Abstract
The states of pregnancy and lactation bring about a range of physiological and behavioral changes in the adult mammal that prepare the mother to care for her young. Cell proliferation increases in the subventricular zone (SVZ) of the female rodent brain during both pregnancy and lactation when compared to that in cycling, diestrous females. In the present study, the effects of maternal behavior induction and pup exposure on neurogenesis in nulliparous rats were examined in order to determine whether maternal behavior itself, independent of pregnancy and lactation, might affect neurogenesis. Adult, nulliparous, Sprague-Dawley, female rats were exposed daily to foster young in order to induce maternal behavior. Following the induction of maternal behavior each maternal subject plus females that were exposed to pups for a comparable number of test days, but did not display maternal behavior, and subjects that had received no pup exposure were injected with bromodeoxyuridine (BrdU, 90 mg/kg, i.v.). Brain sections were double-labeled for BrdU and the neural marker, NeuN, to examine the proliferating cell population. Increases in the number of double-labeled cells were found in the maternal virgin brain when compared with the number of double-labeled cells present in non-maternal, pup-exposed nulliparous rats and in females not exposed to young. No changes were evident in the dentate gyrus of the hippocampus as a function of maternal behavior. These data indicate that in nulliparous female rats maternal behavior itself is associated with the stimulation of neurogenesis in the SVZ.
Keywords: Maternal Care, Cell proliferation, Virgin female rats, Subventricular Zone, Pup Exposure and Nulliparity
Introduction
Adult neurogenesis has been documented in several vertebrate species, including birds (4), rodents (18, 21, 27) nonhuman primates (15) and humans (7). These new cells proliferate in the subventricular zone (SVZ) of the forebrain and most migrate along the rostral migratory stream (RMS) into the olfactory bulb where the majority of cells develop into neurons (29, 34). These new olfactory neurons disperse and differentiate into granule or periglomerular cells in the main olfactory bulb (26, 34) or into granule cells in the accessory olfactory bulb (2, 34). Recently, newly proliferated cells have been visualized in other brain regions, including the preoptic area (POA), thalamus, hypothalamus, and hippocampus (7, 15, 18). In mice, it was reported that the production of neuronal progenitors is stimulated in the forebrain SVZ during pregnancy by the hormone prolactin (44). In a previous study we identified increases in cell proliferation during pregnancy in the SVZ of the rat (13), confirming earlier findings that found increased neurogenesis during both pregnancy and lactation in the SVZ in mice (44).
Several species of mammals include the mouse (24), hamster (38), monkey (40) and human, non-pregnant nulliparous females display components of maternal behavior. In rats, maternal behavior, including pup retrieval, grouping of young and crouching, can be induced by continuously exposing inexperienced, nulliparous females to foster young for 5 to 7 days (3, 39). The maternal behaviors displayed by nulliparous rats are similar to those displayed by lactating rats (12, 5, 43).
In the present study, the effects of maternal behavior induction and pup exposure on neurogenesis in nulliparous rats were examined in order to determine whether maternal behavior itself, independent of pregnancy and lactation, might affect neurogenesis in the SVZ, a known region of neurogenesis in adult rodents (25, 26, 33). In addition, to evaluate the neural specificity of possible effects of maternal behavior on neurogenesis, responses in the SVZ were compared with those in the dentate gyrus of the hippocampus.
Materials and Methods
Animals
Twenty nulliparous female Sprague-Dawley rats arrived from Charles River Breeding Laboratory (Kingston, NY). They were double-housed in a light (on 0500 – 1900 hr) and temperature (21°C –24°C) -controlled room. The animals used in these experiments were maintained in accordance with the National Research Council (NRC) Guide for the Care and Use of Laboratory Animals (© 1996, National Academy of Science). The research protocol was approved by Tufts University - Cummings School of Veterinary Medicine’s Institutional Animal Care and Use Committee.
Experimental Groups
Subjects were assigned to one of three groups. Group 1 and 2 females were single-housed in polypropylene test cages (45×25×20) three days prior to the start of maternal behavior testing. At this time, Plexiglas dividers were placed on the floor of each cage to divide the cage into 4 equal quadrants. The remaining six rats that comprised Group 3 were singly housed, but were not exposed to pups and hence tested for maternal behavior.
Behavior Testing
Animals in Groups 1 and 2 were tested daily for maternal behavior between 0900 and 1100 h daily by exposing them to 3 foster pups, 3 to 9 days of age, that were recently fed by lactating donor mothers. Each test day the position of the test female was recorded prior to the start of testing. To initiate a test session, a test pup was placed in each quadrant of the home cage, except the quadrant where the nest was located. For the next 15 min, each female was observed continuously and the behavioral responses of the test animal recorded. Typically, 6 to 10 subjects were tested at one time. The occurrence and latency to display the following behaviors were recorded: retrieval of each pup, grouping all pups in the nest, and crouching over them. After the first 15-min observation period, test animals were spot checked at the 30, 45, and 60 minute time points. Pups remained with the subjects until the following test session. Testing was conducted once daily until a female reached the criterion for full maternal behavior or until such time that a non-responding female was yoked to a maternal subject.
Full maternal behavior was defined as the retrieval of all three-test pups to the nest, grouping them together and crouching over them during the one-hour test period for 2 consecutive days. When a female responded maternally (M) on the second day, she was yoked when possible to two other females - one that was exposed to pups (non-maternal pup exposure, NMP), but did not display any maternal behaviors and another that had received no pup exposure (NE). The yoked NMP female was one that had been tested for maternal behavior for the same number of days as the maternal female, but had not responded maternally (i.e. had never retrieved any pups).
Immunocytochemistry
At the end of the one-hour testing period on the second day of full maternal behavior, M females as well as the yoked NMP and NE females were anesthetized with ketamine/xylazine. Each subject was injected with BrdU (90 mg/kg in EDTA) via the jugular vein. One hour after injections, animals were given an overdose of ketamine/xylazine and perfused with a solution of 2% paraformaldehyde and 4% acrolein in 0.1M phosphate buffer. Brains were post-fixed in the perfusion solution for 24 hours at 4°C and cryoprotected for at least 24 hours in 30% sucrose in 0.1M phosphate buffer prior to being frozen at −80°C. Free-floating 30µm sections were subsequently collected at −20°C through the SVZ and hippocampus. In order to examine the labeling of both BrdU and NeuN, sections were incubated simultaneously for 24 h at 4°C in primary mouse monoclonal anti-BrdU antibody (Becton Dickinson) and mouse anti-NeuN antibody (Chemicon) at dilutions of 1:250. Bound NeuN antibody was detected with biotinylated anti-mouse IgG at a dilution of 1:200 (Vector Lab., Burlingame, CA) for 60 minutes and streptavidin-FITC at a dilution of 1:330 (Vector Lab., Burlingame, CA) for 120 minutes. BrdU labeling was visualized using donkey anti-rat CY3-fluorescent IgG antibody at dilution of 1:400 (Jackson Immuno Research) for 60 minutes (23). Double-labeled cells were confirmed and photographed by fluorescence microscopy (Nikkon, PCM-2000)
Histological Analysis
The numbers of BrdU-labeled cells were counted in brain areas using fluorescence microscopy (20X). The counter was unaware of the experimental group. Areas examined included the SVZ and dentate gyrus (DG) of the hippocampus. The SVZ was divided into anterior (SVZ-I), central (SVZ-II) and posterior (SVZ-III) components, since these areas have been identified as regions of neurogenesis in adult rodents. The numbers of BrdU-labeled cells in the right and left hemispheres of 12 sections each area of per rat was counted. The sum of BrdU-labeled cells per area was then calculated for each rat, and the average number of cells per group was determined for each area (i.e. SVZ-I, SVZ -II, SVZ-III, entire SVZ and DG).
Data from each area were analyzed by ANOVA followed by Fisher’s protected LSD test for post-hoc differences. Differences were considered significant when P<0.05.
Results
Maternal Behavior
The average latency to the onset of full maternal behavior for the M animals was 7.9 ± 0.7 days with a range of 5 to 11 days (see table 1). The average latency of the pup exposure for the NMP animals was SEM 6.2 ± 0.6 days with a range of 5 to 8 days (see table 1).
Table 1.
The number of double labeled cells in the entire SVZ
| Group | Mean Cells | ± SEM (N) |
|---|---|---|
| NE | 1121.7 | ±151.7 (6) |
| NMP | 1211.0±171.6 (5) | |
| M | 1770.5±172.3 (8) | |
| Double-labeled cells as a function of response latency in group M rats |
Response day | Number of Cells |
| day 5 | 2146 | |
| day 6 | 2456 | |
| day 7 | 1556 | |
| day 8 | 2066, 1261 | |
| day 9 | 1019, 1622 | |
Number of BrdU-immunoreactive NeuN-immunoreactive labeled cells in the entire SVZ. Individual cell counts for rats in the M group are also listed as a function of their maternal response latencies.
Immunocytochemistry
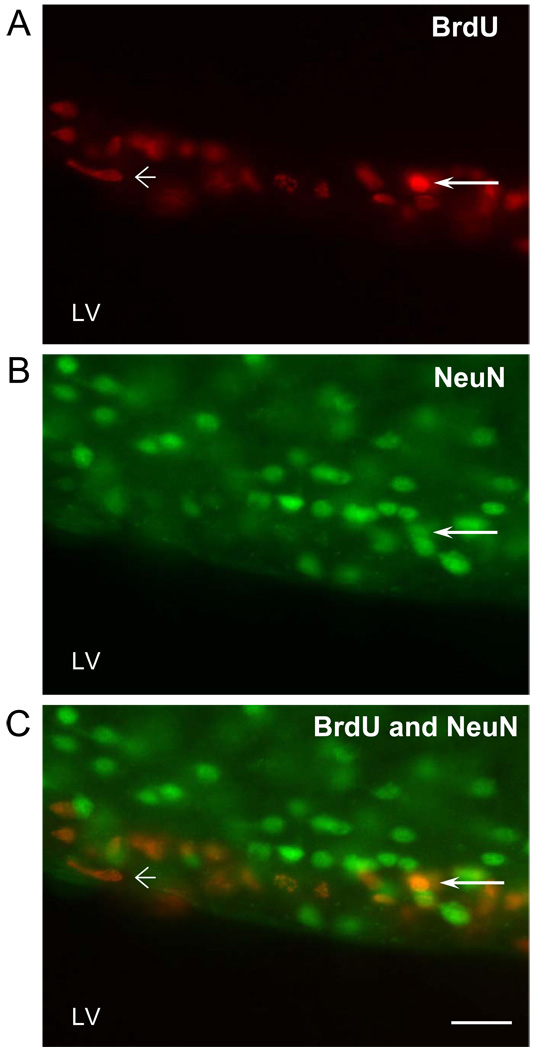
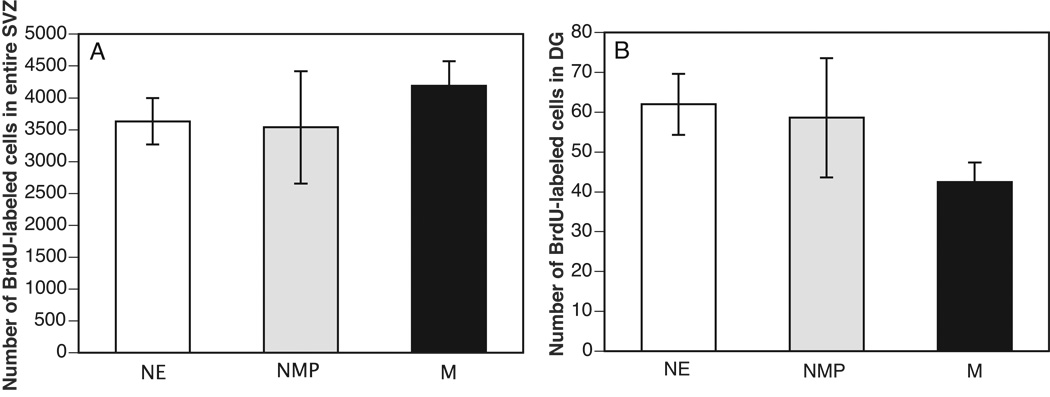
Representative microphotographs of BrdU-labeling (a measure of cell proliferation) and NeuN-labeling (a neural marker) together with double-labeled cells in the subventricular zones of three experimental groups are shown in Figure 1. As shown in Figure 2, significant differences in the number of BrdU-labeled cells were not detected in the entire SVZ (P=0.624) and the DG (P=0.23). Likewise, analysis of BrdU-labeling in the divisions of the SVZ failed to detect any differences among the treatment groups (SVZ-I, P=0.59; SVZ-II, P=0.29; SVZ-III, P=0.98).
Figure 1.
Representative photomicrographs of BrdU-immunoreactive cells (red; upper panel), NeuN-immunoreactive cells, neural marker (green; middle panel) and colocalization of BrdU-immunoreactive cells with NeuN-immunoreactive cells (yellow-orange; lower panel) in the subventricular zone (coronal section) of a NE subject. Scale bar = 50µm, LV - lateral ventricle. Arrows identify co-localized cells.
Figure 2.
The mean ± SEM number of BrdU-labeled cells in the (A) entire SVZ and (B) DG in the M (N=8), NMP (N=5), and NE (N=6) groups.
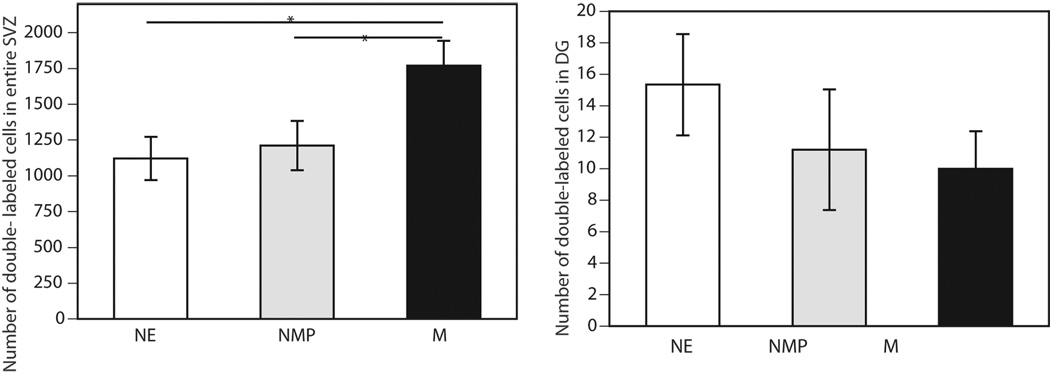
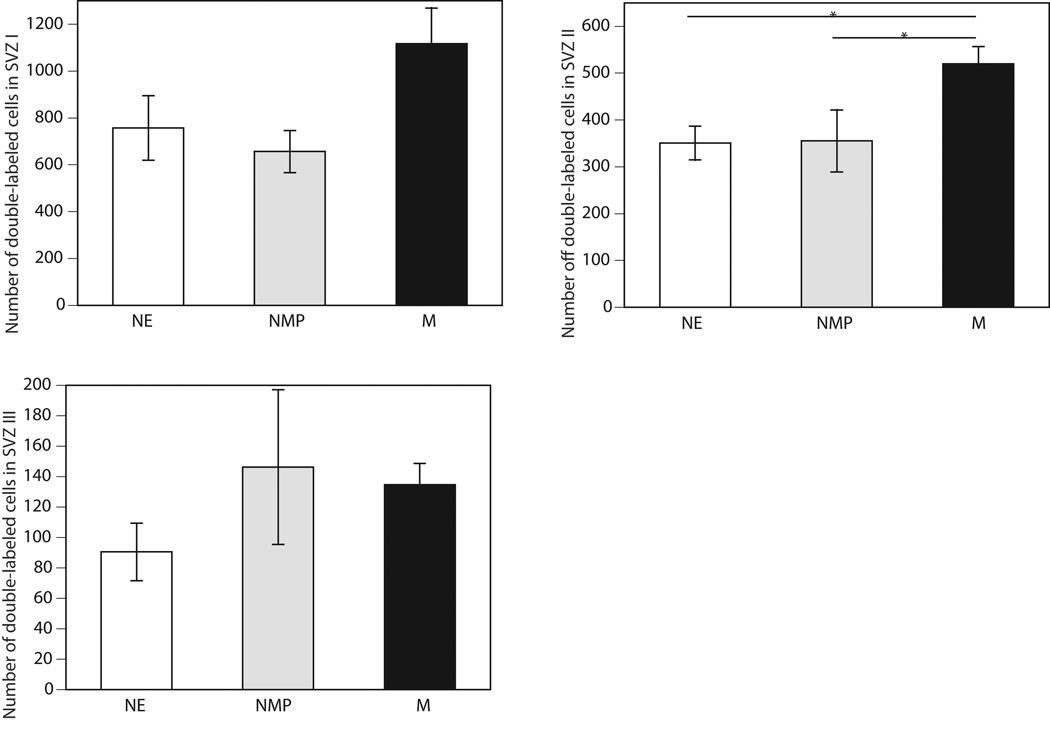
In contrast, as shown in figure 3, when double-labeled cells were analyzed within the SVZ, significant overall differences were found in the number of the double-labeled cells (P<0.05). No treatment effect was found within the DG (P=0.43). Further analysis of the subdivisions of the SVZ (see Figure 4) revealed a significant increase in the number of double-labeled cells in the SVZ-II (P<0.05), but not the SVZ-I (P=0.074) or SVZ-III (P=0.36). Post-hoc analysis indicated that the number of double-labeled cells in the SVZ-II in M group was significantly greater than that in the NMP or NE groups (P<0.05). Likewise, comparisons between the three groups in the entire SVZ revealed that there were a greater number of double-labeled cells in the entire SVZ in M group than in either control group (P<0.05).
Figure 3.
The mean number of double-labeled cells in the (A) entire SVZ and (B) DG in the M (N=8), NMP (N=5), and NE (N=6) groups.
Figure 4.
The mean ± SEM number of immunoreactive cells in the (A) SVZ-I, (B) SVZ-II and (C) SVZ-II double-labeled BrdU and NeuN in the M (N=8), NMP (N=5), and NE (N=6) groups.
Table 1 shows the number of double-labeled cells in the entire SVZ as a function of the latency for M group to display full maternal behavior. Whereas females with maternal latencies of 5 and 6 days had a tendency to have greater numbers of double-labeled cells when compared with those in the control groups, the sample size on these days was limited and precluded thorough statistical analysis.
Discussion
The results of the present study demonstrate that increases in double-labeled or merged cells occur in the brains of nulliparous female rats that are induced to show maternal behavior independent of pregnancy and lactation. Significant increases in BrdU/NeuN merged cells were identified in the SVZ of maternal virgin rats when compared with labeling present in both non-maternal, nulliparous rats exposed to pups and non-exposed, cycling diestrous controls. No changes were evident in the number of merged cells in the dentate gyrus of the hippocampus as a function of maternal behavior.
What factors might stimulate neurogenesis in the maternal virgin rats? A recent research study found that neurogenesis during pregnancy and lactation is stimulated by prolactin (PRL) acting through the PRL receptor at the level of the SVZ (44). Is it possible that the increase in neurogenesis associated with the induction of maternal behavior in the nulliparous rats in the present study is also stimulated by PRL? It appears unlikely that circulating PRL released from the pituitary gland contributes to neurogenesis in the maternal virgin rat, since PRL levels are unchanged in pup-induced maternal rats (41). This, however, does not exclude the possibility that the induction process may affect neural-derived PRL that in turn could act to stimulate neurogenesis within the SVZ. Recent studies in lactating rats found that brain-derived PRL functions in the modulation of stress responses (47). Moreover, pup contact and related stimuli received by maternal, nulliparous rats have been reported to induce the expression of the long, but not short, form of PRL-R mRNA in brain (45). Several approaches have been also shown PRL-R is involved in the signaling leading to cell-cycle control and differentiation (48). Thus, it is possible that a so-called "neural PRL system" may be activated in maternal virgins that contribute to enhanced neurogenesis in the nulliparous female. This possibility is the topic of future studies.
What cellular mechanism underlies the increase in double-labeling of cells in the SVZ-II? In vitro studies have revealed that the adult SVZ can be induced to generate both neurons and glia under the influence of growth factors (16, 33, 35, 36, 37). Furthermore, the in vivo exposure of the adult forebrain SVZ to neurotrophins induces an increase in the number of progenitor cells, as well as the production of newly generated neurons (6, 23, 49). It was suggested that the brain-derived neurotrophic factor, BDNF may influence the development of large range of neuronal cell types (1, 20, 22, 28, 42). In vitro studies of adult and old-aged rats demonstrated that BDNF promotes the survival of neurons generated from SVZ progenitor cells, suggesting that BDNF may also exert an effect on neurogenesis in the maternal virgin rat. Moreover, the BDNF gene contains an estrogen response element like motif capable of binding estrogen receptors, alpha and beta (19), and estrogen treatment increases both BDNF mRNA and protein (14) in the female rat olfactory bulbs. Therefore, it seems tenable that maternal behavior itself may stimulate increased BDNF activity in parts of the SVZ.
The destinations and phenotypes of new cells in the maternal virgin rat are not known. While many of the newly generated cells in mice emerge from the SVZ and then migrate along the RMS to form new interneurons in the olfactory bulb, other newly divided SVZ cells undergo apotosis or generate glial cells that migrate to the corpus callosum (31). Other newly cell proliferated cells have been visualized in the neocortex, POA, central gray, thalamus, hypothalamus and hippocampus (7,15,18).
Whereas in the present study the overall numbers of BrdU-labeled cells in the SVZ or the dentate gyrus were not found to differ among treatment groups, the number of double–labeled cells in the SVZ of the maternal group, especially the SVZ-II, was significantly higher than either that found in the non-maternal pup-exposed or non-exposed groups. The percentage of double-labeled cells in the SVZ of these two latter groups ranged from 31–33% of the BrdU labeled cells, while this number approached 41% in the maternal group. It is noted that the percentage of double-labeled cells in the SVZ was more pronounced in the rostral portions of the SVZ, i.e. SVZ-I and SVZ-II, where it ranged from 47–54%. One possible explanation for these findings is that maternal experience might accelerate the rate of cell proliferation and maturation of neurons, possibly as a function of the female’s endocrine status (44). Future studies, however, are needed to account for these shifts in cell distributions and identify factors mediating these changes.
The functional significance of possible maternal behavior-associated neurogenesis in the virgin rat remains to be determined. It is of interest that in mice neurogenesis in the SVZ results in enhanced migration of cells to the olfactory bulb (44). Olfactory pathways are known to play important roles in maternal care in numerous mammals (9, 10). Therefore, neurogenesis may result in the functional integration of new neurons in olfactory areas or possibly the POA sites are of particular physiological importance not only to the parturient rat, but also to the inexperienced, virgin rat (32). The possible relationship between the amygdala and neurogenesis in the maternal virgin rat is of interest in light of the involvement of the amygdala in maternal behavior in virgin rats. It is established that pup exposure elicits withdrawal and avoidance responses in inexperienced, virgin female rats. These odor-mediated behavioral responses are transduced to the amygdala via the vomeronasal pathway. Moreover, maternal behavior in virgin rats is stimulated when this inhibitory input is blocked by either rendering the females anosmic (10) or lesioning the amygdala (11, 32). Amygdaloid kindling, which enhances fearfulness in rat, also increases neophobia and decreases approaches to pup-related stimuli in virgin rats (30). The possible reciprocal relationships between the amygdala and SVZ the expression of maternal behavior merit investigation.
The extent of neurogenesis induced in maternal virgin rats in the present study appears less pronounced that found in late pregnant rats (13). What might help account for this apparent difference in neurogenesis? One possibility may be that pregnant rats are exposed to high levels of prolactin and other lactogenic hormones during gestation that can stimulate neurogenesis (13), whereas circulating PRL hormone levels are generally unaffected by pup exposure in virgin rats (41). Hence, this differential hormonal exposure would be expected to alter the degree of neurogenesis. Another possibility, albeit more speculative, is that the state of pregnancy that is accompanied by fetal microchimerism (46) might contribute to the increase in cell proliferation during late pregnancy is unknown. However, the precise role that these fetal cells as well as hormones play in neurogenesis during pregnancy and the reproductive success of the female remain to be delineated.
For virgin females, pups elicit withdrawal and avoidance associated with odor cues transduced via olfactory system. The vomeronausal projections arise via the amygdala. Thus, anosmic females are more readily maternal (10) and lesions of the amygdala enhance maternal behavior in virgin rats (11, 32). These findings suggest that the cues which elicit withdrawal are transmitted through the amygdala. The amygdaloid kindling, which enhances fearfulness in rat, increases neophobia and decreases approaches to pup-related stimuli in virgin rats (30). These findings suggest that olfactory system via amygdala and increases neurogenesis in the SVZ is included if virgin female are maternal. A part of these increases neurogenesis may be also migrate to the amygdala in non-maternal virgin rats.
The possible causal relationship between the expression of maternal behavior and the increase in the number of double-labeled cells has not been determined. While it is possible that the increase in merged cells within the SVZ may help stimulate the onset of maternal care, it is also quite possible that the shift in merged cell numbers is a reflection or outcome of the maternal state of the female. This latter possibility seems more plausible, since new neurons produced within the SVZ would be expected to take 5–10 days to migrate to the olfactory bulb where they might become functionally integrated to alter the olfactory response of the female to pups. Since olfaction appears to be a key determinant governing the expression of maternal behavior (8, 9, 10), it would seem more likely that any shift in SVZ neurogenesis might alter the expression of ongoing or previously established maternal care. These relationships require further study in order to determine the functional significance of these new neurons.
Finally, that neurogenesis occurs in maternal virgins as it does in parous females (13) indicates that the process associated with the induction of maternal care in females independent of the states of pregnancy and lactation are sufficient to induce formation of new neurons. It is intriguing to consider that longer term changes in neural activity result from the responses associated with behavioral motherhood. For example, to what extent to do epigenetic factors during early development or subsequent behavioral states in adulthood, i.e. depression, alter neurogenesis in response to exposure to young and the expression of maternal behavior in the virgin and parous female.
Acknowledgements
This research was funded by PHS grant R37 HD19789 awarded to R.S. Bridges.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
Neither of the authors listed below has any conflict of interest that may impact the publication of the manuscript submitted entitled “Effects of Maternal Behavior Induction and Pup Exposure on Neurogenesis in Adult, Virgin Female Rats” that is being submitted to Behavioral Brain Bulletin.
References
- 1.Alderson RF, Alterman AL, Barde YA, Lindsay RM. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron. 1990;5:297–306. doi: 10.1016/0896-6273(90)90166-d. [DOI] [PubMed] [Google Scholar]
- 2.Bonfanti L, Peretto P, Merighi A, Fasolo A. Newly-generated cells from the rostral migratory stream in the accessory olfactory bulb of the adult rat. Neuroscience. 1997;81:489–502. doi: 10.1016/s0306-4522(97)00090-0. [DOI] [PubMed] [Google Scholar]
- 3.Bridges SR. A quantitative analysis of the roles of dosage, sequence, and duration of estradiol and progesterone exposure in the regulation of maternal behavior in the rat. Endocrinology. 1984;114:930–940. doi: 10.1210/endo-114-3-930. [DOI] [PubMed] [Google Scholar]
- 4.Brown SD, Johnson F, Bottjer SW. Neurogenesis in adult canary telencephalon is independent of gonadal hormone levels. J. Neurosci. 1993;13:2024–2032. doi: 10.1523/JNEUROSCI.13-05-02024.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc. Natl. Acad. Sci. U S A. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craig CG, Tropepe V, Morshead CM, Reynolds BA, Weiss S, van der Kooy D. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J. Neurosci. 2001;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 8.Fleming A, Vaccarino F, Tambosso L, Chee P. Vomeronasal and olfactory system modulation of maternal behavior in the rat. Science. 1979;203:372–374. doi: 10.1126/science.760196. [DOI] [PubMed] [Google Scholar]
- 9.Fleming AS, Rosenblatt JS. Olfactory regulation of maternal behavior in rats. I. Effects of olfactory bulb removal in experienced and inexperienced lactating and cycling females. J. Comp. Physiol. Psychol. 1974a;86:221–232. doi: 10.1037/h0035937. [DOI] [PubMed] [Google Scholar]
- 10.Fleming AS, Rosenblatt JS. Olfactory regulation of maternal behavior in rats. II. Effects of peripherally induced anosmia and lesions of the lateral olfactory tract in pup-induced virgins. J. Comp. Physiol. Psychol. 1974b;86:233–246. doi: 10.1037/h0035936. [DOI] [PubMed] [Google Scholar]
- 11.Fleming AS, Vaccarino F, Luebke C. Amygdaloid inhibition of maternal behavior in the nulliparous female rat. Physiol. Behav. 1980;25:731–743. doi: 10.1016/0031-9384(80)90377-7. [DOI] [PubMed] [Google Scholar]
- 12.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 13.Furuta M, Bridges SR. Gestation-induced cell proliferation in the rat brain. Dev. Brain. Res. 2005;156:61–66. doi: 10.1016/j.devbrainres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Gibbs RB. Treatment with estrogen and progesterone affects relative levels of brain-derived neurotrophic factor mRNA and protein in different regions of the adult rat brain. Brain. Res. 1999;844:20–27. doi: 10.1016/s0006-8993(99)01880-6. [DOI] [PubMed] [Google Scholar]
- 15.Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 16.Gritti A, Frolichsthal-Schoeller P, Galli R, Parati EA, Cova L, Pagano SF, Bjornson CR, Vescovi AL. Epidermal and fibroblast growth factors behave as mitogenic regulators for a single multipotent stem cell-like population from the subventricular region of the adult mouse forebrain. J. Neurosci. 1999;19:3287–3297. doi: 10.1523/JNEUROSCI.19-09-03287.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herzenberg LA, Bianchi DW, Schroder J, Cann HM, Iverson GM. Fetal cells in the blood of pregnant women: detection and enrichment by fluorescence-activated cell sorting. Proc. Natl. Acad. Sci. U S A. 1979;76:1453–1455. doi: 10.1073/pnas.76.3.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang L, De Vries GJ, Bittman EL. Photoperiod regulates neuronal bromodeoxyuridine labeling in the brain of a seasonally breeding mammal. J. Neurobiol. 1998;36:410–420. doi: 10.1002/(sici)1097-4695(19980905)36:3<410::aid-neu8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 19.Jezierski MK, Sohrabji F. Estrogen enhances retrograde transport of brain-derived neurotrophic factor in the rodent forebrain. Endocrinology. 2003;144:5022–5029. doi: 10.1210/en.2003-0724. [DOI] [PubMed] [Google Scholar]
- 20.Johnson D, Lanahan A, Buck CR, Sehgal A, Morgan C, Mercer E, Bothwell M, Chao M. Expression and structure of the human NGF receptor. Cell. 1986;47:545–554. doi: 10.1016/0092-8674(86)90619-7. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- 22.Knusel B, Winslow JW, Rosenthal A, Burton LE, Seid DP, Nikolics K, Hefti F. Promotion of central cholinergic and dopaminergic neuron differentiation by brain-derived neurotrophic factor but not neurotrophin 3. Proc. Natl. Acad. Sci. USA. 1991;88:961–965. doi: 10.1073/pnas.88.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuan CY, Schloemer AJ, Lu A, Burns KA, Weng WL, Williams MT, Strauss KI, Vorhees CV, Flavell RA, Davis RJ, Sharp FR, Rakic P. Hypoxia-Ischemia Induces DNA Synthesis without Cell Proliferation in Dying Neurons in Adult Rodent Brain. J. Neurosci. 2004;24:10763–10772. doi: 10.1523/JNEUROSCI.3883-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leblond CP, Nelson WO. Maternal behavior in hypophysectomized male and female mice. Am. J. Physiol. 1937;120:167–172. [Google Scholar]
- 25.Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 26.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 27.Luskin MB, Boone MS. Rate and pattern of migration of lineally-related olfactory bulb interneurons generated postnatally in the subventricular zone of the rat. Chem. Senses. 1994;19:695–714. doi: 10.1093/chemse/19.6.695. [DOI] [PubMed] [Google Scholar]
- 28.Mansour-Robaey S, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Effects of ocular injury and administration of brain-derived neurotrophic factor on survival and regrowth of axotomized retinal ganglion cells. Proc. Natl. Acad. Sci. U S A. 1994;91:1632–1636. doi: 10.1073/pnas.91.5.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menezes JR, Smith CM, Nelson KC, Luskin MB. The division of neuronal progenitor cells during migration in the neonatal mammalian forebrain. Mol. Cell. Neurosci. 1995;6:496–508. doi: 10.1006/mcne.1995.0002. [DOI] [PubMed] [Google Scholar]
- 30.Morgan HD, Watchus JA, Fleming AS. The effects of electrical stimulation of the medial preoptic area and the medial amygdala on maternal responsiveness in female rats. Ann. N. Y. Acad. Sci. 1997;807:602–605. doi: 10.1111/j.1749-6632.1997.tb51980.x. [DOI] [PubMed] [Google Scholar]
- 31.Morshead CM, van der Kooy D. Postmitotic death is the fate of constitutively proliferating cells in the subependymal layer of the adult mouse brain. J. Neurosci. 1992;12:249–256. doi: 10.1523/JNEUROSCI.12-01-00249.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Numan M, Numan MJ. Expression of Fos-like immunoreactivity in the preoptic area of maternally behaving virgin and postpartum rats. Behav. Neurosci. 1994;108:379–394. doi: 10.1037//0735-7044.108.2.379. [DOI] [PubMed] [Google Scholar]
- 33.Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J. Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peretto P, Merighi A, Fasolo A, Bonfanti L. The subependymal layer in rodents: a site of structural plasticity and cell migration in the adult mammalian brain. Brain Res. Bull. 1999;49:221–243. doi: 10.1016/s0361-9230(99)00037-4. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds BA, Tetzlaff W, Weiss SA. Multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J. Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1646. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 37.Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev. Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- 38.Richards MP. Maternal behaviour in virgin female golden hamsters (Mesocricetus auratus Waterhouse): the role of the age of the test pup. Anim. Behav. 1966;14:303–309. doi: 10.1016/s0003-3472(66)80087-8. [DOI] [PubMed] [Google Scholar]
- 39.Rosenblatt JS. Nonhormonal basis of maternal behavior in the rat. Science. 1967;56:1512–1514. doi: 10.1126/science.156.3781.1512. [DOI] [PubMed] [Google Scholar]
- 40.Rowell TE. Behavior and female reproductive cycle of rhesus macaque. J. Reprod. Fertil. 1963;39:193–203. doi: 10.1530/jrf.0.0060193. [DOI] [PubMed] [Google Scholar]
- 41.Samuels MH, Bridges RS. Plasma prolactin concentrations in parental male and female rats: effects of exposure to rat young. Endocrinology. 1983;113:1647–1654. doi: 10.1210/endo-113-5-1647. [DOI] [PubMed] [Google Scholar]
- 42.Segal RA, Takahashi H, McKay RD. Changes in neurotrophin responsiveness during the development of cerebellar granule neurons. Neuron. 1992;9:1041–1052. doi: 10.1016/0896-6273(92)90064-k. [DOI] [PubMed] [Google Scholar]
- 43.Sheehan TP, Cirrito J, Numan MJ, Numan M. Using c-Fos immunocytochemistry to identify forebrain regions that may inhibit maternal behavior in rats. Behav. Neurosci. 2000;114:337–352. doi: 10.1037//0735-7044.114.2.337. [DOI] [PubMed] [Google Scholar]
- 44.Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, Cross JC, Weiss S. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- 45.Sugiyama T, Minoura H, Toyoda N, Sakaguchi K, Tanaka M, Sudo S, Nakashima KJ. Pup contact induces the expression of long form prolactin receptor mRNA in the brain of female rats: effects of ovariectomy and hypophysectomy on receptor gene expression. J. Endocrinol. 1996;149:335–340. doi: 10.1677/joe.0.1490335. [DOI] [PubMed] [Google Scholar]
- 46.Tan XW, Liao H, Sun L, Okabe M, Xiao ZC, Dawe GS. Fetal microchimerism in the maternal mouse brain: A novel population of fetal progenitor or stem cells able to cross the blood-brain barrier? Stem Cells. 2005;23:1443–1452. doi: 10.1634/stemcells.2004-0169. [DOI] [PubMed] [Google Scholar]
- 47.Torner L, Maloumby R, Nava G, Aranda J, Clapp C, Neumann ID. In vivo release and gene upregulation of brain prolactin in response to physiological stimuli. Eur. J. Neurosci. 2004;19:1601–1608. doi: 10.1111/j.1460-9568.2004.03264.x. [DOI] [PubMed] [Google Scholar]
- 48.Walker AM. Prolactin receptor antagonist. Curr. Opin. Investig. Drugs. 2005;6:378–385. [PubMed] [Google Scholar]
- 49.Zigova T, Pencea V, Wiegand SJ, Luskin MB. Intraventricular administration of BDNF increases the number of newly generated neurons in the adult olfactory bulb. Mol. Cell Neurosci. 1998;11:234–245. doi: 10.1006/mcne.1998.0684. [DOI] [PubMed] [Google Scholar]