Abstract
During Pavlovian conditioning the expression of a conditioned response is typically taken as evidence that an association between a conditioned stimulus (CS) and an unconditioned stimulus (UCS) has been formed. However, learning-related changes in the unconditioned response (UCR) produced by a predictable UCS can also develop. Learning-related reductions in UCR magnitude are often referred to as UCR diminution. In the present study, we examined UCR diminution in the functional magnetic resonance imaging (fMRI) signal by pairing supra and sub-threshold CS presentations with a UCS. UCR diminution was observed within several brain regions associated with fear learning and memory including the insula, inferior parietal lobe, ventromedial prefrontal cortex (PFC), dorsomedial PFC, and dorsolateral PFC. CS perception appeared to mediate UCR diminution within the ventromedial PFC and posterior cingulate cortex. UCRs within these regions were larger when the UCS followed an unperceived compared to a perceived CS. UCS expectancies appeared to modulate UCRs within the dorsomedial PFC, dorsolateral PFC, insula, and inferior parietal lobe. Activity within these regions showed an inverse relationship with participants’ UCS expectancies, such that as UCS expectancy increased UCR magnitude decreased. In addition, activity within the dorsomedial PFC, dorsolateral PFC, and insula showed a linear relationship with unconditioned skin conductance response expression. These findings demonstrate UCR diminution within the fMRI signal, and suggest that UCS expectancies modulate prefrontal cortex responses to aversive stimuli. In turn, prefrontal cortex activity appears to modulate the expression of unconditioned SCRs.
During Pavlovian conditioning, the presentation of a conditioned stimulus (CS) predicts an unconditioned stimulus (UCS). Typically, expression of a conditioned response (CR) to the CS is taken as evidence that an association between the CS and UCS has been learned. An important consequence of learning the CS-UCS relationship is that an individual can respond to the UCS more effectively (Domjan, 2005). For example, prior work has shown that when an aversive stimulus is anticipated, less pain is experienced (Fanselow & Baackes, 1982). Several studies have demonstrated similar learning-related changes in the unconditioned response (UCR) during Pavlovian conditioning (see Domjan 2005 for review). Many of these studies have observed a reduction in UCR magnitude as associative learning develops, and have demonstrated that smaller UCRs are produced when the UCS follows a CS compared to when the UCS is presented alone (Baxter, 1966; Kimmel, 1967). This effect, known as UCR diminution, appears to be mediated by an associative learning process (Baxter, 1966; Kimmel, 1967; Marcos and Redondo, 1999) and is influenced by conscious UCS expectancies (Dunsmoor et al., 2008; Rust, 1976).
Previous conditioning research has investigated diminution of unconditioned skin conductance responses (SCR) using differential training procedures in which one CS is paired with the UCS (CS+) while a second CS is presented alone (CS−). This work has shown that UCR magnitude is reduced when the UCS follows the CS+ compared to when the UCS follows the CS− on test trials (Marcos and Redondo, 1999). These findings indicate the CS+ gains discriminative control over the UCR during Pavlovian conditioning. Other research has shown that UCR diminution is greater when participants expect to receive a UCS, suggesting that conscious UCS expectancies modulate UCR expression (Dunsmoor, et al. 2008; Rust, 1976).
Prior fMRI research has identified a number of brain regions that appear to mediate fear learning and memory processes. These regions include the prefrontal and sensory cortices as well as the amygdala, hippocampus, thalamus, cingulate, and insula (Büchel et al., 1998, 1999; Dunsmoor et al., 2007; Knight, et al., 1999, 2004, 2009; LaBar et al., 1998; Phelps et al., 2004). These studies suggest the amygdala is important for learning CS-UCS associations and expressing CRs (Büchel et al., 1998; Cheng et al., 2003, 2006; Knight et al., 2005; LaBar et al., 1998). In addition, prior work suggests that a network of brain regions including the amygdala, hippocampus, and ventromedial prefrontal cortex mediates extinction-related processes (Kalisch et al., 2006; Milad et al., 2005; Milad et al., 2007; Phelps et al., 2004). This circuit appears to play a role in reducing the expression of learned fear behaviors during extinction, and may mediate the learning-related diminution of UCRs. Further, the dorsolateral and medial prefrontal cortices appear to support conscious, top-down functions that are associated with contingency awareness and influence other learning-related processes (Carter et al., 2006; Dunsmoor et al., 2008; McIntosh et al., 2003). These brain regions may work in concert to diminish UCR magnitude during Pavlovian conditioning.
Prior research investigating the neural substrates of UCR diminution has observed learning-related changes within the amygdala, thalamus, anterior cingulate, inferior parietal lobe, auditory cortex, and dorsolateral PFC (Dunsmoor et al., 2008). Related work suggests that the dorsolateral PFC and insula are more responsive when UCS presentation is uncertain (Dunsmoor et al., 2007), and indicates that UCS expectancies may modulate unconditioned fMRI signal responses (Dunsmoor et al., 2008). Specifically, the magnitude of the unconditioned fMRI signal within the amygdala, anterior cingulate, and dorsolateral PFC decreases as UCS expectancy increases (Dunsmoor et al., 2008). This inverse relationship between UCR magnitude and UCS expectancy suggests that conscious expectations influence brain and behavioral responses to aversive stimuli. Although this prior work indicates that UCR diminution can be observed within several brain regions (Dunsmoor et al., 2008), it remains unclear how these learning-related changes in brain activity influence the unconditioned SCRs that are expressed.
The present study investigated unconditioned fMRI signal responses from a previously published study on explicit and implicit memory processes (Knight et al., 2009). In this study, one tone was paired with a UCS (CS+), while a second tone was presented alone (CS−). These tones were presented at supra and sub-threshold levels. In the present study we investigated the fMRI signal response produced by a UCS paired with these supra and sub-threshold CS+ presentations. In addition, SCR and UCS expectancy were monitored to determine the relationship between these behavioral measures and the fMRI signal.
Materials and Methods
Participants
Fifteen healthy right-handed volunteers (8 male and 7 female; mean age, 28.87±1.69 years; range, 22 to 39 years) participated in this study. All subjects provided written informed consent in compliance with the National Institute of Mental Health Institutional Review Board.
Conditioned and Unconditioned Stimuli
Auditory stimuli were presented via a pneumatic headphone system using passive noise cancellation ear defenders. Two pure tones (700 and 1300 Hz) were presented as CSs (10 s duration, 20 s inter-trial interval) during the training session. The CS+ (60 trials) coterminated with a 500 ms loud (100 dB) white-noise UCS and the CS− (60 trials) was presented alone. The tones that served as the CS+ and CS− were counter-balanced and presented in a pseudo-random order such that no more than 2 trials of the same CS were consecutively presented. CS volume was initially set at 65 dB. The volume of the CS+ and CS− were modulated on a trial-by-trial basis for each subject using an adaptive threshold estimation procedure as described below.
UCS Expectancy
An MRI compatible joystick was used to monitor CS perception and UCS expectancy. CS perception was monitored by instructing subjects to push a button on the joystick immediately upon hearing either tone. In addition, the joystick controlled a rating bar presented throughout training at the bottom of the visual display. Subjects were instructed to rate their UCS expectancy on a continuous scale from 0 to 100 (0 = certain that the UCS will not be presented, 50 = uncertain whether the UCS will be presented, 100 = certain that the UCS will be presented), and were instructed to continuously update (sampled at 10 Hz) their rating to reflect their current UCS expectancy.
SCR
A Contact Precision Instruments (Cambridge, MA) skin conductance monitoring system was used to monitor skin conductance response (SCR) throughout the assessment. SCR was sampled (40 Hz) with a pair of surface gel cup electrodes [silver/silver chloride, 6mm diameter, BIOPAC (Goleta, CA) model TSD203] attached to the distal phalanx of the middle and ring fingers of the nondominant hand.
Procedure
Subjects were informed that two tones would be repeatedly presented and told that the volume of each tone would vary above and below their perceptual threshold. Subjects were instructed to push the button immediately upon hearing a tone, and to update their UCS expectancy accordingly. Unknown to the subjects, the volume of each CS was controlled by their button press responses, such that the volume of the CS was decreased by 5 dB following perceived trials (i.e. when a button press was made). CS volume was increased by 5 dB following unperceived trials (i.e. when a button press was not made). The volume of the CS+ and CS− were modulated independently.
Behavioral Data Analysis
UCS expectancy was calculated as the average (1 s sample; 10 Hz) response beginning 1 s prior to CS termination. Skin conductance responses (SCR) were also monitored during the conditioning session. SCR amplitude was calculated by subtracting the average skin conductance measurement during the baseline period (5 s immediately preceding CS presentation) from the unconditioned response (peak response during the 5 s following UCS presentation). T-test comparisons of UCS expectancy and unconditioned SCR data were completed for perceived versus unperceived CS+ trials. In addition, multiple linear regression was completed for each subject to determine the influence of CS Perception and UCS expectancy on unconditioned SCR magnitude. Beta coefficients were obtained from each subject’s regression analysis and included in a single group T-test to determine whether CS perception or UCS expectancy significantly modulated unconditioned SCRs.
Functional Image Acquisition and Analysis
Structural and functional imaging was completed on a 1.5 Tesla General Electric Signa scanner using a brain-specific RF head coil (Medical Advances, Milwaukee, WI). Functional imaging of the entire brain was conducted using a gradient-echo echoplanar pulse sequence (TR = 2000 ms, TE = 40 ms, FOV = 24 cm, matrix = 64 × 64, slice thickness = 6 mm) during each of four 920 s blocks of stimulus presentations. High-resolution anatomical images (SPGR) were obtained to serve as an anatomical reference. Image processing was performed with the AFNI software package (Cox, 1996). Echo-planar time series data were motion corrected, concatenated, and reregistered to the fifth volume of the first functional imaging scan. Multiple linear regression was performed using a gamma variate hemodynamic response function (HRF) to model CS+, CS−, and UCS presentations. Additional UCS regressors were included to model UCS activity modulated by CS perception and UCS expectancy. Thus, the UCS was modeled with an unmodulated regressor, a regressor modulated by CS perception, and a regressor modulated by UCS expectancy. Similar regressors were included for the post-CS− period to serve as an additional control, as were regressors to account for head motion and motor processes. Functional maps reflecting the beta values for UCS regressors modulated by CS perception and UCS expectancy were converted to a standard stereotaxic coordinate system (Talairach & Tournoux, 1998) and spatially blurred using a 4 mm full-width-at-half-maximum isotropic Gausian filter. Single group T-test comparisons were completed for the functional maps reflecting CS perception and UCS expectancy modulated responses using a significance threshold (p<0.005 uncorrected; t>3.22) that Monte Carlo simulations indicated was significant at a p<0.05 (corrected) level when restricted to clusters of activation larger than 420 mm3. Areas of activation that passed this threshold were then used as regions of interest (ROIs) to further investigate the relationship between the fMRI signal and behavioral measures. This was accomplished by completing a secondary analysis that modeled the hemodynamic response produced by the UCS when binned by participants’ UCS expectancy ratings (i.e. 0–25, 25–50, 50–75, 75–100). The percent area under images two through four (4–10 s after UCS presentation) of the hemodynamic response curve (AUC), which follow UCS presentation on CS+ trials, was compared to a resting baseline (normalized mean activation of fMRI scans) and used as an index of UCR magnitude. A similar analysis was performed for CS− trials to serve as another control. An additional analysis modeled the hemodynamic response produced by the UCS when binned in relationship to participants’ unconditioned SCR magnitude. This analysis compared the fMRI signal associated with four discrete SCR amplitude ranges (i.e. 0–25%, 25–50%, 50–75%, and 75–100%) where 0–25% reflects the smallest 25% of UCRs, 25–50% and 50–75% reflect intermediate UCR amplitude ranges, and 75–100% represents the largest 25% of UCRs for each participant.
Results
Conditioned Stimuli
An independent perceptual threshold was determined for each subject using an adaptive threshold estimation procedure. Subjects pressed a button to index CS perception. Response times averaged 2399±198 ms (average range = 768±53 to 8193±413 ms). CS volume averaged 57±2 dB, approximately 10–15 dB louder than CS volume in similar studies performed without the acoustic noise associated with fMRI (Knight et al., 2003, 2006). By design, the volume of perceived CS presentations was higher than the volume of unperceived CS presentations (t[14]=8.49, p<0.05). The number of perceived (Block 1 = 8.27 ± 0.30, Block 2 = 8.20 ± 0.31, Block 3 = 8.40 ± 0.29, Block 4 = 8.40 ± 0.36; all values reflect mean number of trials ± SEM) and unperceived (Block 1 = 6.73 ± 0.30, Block 2 = 6.80 ± 0.31, Block 3 = 6.60 ± 0.29, Block 4 = 6.60 ± 0.36) CS+ trials were evenly distributed across the conditioning session (F<1.00). However, the total number of perceived CS+ trials (33.47 ± 1.17) was larger than the number of unperceived CS+ trials (26.53 ± 1.17; t[14]=2.77, p<0.05). A cross-correlation analysis showed no temporal correlation between CS type and CS perception (r < ± 0.04 at lags ± 1–5). Additional details regarding CS presentations have been published previously (Knight et al., 2009).
SCR and UCS Expectancy
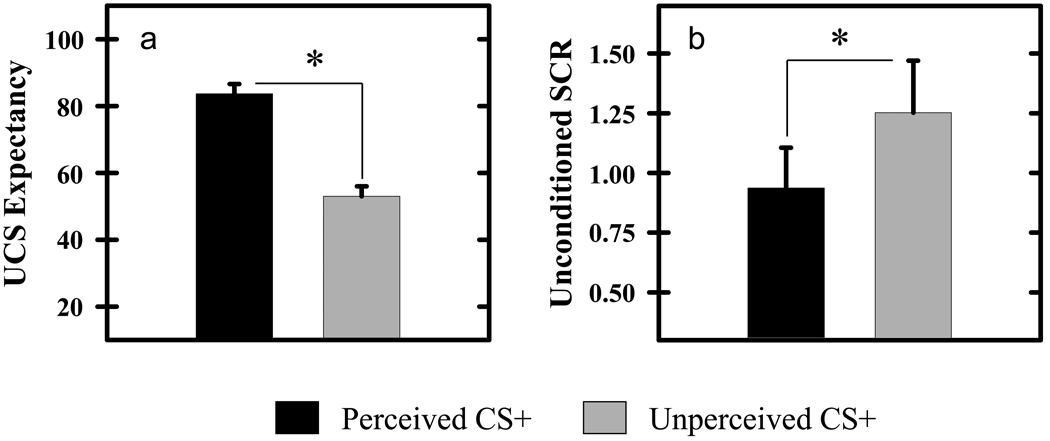
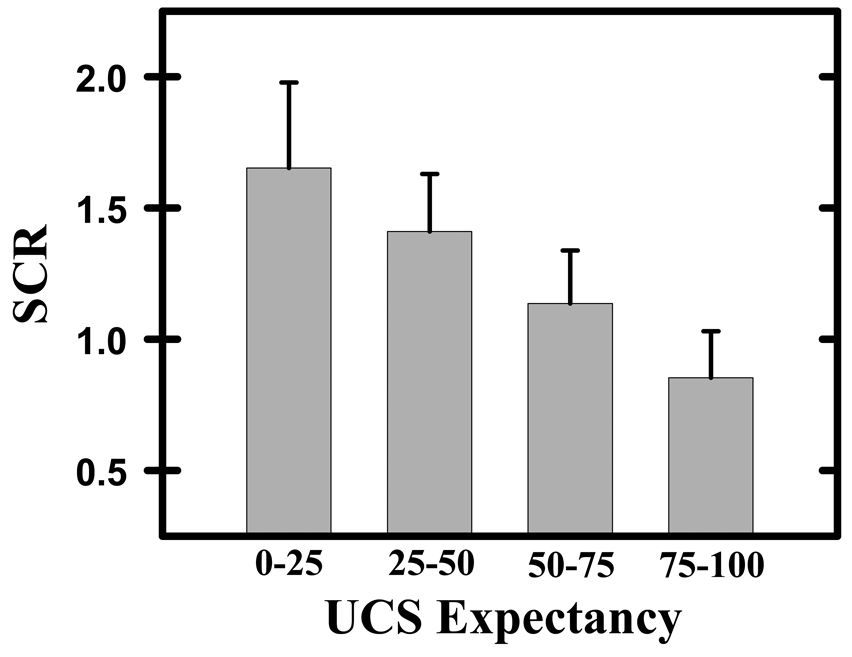
Our behavioral results demonstrate that significant differences in UCS expectancy and unconditioned SCR were expressed during the conditioning session. T-test comparisons revealed significantly greater UCS expectancies on perceived (83.65±2.90) compared to unperceived (52.98±2.96) CS+ trials (t[14]=6.50, p<0.05; Figure 1a). Additional details regarding UCS expectancy data have been published previously (Knight et al., 2009), and can found in supplemental Figure 1. Unconditioned SCRs were larger when the UCS followed an unperceived compared to perceived CS+ presentation (t[14]=2.17, p<0.05; Figure 1b). However, the multiple linear regression analysis revealed that trial-to-trial variations in UCS expectancy (t[14]= −4.28, p<0.05), but not CS perception (t[14]=1.15) had a significant impact on unconditioned SCR magnitude. Figure 2 presents SCR data, binned into 4 distinct UCS expectancy ranges (i.e. 0–25, 25–50, 50–75, 75–100) to demonstrate the relationship between UCS expectancy and unconditioned SCR amplitude. UCS presentations produced large SCRs when UCS expectancy ratings were low, while smaller UCRs were elicited when UCS expectancies were high. These findings are consistent with prior findings of UCR diminution from our laboratory (Dunsmoor et al., 2008).
Figure 1.
UCS expectancy & SCR data. a) Participants expected the UCS during perceived CS+ trials, whereas they were uncertain whether the UCS would be presented on unperceived CS+ trials. b) Unconditioned SCRs were larger when the UCS followed an unperceived compared to perceived CS+. The learning-related reduction in the magnitude of the response produced by a UCS is referred to as UCR diminution. Asterisk indicates significant difference. Error bars represent standard error of the mean.
Figure 2.
UCS expectancy & SCR data. SCR data were grouped in relation to participants’ UCS expectancy ratings (i.e. 0–25, 25–50, 50–75, 75–100). Unconditioned SCR magnitude decreased as UCS expectancy increased. Error bars represent standard error of the mean.
Functional MRI Analysis
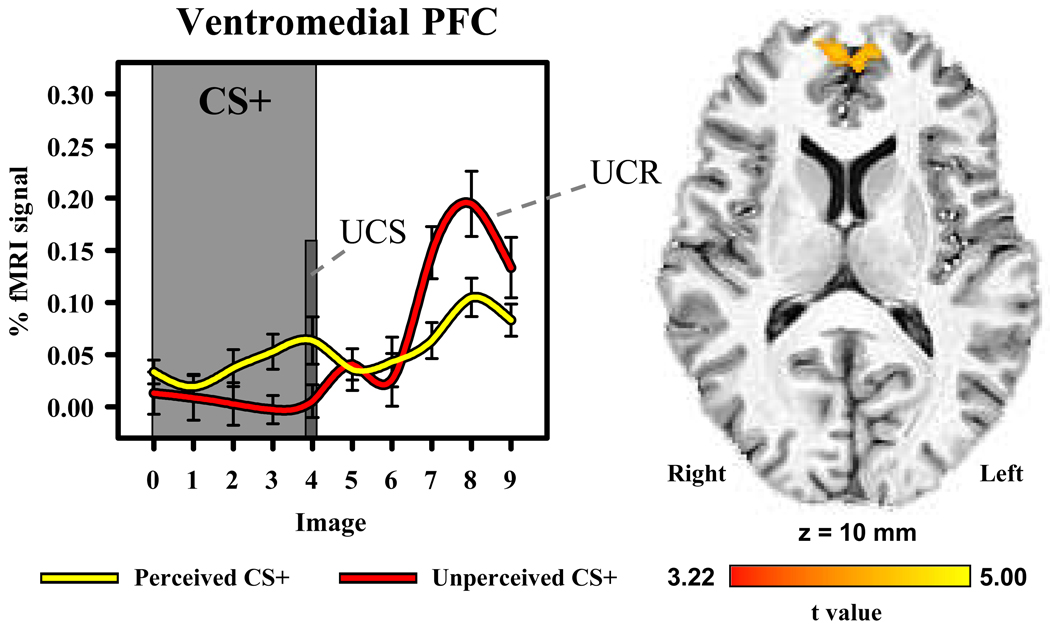
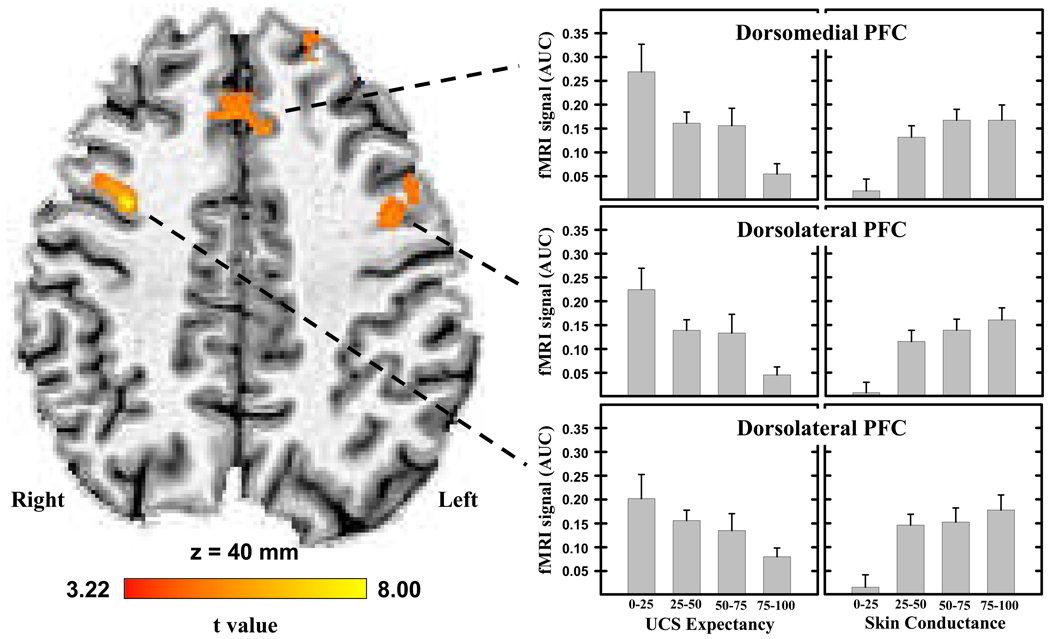
Our whole-brain analysis revealed significant (t[14]>3.22; p<0.05, corrected) diminution of the unconditioned fMRI signal within several brain areas (Table 1). These regions included the ventromedial PFC, dorsomedial PFC, dorsolateral PFC, insula, inferior parietal lobe, and posterior cingulate cortex. UCR magnitude within the ventromedial PFC and posterior cingulate cortex were modulated by perception of the CS that preceded UCS presentation (Figure 3), such that UCR magnitude within these brain regions was larger when the UCS followed an unperceived compared to a perceived CS. An inverse relationship was observed between UCS expectancy and UCR activity within the dorsomedial and dorsolateral PFC, inferior parietal lobe, middle temporal gyrus, and insula (Figure 4). As UCS expectancy increased, UCR magnitude decreased within these brain regions. This analysis did not show a significant relationship between CS perception or UCS expectancy and the fMRI signal following CS- presentation within these areas (Supplemental Figure 2).
Table 1.
Regions showing UCR diminution during Pavlovian conditioning
| Talairach Coordinates | ||||||
|---|---|---|---|---|---|---|
| Region | Hemisphere | Volume (mm3) | RL | AP | IS | t value |
| Regional activity modulated by CS perception | ||||||
| Ventromedial PFC | Left | 787 | −3 | 57 | 14 | 5.07 |
| Posterior Cingulate | Right | 1410 | 8 | −40 | 27 | 4.06 |
| Regional activity modulated by UCS expectancy | ||||||
| Dorsolateral PFC | Left | 1531 | −42 | 8 | 32 | 4.39 |
| Dorsolateral PFC | Right | 459 | 34 | 9 | 41 | 8.62 |
| Dorsomedial PFC | Right | 1338 | 3 | 23 | 44 | 5.30 |
| Superior Frontal Gyrus | Left | 1225 | −14 | 40 | 34 | 6.13 |
| Inferior Parietal Lobe | Left | 1033 | −45 | −55 | 30 | 6.86 |
| Inferior Parietal Lobe | Right | 506 | 45 | −65 | 32 | 5.90 |
| Middle Temporal Gyrus | Left | 794 | −57 | −47 | 6 | 4.61 |
| Insula | Left | 616 | −49 | 10 | 2 | 3.65 |
Locations, volumes, and Talairach coordinates (Talairach and Tournoux, 1988) for the center of mass of areas of activation. RL, right/left; AP, anterior/posterior; IS, inferior/superior.
Figure 3.
UCR diminution within the ventromedial PFC. The unconditioned fMRI signal response was larger when the UCS followed the unperceived compared to perceived CS+. The graph depicts the fMRI time course (% signal change from baseline) for perceived and unperceived CS+ trials. Error bars represent standard error of the mean.
Figure 4.
Area under the hemodynamic response curve (AUC) from brain regions showing UCR diminution modulated by UCS expectancy. The magnitude of dorsomedial and dorsolateral PFC activity decreased as UCS expectancy increased (left side graphs). Functional MRI signal responses within these brain regions also showed a linear relationship with unconditioned SCR magnitude (right side graphs). SCR data were separated into 4 UCR magnitude ranges where 0–25 reflects the lowest 25% of the responses, 25–50 and 50–75 represent intermediate range SCR amplitudes, and 75–100 reflects the largest 25% of the SCRs for each participant. Error bars represent standard error of the mean.
In a secondary analysis, unconditioned fMRI signal responses from these ROIs were binned in relation to the magnitude of each participant’s unconditioned SCRs. Repeated measures ANOVA demonstrated a significant linear relationship between unconditioned SCR magnitude and the amplitude of unconditioned fMRI signal responses within the dorsomedial PFC, dorsolateral PFC, and left insula (F>11.52; p<0.05 corrected; see Figure 4). These data demonstrate that as activity within these brain regions increased, larger unconditioned SCRs were produced.
Discussion
CR expression is typically taken as evidence of associative learning in behavioral and fMRI studies of Pavlovian conditioning. However, the present findings indicate that UCR diminution can also serve as evidence that the CS-UCS association has been formed. In the present study, CS perception was monitored on a trial-by-trial basis, and behavioral and fMRI data were subsequently grouped into perceived and unperceived trial types. UCS expectancy ratings were higher to perceived than unperceived CS+ presentations, indicating that participants expected the UCS on perceived trials, but were uncertain of UCS presentation on unperceived trials. Further, unconditioned SCRs were larger to UCS presentations that followed the unperceived versus perceived CS+. These findings demonstrate unconditioned SCR diminution during Pavlovian conditioning, and generally support the view that UCS expectancies influence UCR production (Dunsmoor et al., 2008; Rust, 1976). The influence of conscious UCS expectancies on UCR magnitude was further investigated by comparing the change in unconditioned SCRs in relation to the UCS expectancy ratings that were reported on each conditioning trial. These data demonstrated that unconditioned SCR magnitude decreased as UCS expectancy increased (see Figure 2), providing further evidence that conscious UCS expectancies modulate the behavioral UCRs produced during Pavlovian conditioning.
UCR diminution was also demonstrated within the fMRI signal from several brain regions. Learning-related changes in UCR activity were observed within the ventromedial PFC, dorsomedial PFC, dorsolateral PFC, inferior parietal lobe, posterior cingulate, and insula. The UCRs within the ventromedial PFC and posterior cingulate cortex were modulated by CS perception. Functional MRI signal responses within these brain regions were larger when the UCS followed the unperceived compared to the perceived CS+ (Figure 3). UCR magnitude within the dorsomedial PFC, dorsolateral PFC, inferior parietal lobe, and insula varied with UCS expectancy. As UCS expectancy increased, activity within these brain regions decreased (see Figure 4). These findings suggest that conscious UCS expectancies modulate UCR magnitude within these brain areas. Further, these imaging findings mirrored the behavioral data (see Figure 2) that showed UCS expectancies modulate unconditioned SCR expression. These findings are consistent with prior work demonstrating UCR diminution in human brain activity (Dunsmoor et al., 2008).
The similarity of learning-related behavioral and fMRI signal responses suggests that brain regions such as the dorsomedial and dorsolateral PFC may mediate the diminution of unconditioned behavioral responses. Therefore, unconditioned fMRI data were sorted in relation to participants’ SCR magnitude to determine the role of these brain regions in the diminution of unconditioned SCRs. Activity within the dorsomedial PFC, dorsolateral PFC, and insula showed a linear relationship with unconditioned SCR amplitude (see Figure 4), suggesting that these brain regions influence the expression of unconditioned SCRs. These findings are consistent with prior work demonstrating a relationship between SCR production and neural activity within each of these brain regions (Critchley et al., 2000; Knight et al., 2005; Patterson et al., 2002). The findings from the present study suggest that UCS expectancies modulate unconditioned fMRI responses within prefrontal brain regions, and in turn, these brain regions provide top-down modulation of behavioral responses (e.g. SCR) to aversive stimuli.
A number of previous studies have suggested that regions of the prefrontal cortex play a role in error detection (Carter et al., 1998, 2007). The pattern of activation observed within this region in the present study is generally consistent with suggestions that prefrontal activity increases when errors are likely to be made (Carter et al., 1998, 2007; Wittfoth et al., 2009). Specifically, dorsomedial and dorsolateral PFC activity was larger on trials that participants reported the lowest UCS expectancy ratings, and thus made the largest UCS prediction errors. To determine whether the observed PFC activity was driven by UCR diminution or error detection we also analyzed the data from CS− trials. If a linear relationship between UCS expectancy and the fMRI signal following CS− presentation were demonstrated, the findings would be consistent with the view that error detection produced the PFC activity observed in this study. However, the analysis of these data determined that the fMRI signal on CS− trials did not vary with UCS expectancy (see supplemental Figure 2). These findings indicate the reduction in UCR magnitude observed within the PFC in the present study is better explained by UCR diminution than an error detection-related process.
The acoustic noise associated with fMRI served as an auditory mask that limited CS perception in the present study. Although, the perception of auditory stimuli may be mediated by somewhat different mechanisms in silent compared to noise-filled environments, the pattern of activation we observed within the dorsomedial PFC, dorsolateral PFC, inferior parietal lobe, and insula generally replicates prior UCR diminution work that has not modulated auditory CS perception (Dunsmoor et al., 2008). However, this prior UCR diminution research has also reported learning-related changes within the amygdala during Pavlovian conditioning (Dunsmoor et al., 2008). We did not observe similar changes within this region in the present study, even when more lenient threshold criteria were applied. Prior work suggests the amygdala is an important component of the neural circuit that mediates fear learning and memory processes (Büchel et al., 1998; Cheng et al., 2003, 2006; Knight et al., 2005, 2009; LaBar et al., 1998; Tabbert et al., 2005). Further, amygdala activity typically shows both a CR and UCR during fMRI studies of Pavlovian conditioning. However, the amygdala is more active during CR, than UCR expression (Knight et al., 2005). Further, amygdala damage disrupts CR, but not UCR production (Bechara et al., 1995). These findings suggest the amygdala may be more important for the production of the CR than the UCR. If so, the amygdala may not be a critical component of the neural circuit that mediates UCR diminution. However, an alternative explanation is that learning-related reductions in UCR amplitude may have developed within the amygdala on both perceived and unperceived conditioning trials. Prior work has shown that both perceived and unperceived CS+ presentations can produce learning-related CRs within the amygdala (Knight et al., 2009; Morris et al., 1998). Therefore, it is possible that amygdala UCRs were equally diminished to UCS presentations following the perceived and unperceived CS+. To investigate this possibility we compared CR and UCR magnitude within the amygdala. No relationship was demonstrated between these responses, suggesting that amygdala CRs do not directly modulate amygdala UCRs. However, these findings do not rule out the possibility that other brain regions modulate UCRs within the amygdala. These issues should be investigated further in future studies by including presentations of the UCS alone. Presentations of the UCS alone were not included in the present study. Therefore we cannot determine if UCR diminution occurred on both perceived and unperceived CS+ trials, or whether instead UCR diminution simply did not occur within this region of the brain. However, the inclusion of a UCS alone condition would help to determine whether amygdala UCRs are equally diminished on both trial types in future studies.
The present study investigated learning-related decreases in UCR magnitude (i.e. UCR diminution) that develop during Pavlovian conditioning. UCS expectancy, fMRI signal, and SCR expression were monitored as supra and sub-threshold auditory CS presentations were paired with a UCS. UCR diminution was observed within several brain regions associated with fear learning and memory including the ventromedial PFC, dorsomedial PFC, dorsolateral PFC, insula, posterior cingulate, and inferior parietal lobe. Activity within a subset of these brain regions showed an inverse relationship with UCS expectancy ratings, such that as UCS expectancy increased UCR magnitude within the dorsomedial and dorsolateral PFC decreased. Further, activity within the dorsomedial and dorsolateral PFC showed a linear relationship with unconditioned SCR expression. These findings suggest that UCS expectancies modulate prefrontal cortex responses to aversive stimuli. In turn, prefrontal activity appears to modulate the expression of unconditioned SCRs.
Supplementary Material
UCS expectancy data. a) Learning-related changes in UCS expectancy were demonstrated on perceived trials only. Larger responses were produced during the CS+ relative to the CS− on perceived, but not unperceived trials. Asterisk indicates significant difference. Error bars represent standard error of the mean.
Area under the hemodynamic response curve (AUC) from brain regions showing UCR diminution. As an additional control, data from CS− trials were analyzed to determine whether the activation observed in this study is consistent with prior research on error detection. No relationship was observed between UCS expectancy and brain activity on CS− trials. In contrast, UCR magnitude decreased within the prefrontal cortex as UCS expectancy increased on CS+ trials (see Figure 4). Data presented in this figure & in figure 4 are more consistent with UCR diminution than an error detection-related process.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Institute of Mental Health.
Abbreviations footnote
- CS
conditioned stimulus
- UCS
unconditioned stimulus
- CS+
CS paired with the UCS
- CS−
CS presented alone
- CR
conditioned response
- UCR
unconditioned response
- SCR
skin conductance response
- PFC
prefrontal cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baxter R. Diminution and recovery of the UCR in delayed and trace classical GSR conditioning. Journal of Experimental Psychology. 1966;71(3):447–451. doi: 10.1037/h0022977. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Büchel C, Dolan RJ, Armony JL, Friston KJ. Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. J. Neurosci. 1999;19(24):10869–10876. doi: 10.1523/JNEUROSCI.19-24-10869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20(5):947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: An update of theory and data. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(4):367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Carter RM, O’Doherty JP, Seymour B, Koch C, Dolan RJ. Contingency awareness in human aversive conditioning involves the middle frontal gyrus. Neuroimage. 2006;29(3):1007–1012. doi: 10.1016/j.neuroimage.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human amygdala activity during Pavlovian fear conditioning: stimulus processing versus response expression. Behavioral Neuroscience. 2003;117:3–10. doi: 10.1037//0735-7044.117.1.3. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Stein EA, Helmstetter FJ. Human amygdala activity during the expression of fear responses. Behav. Neurosci. 2006;120(6):1187–1195. doi: 10.1037/0735-7044.120.5.1187. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. Journal of Neuroscience. 2000;20(8):2033–3040. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domjan M. Pavlovian conditioning: a functional perspective. Annual Review of Psychology. 2005;56:179–206. doi: 10.1146/annurev.psych.55.090902.141409. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC. Neural substrates of UCR diminution during Pavlovian conditioning. NeuroImage. 2008;49:811–817. doi: 10.1016/j.neuroimage.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Knight DC, Bandettini PA. Impact of continuous versus intermittent CS-UCS pairing on human brain activation. Behavioral Neuroscience. 2007;121(4):635–642. doi: 10.1037/0735-7044.121.4.635. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Baackes MP. Conditioned fear-induced opiate analgesia on the formalin test: Evidence for two aversive motivational systems. Learning & Motivation. 1982;13:200–221. [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. Journal of Neuroscience. 2006;26(37):9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel E. Judgments of UCS intensity and diminution of the UCR in classical GSR conditioning. Journal of Experimental Psychology. 1967;73(4):532–543. doi: 10.1037/h0024333. [DOI] [PubMed] [Google Scholar]
- Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. Neural substrates mediating human delay and trace fear conditioning. J. Neurosci. 2004;24(1):218–228. doi: 10.1523/JNEUROSCI.0433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. Expression of conditional fear with and without awareness. Proceedings of the National Academy of Sciences. 2003;100(25):15280–15283. doi: 10.1073/pnas.2535780100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. The role of the human amygdala in the production of conditioned fear responses. Neuroimage. 2005;26(4):1193–1200. doi: 10.1016/j.neuroimage.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. The role of awareness in delay and trace fear conditioning in humans. Cognitive, Affective, and Behavioral Neuroscience. 2006;5(2):157–162. doi: 10.3758/cabn.6.2.157. [DOI] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human Pavlovian fear conditioning: patterns of activation as a function of learning. NeuroReport. 1999;10(17):3665–3670. doi: 10.1097/00001756-199911260-00037. [DOI] [PubMed] [Google Scholar]
- Knight DC, Waters NS, Bandettini PA. Neural substrates of explicit and implicit fear memory. NeuroImage. 2009 doi: 10.1016/j.neuroimage.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20(5):937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Marcos JL, Redondo J. Effects of conditioned stimulus presentation on diminution of the unconditioned response in aversive classical conditioning. Biological Psychology. 1999;50:89–102. doi: 10.1016/s0301-0511(99)00007-1. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Rajah MN, Lobaugh NJ. Functional connectivity of the medial temporal lobe relates to learning and awareness. Journal of Neuroscience. 2003;23(16):6520–6528. doi: 10.1523/JNEUROSCI.23-16-06520.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007;62(5):446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proceedings of the National Academy of Sciences, USA. 2005;102(30):10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Öhman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393(6684):467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Patterson JC, Ungerleider LG, Bandettini PA. Task-independent functional brain activity correlation with skin conductance changes: an fMRI study. Neuroimage. 2002;17(4):1797–1806. doi: 10.1006/nimg.2002.1306. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Rust J. Unconditioned response diminution in the skin resistance response. Journal of General Psychology. 1976;95:77–84. doi: 10.1080/00221309.1976.9710867. [DOI] [PubMed] [Google Scholar]
- Tabbert K, Stark R, Kirsch P, Vaitl D. Hemodynamic responses of the amygdala, the orbitofrontal cortex and the visual cortex during a fear conditioning paradigm. Int. J. Psychophysiol. 2005;57(1):15–23. doi: 10.1016/j.ijpsycho.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Wittforth M, Schardt DM, Fahle M, Herrmann M. How the brain resolves high conflict situations: Double conflict involvement of dorsolateral prefrontal cortex. Neuroimage. 2009;44(3):1201–1209. doi: 10.1016/j.neuroimage.2008.09.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
UCS expectancy data. a) Learning-related changes in UCS expectancy were demonstrated on perceived trials only. Larger responses were produced during the CS+ relative to the CS− on perceived, but not unperceived trials. Asterisk indicates significant difference. Error bars represent standard error of the mean.
Area under the hemodynamic response curve (AUC) from brain regions showing UCR diminution. As an additional control, data from CS− trials were analyzed to determine whether the activation observed in this study is consistent with prior research on error detection. No relationship was observed between UCS expectancy and brain activity on CS− trials. In contrast, UCR magnitude decreased within the prefrontal cortex as UCS expectancy increased on CS+ trials (see Figure 4). Data presented in this figure & in figure 4 are more consistent with UCR diminution than an error detection-related process.