.1016/j.concog.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Habib R, McIntosh AR, Tulving E. Reactivation of encoding-related brain activity during memory-retrieval. Proc Natl Acad Sci USA. 2000;97:11120–11124. doi: 10.1073/pnas.97.20.11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. When more means less: neural activity related to unsuccessful memory encoding. Curr Biol. 2001;11:1528–1530. doi: 10.1016/s0960-9822(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Persson J, Nyberg L. Conjunction analysis of cortical activations common to encoding and retrieval. Microsc Res Tech. 2000;51:39–44. doi: 10.1002/1097-0029(20001001)51:1<39::AID-JEMT4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Rainer G. Neural mechanisms for detecting and remembering novel events. Nat Rev Neurosci. 2003;4:193–202. doi: 10.1038/nrn1052. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Corbetta M, Buckner RL, Fiez JA, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: I. Increases in subcortical structures and cerebellum but not in nonvisual cortex. J Cogn Neurosci. 1997;9:624–647. doi: 10.1162/jocn.1997.9.5.624. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin F, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PSR, Kim ASN, Han H, Moscovitch M, Grady CL. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Stark CEL, Okado Y. Making memories without trying: medial temporal lobe activity associated with incidental memory formation during recognition. J Neurosci. 2003;23:6748–6753. doi: 10.1523/JNEUROSCI.23-17-06748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- Tulving E, Kroll N. Novelty assessment in the brain and long-term memory encoding. Psychon Bull Rev. 1995;2:387–390. doi: 10.3758/BF03210977. [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ, Craik FIM, Habib R, Houle S. Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb Cortex. 1996;6:71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Wagner AD. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol Learn Mem. 2009;91:139–154. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Davachi L. Cognitive neuroscience: forgetting of things past. Curr Biol. 2001;11:R964–R967. doi: 10.1016/s0960-9822(01)00575-9. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural basis of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Petersen SE, Buckner RL. Memory’s echo: vivid remembering reactivates sensory-specific cortex. Proc Natl Acad Sci USA. 2000;97:11125–11129. doi: 10.1073/pnas.97.20.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesmann M, Ishai A. Recollection- and familiarity-based decisions reflect memory strength. Front Syst Neurosci. 2008;2:1. doi: 10.3389/neuro.06.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon C, Feinberg F, Hu P, Gutchess AH, Hedden T, Chen H, Jing Q, Cui Y, Park DC. Category norms as a function of culture and age: Comparisons of item responses to 105 categories by American and Chinese adults. Psychol Aging. 2004;19:379–393. doi: 10.1037/0882-7974.19.3.379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.