Abstract
Lipolysis for the provision of fatty acids (FA) for other organs during times of energy demand occurs uniquely in white adipose tissue (WAT). Recent findings have identified a bona fide TAG hydrolase as well as the major adipose phospholipase A2, AdPLA. By controlling PGE2 levels, AdPLA dominantly regulates lipolysis in an autocrine/paracrine manner. Moreover, recent findings demonstrate that, surprisingly, increasing lipolysis in adipose tissue does not necessarily increase serum FA levels, which are usually correlated with insulin resistance. Rather, increasing lipolysis in adipose tissue causes a shift within adipocytes towards increased FA utilization and energy expenditure and, thus, protects against obesity. Here, we discuss the regulation of lipolysis and its effects on FA utilization within WAT and on insulin resistance.
Trialcylglycerol (TAG) is the major energy storage form in mammals. Excess TAG accumulation resulting in obesity is a major risk factor for metabolic disorders including type 2 diabetes and cardiovascular disease [1]. White adipose tissue (WAT) stores TAG during periods of energy excess and hydrolyzes TAG (lipolysis) to release fatty acids (FA) for use by other tissues during times of energy need [2]. While TAG synthesis occurs in other organs, such as the liver for VLDL production, lipolysis for the provision of FA for use by other organs is unique to adipocytes [3]. Strategies aimed at increasing lipolysis may therefore be useful in preventing obesity. However, elevated lipolysis may increase circulating FFA levels and ectopic TAG storage, which are associated with detrimental metabolic consequences such as insulin resistance. New evidence, however, suggests that adipocytes have the capacity to increase FA utilization in response to increased lipolysis, which may make lipolysis a target for the prevention and treatment of obesity. Here we focus on recent findings in understanding the regulation of lipolysis in adipose tissue and on changes in adipocyte FA metabolism and energy expenditure resulting from increased adipocyte lipolysis.
The lipolytic cascade
TAG in adipose tissue is stored in unilocular cytosolic lipid droplets that are composed of a core of TAG and cholesterol esters, surrounded by a phospholipid monolayer, coated with lipid droplet associated proteins [4]. Many of these lipid droplet associated proteins are characterized by the presence of a conserved amino acid sequence defined as a PAT (perilipin, adipophilin (also called adipocyte differentiation-related protein, ADRP), and tail-interacting protein of 47kDa (TIP47)) domain [5–7]. Several other lipid droplet associated proteins are also found on the lipid droplets, such as fat-specific protein of 27kDa (FSP27)/Cidec, which is required for the formation of unilocular lipid droplets of white adipocytes [7–10]. Although its role is unclear, caveolin, an integral membrane protein associated with caveolae, has also been shown to localize to lipid droplets and regulate lipolysis [11–14]. The relative abundance of lipid droplet associated proteins, as well as their posttranslational modifications and interactions with other cytosolic proteins may control the accessibility of lipases to their TAG substrate and, therefore, affect lipolytic activity [7, 15].
Adipocyte lipolysis proceeds in an orderly and regulated manner, with different enzymes acting at each step. Until recently, hormone-sensitive lipase (HSL) was thought to be the initial enzyme in TAG hydrolysis. However, mice lacking HSL were not obese and had substantial residual TAG hydrolase activity in WAT, accumulating diacylglycerol (DAG) rather than TAG [2]. These findings indicate that although HSL has TAG hydrolase activity in vitro, it functions primarily as a DAG hydrolase in adipose tissue, suggesting the presence of additional unidentified TAG hydrolase(s). Recently, we and others identified a novel TAG hydrolase called desnutrin (also called ATGL, TTS2.2, PNPLA2, or iPLA2ζ) [16–18]. Desnutrin/ATGL exhibits high substrate specificity for TAG, is predominantly expressed in adipose tissue, and is induced during fasting and by glucocorticoids, conditions that favor lipolysis [16, 17]. Its function as a TAG hydrolase is evolutionarily conserved between humans, mice, Drosophila, Saccharomyces cerevisiae, and Arabidopsis, highlighting the importance of this enzyme [17, 19–23]. Although the mechanism is poorly understood, studies indicate that comparative gene identification 58 (CGI-58) serves as a coactivator of desnutrin/ATGL [7]. These findings have led to a new understanding of the lipolytic cascade: Desnutrin/ATGL initiates lipolysis by hydrolyzing a FA from TAG to produce DAG that is subsequently hydrolyzed by HSL to generate a second FA and monoacylglycerol (MAG). MAG lipase then hydrolyzes MAG to yield the final FA and glycerol (Figure 1).
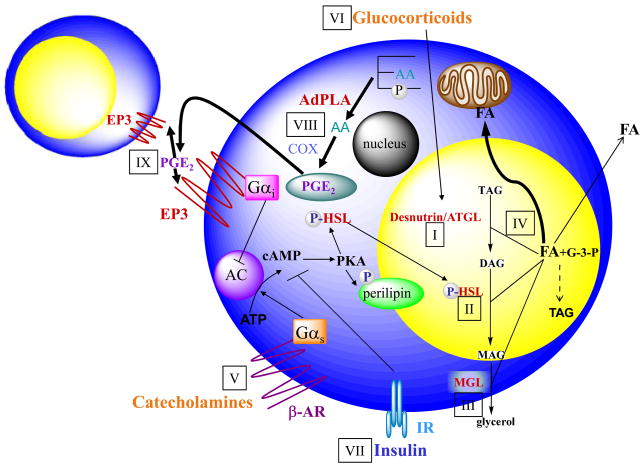
Figure 1. Regulation of lipolysis in adipocytes.
(i) Desnutrin/ATGL performs the initial step of TAG hydrolysis, resulting in diacylglycerol (DAG). (ii) DAG is hydrolyzed by hormone-sensitive lipase (HSL) to monoacylglycerol (MAG), which is subsequently hydrolyzed by (iii) monoglyceride lipase (MGL) to generate glycerol and three fatty acids (FA). (iv) The FAs generated during lipolysis can be released into the circulation for use by other organs or re-esterified to TAG. Glycerol 3-phosphate (G-3-P) can be used as a backbone for TAG synthesis. FA released from lipolysis can also be oxidized within the adipocyte. Lipolysis is under tight endocrine regulation. (v) During fasting, catecholamines by binding to Gαs-coupled β-adernergic receptors (β-AR), activate adenylate cyclase (AC) to increase cAMP and thus activate protein kinase A (PKA). PKA phosphorylates hormone sensitive lipase (HSL), resulting in translocation of HSL from the cytosol to the lipid droplet and increased lipolysis. PKA also phosphorylates the lipid droplet associated protein perilipin. (vi) Additionally, during fasting, glucocorticoids increase the expression of desnutrin/ATGL. (vii) In the fed state, insulin binding to the insulin receptor (IR) results in decreased cAMP levels and decreased lipolysis. Recent findings revealed that lipolysis is dominantly regulated by prostaglandin E2 (PGE2) through adipose-specific phospholipase A2 (AdPLA). (viii) AdPLA hydrolyzes the sn-2 position of phospholipids to generated arachidonic acid (AA), which via cylcooxygenase (COX) produces PGE2. (ix) PGE2 secreted by adipocytes can act locally by binding to the Gαi-coupled EP3 present in adipocytes, resulting in inhibition of AC and decreased lipolysis.
Together HSL and desnutrin/ATGL are responsible for over 95% of TAG hydrolase activity in murine WAT in vitro [24]. Desnutrin/ATGL null mice do not efficiently mobilize TAG stores and have massive TAG accumulation in multiple tissues, most notably the heart, causing cardiac failure and premature death [25]. The adipose-specific role of desnutrin/ATGL is therefore unclear in these mice. However, recent findings in transgenic mice overexpressing desnutrin in adipose tissue have provided further insight into the adipose-specific role of desnutrin/ATGL [26]. Interestingly, while overexpression of desnutrin/ATGL in adipose tissue increases lipolysis, it does not increase circulating FA levels. Overexpression of desnutrin/ATGL, however, promotes FA utilization within WAT, which is evidenced by increased cycling between DAG and TAG, increased expression of genes involved in FA oxidation and thermogenesis, and higher FA oxidation in WAT. As a result, desnutrin/ATGL mice have elevated energy expenditure and body temperatures, and are resistant to diet-induced obesity [26].
Regulation of lipolysis
Regulation of lipolysis that presumably occurs at the surface of lipid droplets is under tight hormonal regulation. During fasting, catecholamines bind to Gαs-coupled β-adernergic receptors to activate adenylate cyclase, which increases cAMP levels activating protein kinase A (PKA) [1]. PKA phosphorylates HSL, causing it to translocate from the cytosol to its site of action on the lipid droplet. PKA also phosphorylates the lipid droplet associated protein, perilipin, translocating it away from the lipid droplet, exposing a greater surface to hydrolytic attack by lipases [7, 27]. Glucocorticoids, which are elevated during fasting, also upregulate desnutrin/ATGL transcription [17]. During the fed state, insulin by binding to its receptor in adipose tissue initiates a cascade of signaling events that inhibit lipolysis. The anti-lipolytic effect of insulin is mediated primarily through phosphorylation/activation of phosphodiesterase 3B, which decreases cAMP and thus PKA activity, causing reduced HSL/perilipin phosphorylation, and decreased lipolysis [1] (Figure 1). Other signaling pathways also regulate lipolysis including regulation by cytokines, growth hormones, AMP-activated protein kinase, nicotinic acid, to name a few. Additionally, regulation of lipolysis by naturietic peptides through a cGMP dependent protein kinase (PKG) has been shown to also exist in humans. However, further investigations are required to establish the relative importance of these pathways in regulating lipolysis [28–34].
While the classic model of endocrine regulation of lipolysis by catecholamines and insulin has been extensively studied, local regulation of lipolysis in adipose tissue by autocrine/paracrine factors is not well understood. Adipocytes secrete several factors that can regulate lipolysis locally, such as TNF-α which stimulates lipolysis [1, 35–37]. Depending on the concentration and species tested, prostaglandins have also been reported to inhibit, stimulate, or have no effect on lipolysis [1]. Furthermore, while stimulation of lipolysis by catecholamines through β-adrenergic receptors, coupled to Gαs, has been well studied, inhibition of lipolysis through receptors coupled to Gαi is less well understood. Factors such as neuropeptide Y/peptide YY, adenosine and nicotinic acid have been shown to inhibit lipolysis via various Gαi-coupled receptors [36]. Recently, however, identification of adipose-specific phospholipase A2 (AdPLA) revealed a surprisingly important and dominant role for adipocyte-derived PGE2 in the autocrine/paracrine regulation of lipolysis [38, 39]. AdPLA is a membrane-associated, calcium-dependent PLA2, that represents a new group of PLA2s, group XVI [39]. AdPLA is the major PLA in adipose tissue and is highly expressed only in adipocytes [39]. As a PLA2, AdPLA catalyzes the release of fatty acids from the sn-2 position of phospholipids that is typically enriched in arachidonic acid, providing substrate for the initial, rate-limiting step in the synthesis of eicosanoids. In adipose tissue, PGE2 is the predominant prostaglandin produced, and loss of AdPLA in AdPLA null mice causes an >85% fall in PGE2 levels in WAT.
Among the cognate receptors for PGE2, Gαi-coupled EP3 is expressed at >10-fold higher levels than the other EP receptors in WAT [38]. A fall in PGE2 levels induced by loss of AdPLA decreases EP3 signaling, resulting in loss of inhibition of adenylate cyclase activity. This, in turn, elevates cAMP levels, activating lipolysis through PKA-mediated phosphorylation of HSL (Figure 1). AdPLA is induced during feeding and by insulin, suggesting that autocrine/paracrine regulation by PGE2 through AdPLA is an integral part of the coordinated suppression of lipolysis that occurs in response to anabolic stimuli [38]. The physiological importance of AdPLA/PGE2 in WAT is highlighted by the unrestrained adipocyte lipolysis and extremely lean phenotype of AdPLA null mice [38]. Importantly, these observations raise the notion that regulation of adipocyte lipolysis may alter FA metabolism and energy expenditure and thus may be a critical factor in the development of obesity.
Energy metabolism and fatty acid utilization in WAT
AdPLA null mice exhibit unrestrained lipolysis and are resistant to both diet-induced and genetic obesity induced by leptin deficiency [38]. Notably, serum FA levels are not elevated in AdPLA null mice despite drastically increased WAT lipolysis, a phenotype similar to desnutrin/ATGL transgenic mice. Two main factors appear to contribute to this phenomenon. First, because WAT mass is substantially reduced, net liberation of FA from WAT is likely lower than would be expected from these mice with elevated lipolytic rates. Second, increased lipolysis in WAT, accompanied by increased energy expenditure, suggests increased oxidation of fatty acids. However, hepatic and skeletal muscle FA oxidation are not increased in these mice. Instead, similar to desnutrin/ATGL transgenic mice, there is a substantial upregulation of genes encoding proteins involved in oxidation and thermogenesis in WAT such as peroxisome proliferator-activated receptor delta (PPARδ) and uncoupling protein 1 (UCP-1). Moreover, FA oxidation in adipocytes is, in fact, significantly higher. In this regard, several other mouse models including perilipin null mice and FSP27 null mice have been reported to be lean with elevated lipolysis, no change in serum FA levels, and increased FA oxidation in WAT [10, 40–43]. Although FA oxidation has not been considered to be a major metabolic pathway in adipocytes, these findings demonstrate that increased adipocyte FA oxidation may play a significant role in energy metabolism and adiposity [36].
Higher UCP-1 expression in WAT is another striking effect observed in models of increased lipolysis [26, 38]. Several recently generated mouse models including corepressor receptor interacting protein 140 (RIP 140) null mice, translation initiation factor elF4E null mice, as well as adipose-specific knockout mice of an essential component of mammalian TOR complex 1 (mTORC1), raptor, all exhibit higher UCP-1 expression in WAT resulting in an enhanced metabolic rate and leanness, evidence that increased mitochondrial uncoupling in WAT protects against obesity [44, 50]. In fact, UCP-1 overexpression in WAT of mice increases mitochondrial respiratory uncoupling specifically in WAT, resulting in a drastically leaner phenotype [51–53]. Taken together, these findings suggest that increased lipolysis may cause a shift within adipocytes towards increased FA utilization and energy expenditure resulting in resistance to obesity.
Insulin resistance in adipose tissue
Elevated circulating FAs are associated with obesity and thought to mediate insulin resistance. High serum FA levels can result in deposition of FA in non-adipose tissues leading to ectopic TAG storage in organs such as the liver and skeletal muscle, contributing to insulin resistance [54]. It is also postulated that other lipid metabolites such as DAG and ceramide, by activating serine kinases, may cause insulin resistance [55–56]. Furthermore, macrophage infiltration in adipose tissue may cause increased inflammatory cytokine production resulting in insulin resistance [56–57]. It has been reported that FA released from adipocytes themselves can activate toll-like receptors on adipocytes and macrophages, inducing inflammatory pathways and reducing insulin signaling [56, 58].
What are the effects of increasing lipolysis in WAT on whole body insulin sensitivity? It may be predicted that elevated lipolysis in WAT would increase circulating FA levels and promotes insulin resistance. However, as discussed above, several mouse models with elevated lipolysis have shown that increasing lipolysis in WAT promotes energy dissipation and FA oxidation in WAT and does not necessarily increase circulating FA levels. According to the Randle hypothesis that states fat oxidation and carbohydrate oxidation are mutually inhibitory, one would expect increased FA oxidation in WAT to prevent glucose oxidation and lead to insulin resistance [59]. However, these mouse models with elevated lipolysis all have higher FA oxidation in WAT, with varying phenotypes in regards to insulin resistance. Desnutrin/ATGL transgenic mice and FSP27-null mice have improved insulin sensitivity, while AdPLA null mice and perilipin null mice are insulin resistant [10,26,38,40]. While further investigation is required, these differences in insulin sensitivity do not appear to be due to differences in macrophage infiltration into adipose tissue since AdPLA null mice show no changes in a number of markers of inflammation in both the serum and WAT [38]. However, differences between these models can be partially explained by differences in WAT mass. AdPLA null have an extreme reduction in adiposity compared to the more modest reduction observed in aP2-desnutrin mice [26, 38]. In this regard, although glucose uptake in WAT of AdPLA null mice is actually higher on a per gram basis compared to wild-type mice because the total mass of adipose tissue was drastically reduced, total glucose uptake by adipose tissue was lower than in wild-type mice, highlighting the importance of an appropriate amount of WAT in maintaining glycemic control [38]. Furthermore, when FA are generated at a rate that exceeds the oxidative capacity of adipocytes, this can result in the deposition of FA in other organs and promote insulin resistance, as seen in AdPLA null mice as well as in lipodystrophy models. Therefore, the total mass as well as the oxidative capacity of WAT could contribute to insulin resistance. Regardless, the relative contribution of WAT to whole body insulin-stimulated glucose uptake is controversial. While WAT accounts for less than 10% of whole-body glucose uptake in mice, adipose tissue-specific ablation of Glut4 results in impaired glucose homeostasis, indicating the importance of adipose tissue in regulating glucose homeostasis [60]. However, adipose tissue-specific ablation of the insulin receptor causes selective insulin resistance in only adipose tissue but does not impair whole-body glucose metabolism [61]. Secretion of adipokines as well as other unknown factors may also contribute to the different phenotypes in these models with altered insulin sensitivity [54]. However, it is clear that increased lipolysis resulting in elevated FA utilization within adipocytes can be a factor affecting insulin sensitivity.
Conclusion
Recently, many exciting advances have been made in understanding lipolysis and FA utilization in adipose tissue. Identification/characterization of the TAG hydrolase, desnutrin/ATGL has firmly established the lipolytic cascade. While the endocrine regulation of lipolysis by catecholamines and insulin has been extensively studied, the local regulation of lipolysis in adipose tissue by autocrine/paracrine factors may also be critical. Recent identification of AdPLA has revealed a new autocrine/paracrine regulation of lipolysis in adipose tissue. By controlling PGE2 levels, AdPLA dominantly inhibits lipolysis and plays a critical role in the development of obesity. Moreover, recent findings demonstrate that, surprisingly, increasing lipolyisis in adipose tissue does not necessarily increase serum FA levels, which are usually correlated with insulin resistance. Rather, it appears that increasing lipolysis in adipose tissue causes a shift within adipocytes towards increased FA utilization and energy expenditure and, thus, protect against obesity.
Important questions still remain. Increasing lipolysis in WAT promotes FA utilization. Conversely, what are the metabolic consequences when lipolysis is decreased in WAT? How does increasing lipolysis promote FA oxidation in adipose tissue? Do FAs resulting from lipolysis act as ligands for peroxisome proliferator-activated receptors (PPARs) or HNF4 and promote expression of genes in oxidative metabolism [62, 63]? Can increasing lipolysis in human WAT prevent obesity without causing significant adverse effects in other organs or on insulin sensitivity? What is the pathological syndrome of humans with single nucleotide polymorphisms detected in genes encoding for proteins involved in lipolysis, such as the AdPLA (PLA2G16) gene? Answers to these questions will help to develop strategies to target lipolysis as a treatment for obesity and its related pathologies.
Acknowledgments
Thanks to James V. Chithalen for assistance with graphics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jaworski K, et al. Regulation of triglyceride metabolism. IV. Hormonal regulation of lipolysis in adipose tissue. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1–4. doi: 10.1152/ajpgi.00554.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duncan RE, et al. Regulation of lipolysis in adipocytes. Annu Rev Nutr. 2007;27:79–101. doi: 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmadian M, et al. Triacylglycerol metabolism in adipose tissue. Future Lipidol. 2007;2:229–237. doi: 10.2217/17460875.2.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2006;7:373–378. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 5.Wolins NE, et al. TIP47 associates with lipid droplets. J Biol Chem. 2001;276:5101–5108. doi: 10.1074/jbc.M006775200. [DOI] [PubMed] [Google Scholar]
- 6.Brasaemle DL, et al. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res. 1997;38:2249–2263. [PubMed] [Google Scholar]
- 7.Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Brasaemle DL, et al. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J Biol Chem. 2004;279:46835–46842. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- 9.Guo Y, et al. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 2008;453:657–661. doi: 10.1038/nature06928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishino N, et al. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J Clin Invest. 2008;118:2808–2821. doi: 10.1172/JCI34090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen AW, et al. Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes. 2004;53:1261–1270. doi: 10.2337/diabetes.53.5.1261. [DOI] [PubMed] [Google Scholar]
- 12.Fujimoto T, et al. Caveolin-2 is targeted to lipid droplets, a new “membrane domain” in the cell. J Cell Biol. 2001;152:1079–1085. doi: 10.1083/jcb.152.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pol A, et al. Dynamic and regulated association of caveolin with lipid bodies: modulation of lipid body motility and function by a dominant negative mutant. Mol Biol Cell. 2004;15:99–110. doi: 10.1091/mbc.E03-06-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ortegren U, et al. A new role for caveolae as metabolic platforms. Trends Endocrinol Metab. 2007;18:344–349. doi: 10.1016/j.tem.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Wolins NE, et al. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006;580:5484–5491. doi: 10.1016/j.febslet.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann R, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 17.Villena JA, et al. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem. 2004;279:47066–47075. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins CM, et al. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem. 2004;279:48968–48975. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- 19.Fischer J, et al. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat Genet. 2007;39:28–30. doi: 10.1038/ng1951. [DOI] [PubMed] [Google Scholar]
- 20.Gronke S, et al. Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab. 2005;1:323–330. doi: 10.1016/j.cmet.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Kurat CF, et al. Obese yeast: triglyceride lipolysis is functionally conserved from mammals to yeast. J Biol Chem. 2006;281:491–500. doi: 10.1074/jbc.M508414200. [DOI] [PubMed] [Google Scholar]
- 22.Kurat CF, et al. Cdk1/Cdc28-dependent activation of the major triacylglycerol lipase Tgl4 in yeast links lipolysis to cell-cycle progression. Mol Cell. 2009;33:53–63. doi: 10.1016/j.molcel.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Eastmond PJ. SUGAR-DEPENDENT1 encodes a patatin domain triacylglycerol lipase that initiates storage oil breakdown in germinating Arabidopsis seeds. Plant Cell. 2006;18:665–675. doi: 10.1105/tpc.105.040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schweiger M, et al. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem. 2006;281:40236–40241. doi: 10.1074/jbc.M608048200. [DOI] [PubMed] [Google Scholar]
- 25.Haemmerle G, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 26.Ahmadian M, et al. Adipose overexpression of desnutrin promotes fatty acid use and attenuates diet-induced obesity. Diabetes. 2009;58:855–866. doi: 10.2337/db08-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granneman JG, Moore HP. Location, location: protein trafficking and lipolysis in adipocytes. Trends Endocrinol Metab. 2008;19:3–9. doi: 10.1016/j.tem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Tunaru S, et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med. 2003;9:352–355. doi: 10.1038/nm824. [DOI] [PubMed] [Google Scholar]
- 29.Birkenfeld AL, et al. Lipid mobilization with physiological atrial natriuretic peptide concentrations in humans. J Clin Endocrinol Metab. 2005;90:3622–3628. doi: 10.1210/jc.2004-1953. [DOI] [PubMed] [Google Scholar]
- 30.Langin D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol Res. 2006;53:482–491. doi: 10.1016/j.phrs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Arner P, et al. FGF21 attenuates lipolysis in human adipocytes - a possible link to improved insulin sensitivity. FEBS Lett. 2008;582:1725–1730. doi: 10.1016/j.febslet.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 32.Daval M, et al. Anti-lipolytic action of AMP-activated protein kinase in rodent adipocytes. J Biol Chem. 2005;280:25250–25257. doi: 10.1074/jbc.M414222200. [DOI] [PubMed] [Google Scholar]
- 33.Taggart AK, et al. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem. 2005;280:26649–26652. doi: 10.1074/jbc.C500213200. [DOI] [PubMed] [Google Scholar]
- 34.Lafontan M, et al. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol Metab. 2008;19:130–137. doi: 10.1016/j.tem.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Wang S, et al. Lipolysis and the integrated physiology of lipid energy metabolism. Mol Genet Metab. 2008;95:117–126. doi: 10.1016/j.ymgme.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 36.Lafontan M. Advances in adipose tissue metabolism. Int J Obes (Lond) 2008;32(Suppl 7):S39–51. doi: 10.1038/ijo.2008.237. [DOI] [PubMed] [Google Scholar]
- 37.Langin D, Arner P. Importance of TNFalpha and neutral lipases in human adipose tissue lipolysis. Trends Endocrinol Metab. 2006;17:314–320. doi: 10.1016/j.tem.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Jaworski K, et al. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat Med. 2009;15:159–168. doi: 10.1038/nm.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duncan RE, et al. Identification and functional characterization of adipose-specific phospholipase A2 (AdPLA) J Biol Chem. 2008;283:25428–25436. doi: 10.1074/jbc.M804146200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saha PK, et al. Metabolic adaptations in the absence of perilipin: increased beta-oxidation and decreased hepatic glucose production associated with peripheral insulin resistance but normal glucose tolerance in perilipin-null mice. J Biol Chem. 2004;279:35150–35158. doi: 10.1074/jbc.M405499200. [DOI] [PubMed] [Google Scholar]
- 41.Puri V, et al. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol Chem. 2007;282:34213–34218. doi: 10.1074/jbc.M707404200. [DOI] [PubMed] [Google Scholar]
- 42.Martinez-Botas J, et al. Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat Genet. 2000;26:474–479. doi: 10.1038/82630. [DOI] [PubMed] [Google Scholar]
- 43.Tansey JT, et al. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci U S A. 2001;98:6494–6499. doi: 10.1073/pnas.101042998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tiraby C, Langin D. Conversion from white to brown adipocytes: a strategy for the control of fat mass? Trends Endocrinol Metab. 2003;14:439–441. doi: 10.1016/j.tem.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Polak P, et al. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 2008;8:399–410. doi: 10.1016/j.cmet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Christian M, et al. Metabolic regulation by the nuclear receptor corepressor RIP140. Trends Endocrinol Metab. 2006;17:243–250. doi: 10.1016/j.tem.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 47.Christian M, et al. RIP140-targeted repression of gene expression in adipocytes. Mol Cell Biol. 2005;25:9383–9391. doi: 10.1128/MCB.25.21.9383-9391.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parker MG, et al. The nuclear receptor co-repressor RIP140 controls the expression of metabolic gene networks. Biochem Soc Trans. 2006;34:1103–1106. doi: 10.1042/BST0341103. [DOI] [PubMed] [Google Scholar]
- 49.Tsukiyama-Kohara K, et al. Adipose tissue reduction in mice lacking the translational inhibitor 4E-BP1. Nat Med. 2001;7:1128–1132. doi: 10.1038/nm1001-1128. [DOI] [PubMed] [Google Scholar]
- 50.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kopecky J, et al. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest. 1995;96:2914–2923. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Orci L, et al. Rapid transformation of white adipocytes into fat-oxidizing machines. PNAS. 2004;101:2058–2063. doi: 10.1073/pnas.0308258100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamada T, et al. Signals from intra-abdominal fat modulate insulin and leptin sensitivity through different mechanisms: neuronal involvement in food-intake regulation. Cell Metab. 2006;3:223–229. doi: 10.1016/j.cmet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Guilherme A, et al. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Savage B, et al. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schenk S, et al. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cinti S, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen MT, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem. 2007;282:35279–92. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 59.Randle PJ, et al. The glucose fatty acid cycle in obesity and maturity onset diabetes mellitus. Ann NY Acad Sci. 1965;131:324–33. doi: 10.1111/j.1749-6632.1965.tb34800.x. [DOI] [PubMed] [Google Scholar]
- 60.Herman MA, Kahn BB. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J Clin Invest. 2006;116:1767–1775. doi: 10.1172/JCI29027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bluher M, et al. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- 62.Michalik L, et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev. 2006;58:726–741. doi: 10.1124/pr.58.4.5. [DOI] [PubMed] [Google Scholar]
- 63.Palanker L, et al. Drosophila HNF4 regulates lipid mobilization and beta-oxidation. Cell Metab. 2009;9:228–239. doi: 10.1016/j.cmet.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]