Abstract
Despite an increased understanding of the pharmacology and long-term cognitive effects of cannabis in humans, there has been no research to date examining its chronic effects upon reward processing in the brain. Motivational theories regarding long-term drug use posit contrasting predictions with respect to how drug users are likely to process non-drug incentives. The reward deficiency syndrome (RDS) of addiction posits that there are deficits in dopamine (DA) motivational circuitry for non-drug rewards, such that only drugs of abuse are capable of normalizing DA in the ventral striatum (VS). Alternatively, the opponent process theory (OPT) holds that in individuals prone to drug use, there exists some form of mesolimbic hyperactivity, in which there is a bias towards reward-centred behaviour concomitant with impulsivity. The current study examined BOLD responses during reward and loss anticipation and their outcome deliveries in 14 chronic cannabis users and 14 drug-naïve controls during a monetary incentive delay (MID) task. Despite no significant behavioural differences between the two groups, cannabis users had significantly more right VS BOLD activity during reward anticipation. Correlation analyses demonstrated that this right VS BOLD response was significantly correlated with life-time use and reported life-time cannabis joints consumed. No correlations between cannabis abstinence and BOLD responses were observed. We also observed a number of group differences following outcome deliveries, most notably hypoactivity in the left insula cortex in response to loss and loss avoidance outcome notifications in the cannabis group. These results may suggest hypersensitivity during instrumental response anticipation for non-drug rewards and a hyposensitivity to loss outcomes in chronic cannabis users; the implications of which are discussed with respect to the potentially sensitizing effects of cannabis for other rewards.
Introduction
The long-term use of cannabis has been linked to deficits in learning, memory and executive functioning in humans (Nestor et al, 2008; Grant et al, 2003; Solowij et al, 2002; Bolla et al, 2002). Critically it has been proposed that compromised cognitive processing in chronic cannabis users may promote continued drug consumption, which may be exacerbated following drug withdrawal (Goldstein and Volkow, 2002; Volkow et al, 2002). Subjective reports concerning the effects of cannabis suggest that its reinforcing properties, like other drugs of abuse, are related to its effects on the ventral striatal (VS) “reward circuitry” (Huestis et al, 2007; D’Souza et al, 2008; Hunault et al, 2008), where inhaled cannabis has been shown to induce dopamine (DA) release (Bossong et al, 2009). Despite an increased understanding of the cognitive and psychopharmacological effects of cannabis, little is known regarding its chronic effects upon reward processing in the human brain.
Despite significant evidence of VS responses to drug-associated stimuli in substance dependence (London et al, 1999; Grusser et al, 2004; Sinha and Li, 2007), there is little evidence that drug users are abnormal in their responses to stimuli that predict non-drug rewards. Motivational theories regarding drug use make contrasting predictions with respect to how drug users may differentially recruit the VS in response to cues that signal non-drug incentives (Bjork et al, 2008). The reward deficiency syndrome (RDS) and the allostatic hypotheses (AH), for example, view addiction as a deficit in DA motivational circuitry for non-drug rewards, such that only drugs of abuse are able to normalize DA at the VS (Blum et al, 2000; Koob et al, 2004). The incentive salience hypothesis (ISH) attributes compulsive drug-use to alterations in striatal functioning, in which drug-cues reputedly acquire increased incentive-motivational value (Robinson and Berridge, 1998, 2001).
Substance-dependent patients have also been shown to exhibit both impulsive and reward-centred choice behaviour under laboratory conditions, with cocaine and alcohol dependence associated with an increased preference for small immediate over larger delayed rewards (Bickel et al, 2001; Bechara et al, 2001; Heil et al, 2006; Bjork, 2004). This may suggest that in individuals who are both prone to, and engage in chronic drug use, there exists some combination of both mesolimbic reward hyperactivity and hypoactive frontocortical punishment-avoidance circuitry (Solomon and Corbit, 1973; Bechara et al, 2005; Bickel et al, 2007). Therefore, drugs such as cannabis, capable of engaging the VS, as described above, may induce a heightened and indiscriminate response to cues which signal all forms of potential reward, an effect which is related to the magnitude of life-time drug use.
Given that cannabis increases DA in the VS (Bossong et al, 2009) and that the anticipation of non-drug incentives reliably activates (Schultz et al, 1997; Knutson et al, 2001; O’Doherty 2004) and increases DA release in this region (Schott et al, 2008) the current investigation hypothesised that 1) chronic cannabis use would be associated with altered VS functioning during reward anticipation, together with differential neural activity in limbic and paralimbic regions during outcome delivery and 2) that VS, limbic and paralimbic activity would be related to life-time cannabis exposure, but not abstinence.
Material and Methods
Participants
Fourteen cannabis users and 14 controls were recruited from the general public and academic institutions around Dublin. A semi-structured interview was conducted to screen participants for past or present history of psychiatric or neurological illness. Information pertaining to any form of treatment (counselling, psychological, psychiatric), past or present, was carefully detailed, with any potential participant describing any major life-time psychiatric event or brain injury (e.g., head trauma resulting in a loss of consciousness, seizure or stroke) considered ineligible for the study. Also, any reports of familial psychiatric history (i.e. sibling, parent, grand parent) meant that people were additionally considered ineligible to participate. All participants completed inventories for mood (Beck Depression Inventory - BDI II) and drug use (questionnaire taken from the Addiction Severity index Lite-CF; see questionnaires section below) to screen for depression and past or concurrent abuse of other substances (e.g., alcohol, amphetamines, benzodiazepines, cocaine, MDMA, hallucinogens and opiates) during a subsequent in-person screening session (Beck et al, 1996; McLellan et al, 1992). Information concerning alcohol, nicotine and cannabis use in each participant was indexed in number of years (life-time) and occasions of recent use (last 30 days). Other drug use information on each participant was indexed by the total number of separate occasions (life-time) and the total number of recent separate occasions (last 30 days).
Cannabis group participants were required to have regularly consumed cannabis (5–7 days/week) for the previous 2 years in order to be eligible for the study. Participants in the cannabis group were additionally required to have smoked a minimum of 500 joints in their life-time, in order to exclude potential participants with relatively low cannabis use. All cannabis users were required to provide a positive urine sample for Δ9-tetrahydrocannabinol (Δ9THC), with additional screening for methadone, benzodiazepines, cocaine, amphetamines, opiates, barbiturates and tricyclic antidepressants (Cozart® RapiScan, UK) taking place. Control group participants were also tested for Δ9THC and the above adulterants. While the identification and quantification of cannabis metabolites in urine may have proved advantageous as a potential predictor of brain functioning, past studies have shown that estimates of recent use, life-time use and age of onset of use, are reliable predictors of behavioural performance and BOLD activity in cannabis users (Solowij et al, 2002; Bolla et al, 2002, 2005; Block et al, 1993; Pope et al, 2003; Pope and Yurgelun-Todd, 1996; Chang et al, 2006). Therefore, urinalyses were conducted merely to verify the presence of Δ9THC in cannabis participants only and the absence of all other drug metabolites in both control and cannabis participants. Following confirmation of eligibility in both cannabis users and controls, people were invited to participate in the study.
Cannabis users qualifying to participate reported, on average: 6.1 years (range = 2.5–17) of life-time cannabis use; the consumption of 7258 life-time cannabis joints (range = 700–34,403); 20 days use in the last 30 days (range = 6–30); the consumption of 64 cannabis joints in the last 30 days (range = 15–140) and 108 hours of cannabis abstinence prior to testing (range = 12–504). We deliberately recruited cannabis users at varying stages of abstinence in order to test whether BOLD responses in the VS during reward processing would be associated with the subacute effects of, and withdrawal from, cannabis – our hypothesis being that lifetime exposure, rather than recent use, would determine neural activity. All participants were right-handed as confirmed by the Edinburgh Handedness Inventory (Oldfield, 1971). All participants’ structural brain scans were examined and cleared of structural abnormalities by a registered radiologist. All research participants provided informed consent and were financially compensated.
Monetary Incentive Delay Task (MID)
We used a “monetary incentive delay task” (MID), which was based on that originally employed by Knutson (Knutson et al, 2001). The version used in the current study, however, differed from that originally developed on two levels. First, we did not use differential magnitudes of financial reward and punishment. Second, in the original version of the task, the anticipatory cue and trial outcome periods were time-invariantly yoked. To overcome any possible cross-contamination between anticipatory cue and outcome–related activation, we used extended temporal jittering between cue periods and target responses, and between outcome notifications and the commencement of the next trial. The temporal jittering enabled the separation of the BOLD signal related to instrumental response anticipation from outcome deliveries using deconvolution analyses. This was confirmed when designing the task by using the image analysis software to screen for multicollinearity between the anticipation and outcome regressors.
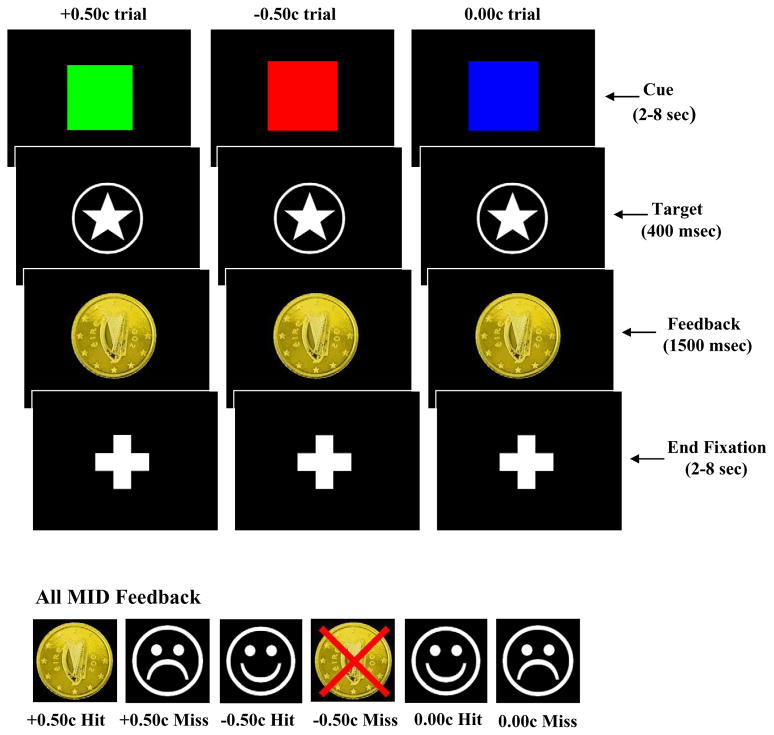
A condition of the approval we received from our institutional review board was the requirement that all participants were paid the same amount of money upon concluding the experiment (40 Euro money voucher for participation). During the practice session, prior to scanning, however, participants were informed that they would receive the total amount of money won during the experiment. Therefore, participants believed their winnings would be contingent upon their MID performance. All participants practiced the task prior to scanning, clearly demonstrating the ability to discriminate between the significance of the three “cues” used to indicate trial type (See below for cue description). While being scanned participants performed the MID task, during which they anticipated potential monetary gain, loss or no potential monetary outcome. During each trial, participants viewed one of three coloured squares (cue) that indicated the potential to win fifty cent (green square), lose fifty cent (red square) or experience no financial outcome (blue square) following their response to an upcoming visual target (See Fig 1 below). Each cue was presented for a variable duration (2–8 sec), after which participants made a button press response upon the presentation of a visual target (star located within a circle). Participants received feedback (1500 ms) following their response to the visual target, after which there was an end fixation period (2–8 sec) before the commencement of the next trial. Responses to the visual target falling within (“hits”) or outside (“misses”) a 400ms response deadline received feedback appropriate for that particular trial (See Fig 1 below). We chose this 400 ms time frame in order to yield accuracy levels at around 50%, which would serve to maintain the participant’s interest in the MID task. Therefore, participants had four hundred milliseconds to respond to the visual target in order to be successful on a “win”, “loss” or “no-outcome” trial. There were a total of 27 trials in each epoch (nine “win”, nine “loss” and nine “no-outcome”), with each trial lasting between six and eighteen seconds. The MID was composed of three epochs, with each epoch lasting 340 seconds. The order of cue presentation within each epoch was randomised across the three epochs. Dependent measures derived from the data included percentage accuracy and reaction time for “win”, “loss” and “no-outcome” conditions. The task was programmed and run using E-Prime version 1.1 (Psychology Software Tools, Pittsburgh, USA).
Figure 1.
Monetary Incentive Delay (MID) task structure. Participants were cued (2–8 sec) regarding a potential financial “win”, “loss” or “no-outcome” using one of three coloured squares. Participants were then required to respond to a target stimulus following cue presentation. The target stimulus was presented for 400ms, during which participants were required to respond, as quickly as possible, with a button press on a hand-held key pad. Following this, participants received feedback regarding their response (1500ms), before the presentation of an end fixation period (2–8 sec), during which they saw a centrally located crosshair.
Questionnaires
The Beck Depression Inventory-II and National Adult Reading Test (NART) were administered to all participants prior to scanning (Beck et al, 1996; Nelson and O’Connell, 1978). Information concerning alcohol and drug use (See Table 1) was obtained from all participants using a questionnaire taken from the Addiction Severity index Lite-CF McLellan et al, 1992). Prior to scanning, cannabis users also provided information concerning withdrawal and cannabis craving (Brower et al, 1988; Heishman et al, 2001). Information regarding cannabis withdrawal, modified from a cocaine withdrawal checklist, was obtained using a thirty two-item checklist, on which cannabis group participants were required to rate, on a scale of 0 (none) to 3 (severe), symptoms they had experienced in the previous 24 hours. The Marijuana Craving Questionnaire is made up of 12 statements, which the participant rates according to a seven-point Likert-type scale from “strongly disagree” to “strongly agree”. Responses to the questionnaire are then divided into four specific constructs made up of compulsivity (inability to control cannabis use), emotionality (the use of cannabis in anticipation of relief from withdrawal or negative mood), expectancy (the anticipation of positive outcomes from smoking cannabis) and purposefulness (the intention and plan to use cannabis for positive outcomes), as they relate to cannabis use.
Table 1.
Mean and SEM for control and cannabis groups on demographic and drug use history.
| Control (n=14) | Cannabis (n=14) | |
|---|---|---|
| Age | 23.1 ± 1.2 | 22.1 ± 1.2 |
| Years of Education | 16.1 ± 0.4 | 17.1 ± 0.6 |
| Verbal Intelligence Score (NART) | 123.0 ± 0.8 | 123.9 ± 0.8 |
| Beck Depression Inventory II Score | 7.2 ± 2.1 | 8.1 ± 2.1 |
| Females/Males | 3/11 | 2/12 |
| Years of Alcohol Use | 6.9 ± 1.2 | 6.1 ± 1.2 |
| Alcohol Use in Last Month (no. days) | 7.8 ± 1.4 | 8.3 ± 1.8 |
| Alcohol Use Age Onset (Years) | 16.2 ± 0.5 | 15.6 ± 0.5 |
| Years of Nicotine Use | 7.9 ± 1.7 | 5.2 ± 1.3 |
| Cigarettes/Day | 10.0 ± 2.4 | 10.0 ± 2.8 |
| Nicotine Use in Last Month (no. days) | 15.0 ± 4.2 | 12.9 ± 4.1 |
| Number of Packs in Last Month | 11.8 ± 3.6 | 12.0 ± 3.8 |
| Nicotine Use Age Onset (Years) | 16.2.0 ± 0.6 | 16.4 ± 1.0 |
| Amphetamine Use (no. times) | 3.3 ± 1.2 | 3.0 ± 1.8 |
| Amphetamine Use in Last Month (no. times) | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Cocaine Use (no. times) | 4.8 ± 0.3 | 6.1 ± 1.2 |
| Cocaine Use in Last Month (no. times) | 0.0 ± 0.0 | 0.0 ± 0.0 |
| MDMA Use (no. times) | 3.6 ± 0.9 | 4.5 ± 0.6 |
| MDMA Use in Last Month (no. times) | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Hallucinogenic Use (no. times) | 2.0 ± 0.4 | 3.2 ± 0.4 |
| Hallucinogenic Use in Last Month (no. times) | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Cannabis Use (Years) | 0.0 ± 0.0 | 6.1 ± 1.1 |
| Life-time Joints (number) | 3.0 ± 0.6 | 7258.6 ± 2512.8 |
| Heavy Cannabis Use (Years) | 0.0 ± 0.0 | 4.5 ± 1.1 |
| Days of Use in Last Month (number) | 0.0 ± 0.0 | 20.1 ± 2.5 |
| Joints in Last Month (number) | 0.0 ± 0.0 | 64.8 ± 10.7 |
| Cannabis Use Age Onset (years) | 17.0 ± 0.3 | 16.1 ± 0.4 |
| Cannabis Abstinence (hours) | 108.0 ± 39.7 | |
| 11.6 ± 2.1 | ||
| Cannabis Withdrawal Score (out of 32) | ||
| Cannabis Craving Scores (each item out of 21) | ||
| Compulsivity | 5.9 ± 0.8 | |
| Emotionality | 7.1 ± 1.2 | |
| Expectancy | 10.6 ± 1.0 | |
| Purposefulness | 12.1 ± 1.5 | |
fMRI acquisition
All scanning was conducted on a Philips Intera Achieva 3.0 Tesla MR system (Best, The Netherlands) equipped with a mirror that reflected the visual display, which was projected onto a panel placed behind the participants’ head outside the magnet. The mirror was mounted on the head coil in the participants’ line of vision.
Each scanning sequence began with a reference scan to resolve sensitivity variations. A parallel Sensitivity Encoding (SENSE) approach with a reduction factor of 2 was utilised for all T1-weighted image acquisitions (Pruessmann et al, 1999). 180 high-resolution T1-weighted anatomic MPRAGE axial images (FOV 230 mm, thickness 0.9 mm, voxel size 0.9 × 0.9 × 0.9) were then acquired (total duration 325 seconds), to allow subsequent activation localization and spatial normalization.
Functional data were acquired using a T2* weighted echo-planar imaging sequence collecting 32 non-contiguous (10% gap) 3.5 mm axial slices covering the entire brain (TE = 35 ms, TR = 2000 ms, FOV 224 mm, 64 × 64 mm matrix size in Fourier space). The functional scans had a total duration of 340 seconds per run.
Data processing and analyses
All analyses were conducted using AFNI software (Cox, 1996). Following image reconstruction, the three 3-D time series (epochs 1, 2 and 3) were concatenated and motion-corrected using 3-D volume registration (least-squares alignment of three translational and three rotational parameters). No participant’s head moved > 2 mm in any direction during the functional acquisitions. Activation outside the brain was removed using edge detection techniques. A single deconvolution analysis calculated activation for the cue and outcome periods.
i) Cue analyses
A regression analysis estimated activation for the “win”, “loss” and “no-outcome” cue periods. The three cue-period regressors were convolved with a standard haemodynamic response to accommodate the lag time of the blood oxygen level-dependent (BOLD) response. Multiple regression analyses calculated activation as a percentage change relative to the baseline, which consisted of the fixation period at the end of each trial.
The percentage change activation maps were re-sampled to 1 mm3 resolution, warped into standard Talairach space (Talairach and Tournoux, 1988) and spatially blurred with a 3-mm isotropic rms Gaussian kernel. Group activation maps for each condition of the task (“win”, “loss” and “no-outcome” cues) were determined with one-sample t-tests against the null hypothesis of zero activation change (i.e. no change relative to the between-trial fixation periods). Using a voxelwise statistical threshold (t = 3.4, p≥.005), a series of Monte Carlo simulations were then conducted (1000 iterations) to determine the frequency of clusters of significant voxels produced purely by chance. From this frequency distribution, we then selected the cluster size (276μl given our parameters) that occurred less than 5% of the time by chance, which, when combined with the voxelwise statistical threshold, resulted in a clusterwise threshold of p≥.05 (corrected).
The thresholded group t-test maps for the “win”, “loss” and “no-outcome” cue periods were combined within each group to form group OR maps. Following this, group OR maps were combined to form an overall OR map. Thus, the overall OR map included those significant voxels from the “win”, “loss” or “no-outcome” cue periods in either the control or cannabis groups. This final map yielded functionally-defined regions of interest for between-group and between-condition comparisons. The approach taken here, wherein we determine activated areas in which to conduct cluster-level between-group comparisons, was chosen because the cluster-level analysis tends to be more statistically robust than between-group voxelwise comparisons.
ii) Outcome analyses
We observed very large areas of significant activation during all outcome periods in both groups, which included the anterior cingulate (ACC), insula [BA13], dorsolateral prefrontal cortex (dlPFC) and nucleus accumbens (NAcc). Due to the volume of these activations, we opted for a whole brain, between groups voxelwise analysis. Analyses were conducted using the same voxelwise threshold (p≥.005) and cluster criterion (276 ul) used for the cue analyses described above.
All between-group analyses of mean cluster activation for the cue and outcome periods were initially conducted using univariate analyses of variance. Significant group x condition interactions were decomposed using independent t-tests, given an a priori interest in BOLD differences between the two groups during specific conditions (i.e. “loss” and “win”).
Results
Demographics and drug use
Table 1 shows the group demographic and drug use history for both samples. The groups did not significantly differ on age, years of education, verbal intelligence, BDI scores or indices of alcohol, nicotine and other drug use. Cannabis use data (no. lifetime joints) was found to be significantly skewed in the cannabis sample, and therefore, log transformed (log10) for further analyses.
MID Performance
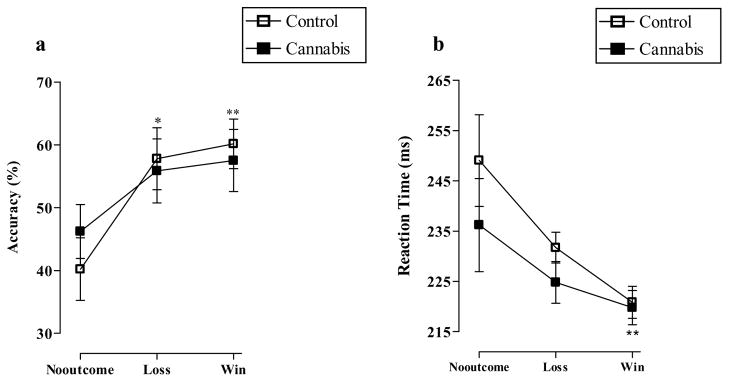
Figure 2a shows the MID accuracy (% “hits”) for the three conditions in the control and cannabis groups. A two (group) by three (condition) univariate analysis of variance showed that there was a significant effect of condition (F2, 78 = 6.5, p<0.01), no effect of group (F1, 78 = 0.02, p=0.9) and no condition x group interaction (F2, 78 = 0.5, p=0.6). Pairwise comparisons confirmed a significant difference in accuracy between the “no-outcome” and “loss” (p<0.05), “no-outcome” and “win” (p<0.01), but not the “loss” and “win” conditions (p=1.0). Figure 2b demonstrates MID reaction time (milliseconds) on “hit” trials on the three conditions for both groups. Here, there was a significant effect of condition (F2, 78 = 5.8, p<0.01), no effect of group (F1, 78 = 3.1, p=0.08) and no condition x group interaction (F2, 78 = 0.2, p=0.8). Pairwise comparisons indicated a significant difference in reaction time between the “no-outcome” and “win” (p<0.01), but not between the “no-outcome” and “loss” (p=0.06) conditions.
Figure 2.
a) Mean percentage accuracy (*p<0.05 “loss” versus “no-outcome”, **p<0.01 “win” versus “no-outcome”) and b) mean reaction time (milliseconds) (**p<0.01 “win” versus “no-outcome”) in the controls and cannabis users on the MID task (means and standard errors). Data were analyzed using two (group) x three (cue) univariate analyses
fMRI
Cue analyses
Figure 3 demonstrates the pattern of activation in both the cannabis and control groups during the “win” cue period of the MID task. Table 2 lists the areas of significant activity during the “win”, “loss” and “no-outcome” cue presentation periods in both the control and cannabis groups. All data across conditions and between groups in each brain region were found to be normally distributed.
Figure 3.
Activation t-test maps (combined) for the cannabis and control groups (p≥0.005) showing coronal sections during “win” cue anticipation across the whole brain.
Table 2.
Brain activations elicited by win, loss and no-outcome cue periods. Positive values for x, y and z Talairach coordinates denote, respectively, locations that are left, posterior and inferior relative to the anterior commissure.
| Structure | BA | HS | Vol (μl) | x | y | z | Post hoc unpaired t-tests t & p values | Direction of significance |
|---|---|---|---|---|---|---|---|---|
| Medial frontal gyrus | 6 | L | 668 | −5 | −13 | 62 | ||
| Medial frontal gyrus | 6 | R | 394 | 10 | −9 | 57 | Ctrl>THC | |
| Fusiform gyus | 37 | L | 468 | −35 | −46 | −10 | 2.7, 0.02 | Ctrl>THC [W] |
| Cingulate gyrus | L | 2220 | −2 | 4 | 43 | |||
| Cuneus | 18 | R | 276 | 2 | −88 | 14 | THC>Ctrl | |
| Ventral striatum | R | 2008 | 20 | 8 | −4 | 2.8, 0.01 | THC>Ctrl [W] | |
| Declive of vermis | 18 | R | 16868 | 1 | −77 | −19 | 2.5, 0.02; 2.1, 0.05 | THC>Ctrl [L] & [W] |
Table abbreviations indicate: L=left; R=right; Ctrl=controls; THC=cannabis; W= “win”; L= “loss” NO= “no-outcome”; FG=frontal gyrus. The t and p values indicate group differences in BOLD activity using post hoc two-sample t-tests upon the observation of a cue x group interaction.
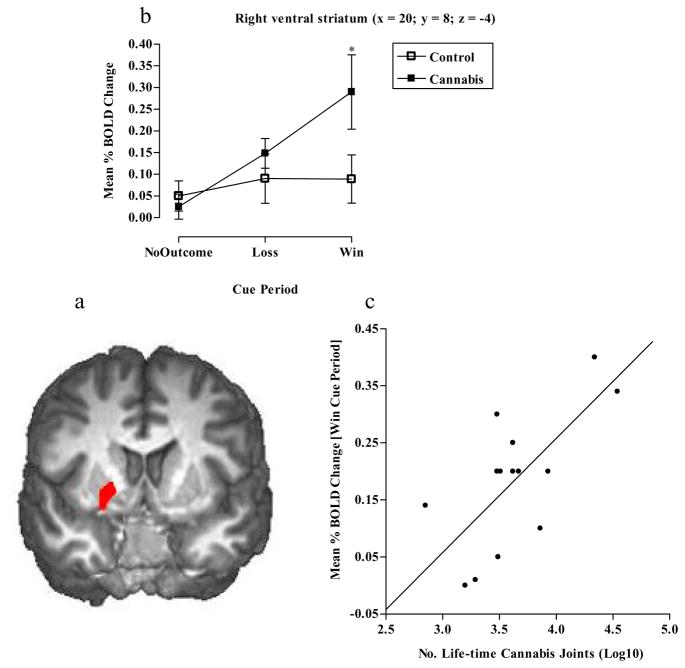
Regions of significant activity for both groups were observed in both left and right hemispheres and included the cerebellum, cingulate gyrus, ventral striatum, medial frontal gyrus, fusiform gyrus and cuneus. Two (group) x three (cue) univariate analyses demonstrated significant BOLD activity differences between the two groups in a number of areas. In the right VS there was no effect of cue (F2, 78 = 2.3, p=0.1), a significant effect of group (F1, 78 = 6.0, p<0.05), in which the cannabis group had significantly more activity than controls, and a significant cue x group interaction (F2, 78 = 4.5, p<0.05). Post hoc tests indicated greater BOLD activity in the cannabis group during the “win” cue (p<0.05) period only (See Figs 4a and b). We also observed a significant positive correlation between BOLD activity in this region during the “win” cue period and the number of reported life-time cannabis joints consumed (r=.6, p<0.05) (See Fig 4c).
Figure 4.
a) Mean brain activity in the right VS (x = 20, y = 8, z = −4), b) graph showing cannabis and control groups significantly differed in right VS BOLD activity during “win” cue periods (*p<0.05 Independent t-tests) and c) correlation between right VS BOLD activity during the “win” cue period and the number of reported lifetime cannabis joints smoked (r=.6, p<0.05).
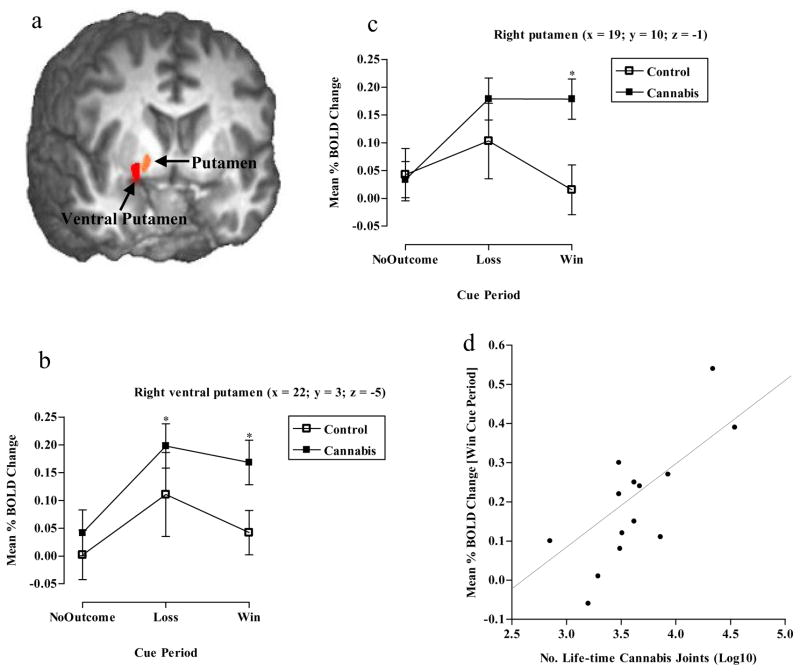
Due to the large size of the observed right VS cluster (2008μl, encompassing the caudate, lentiform nucleus and putamen), we additionally perform a post hoc analysis using a higher voxelwise threshold (t = 4.2, p≥0.001) to determine the exact signal location for the observed group BOLD difference during the “win” cue period. This produced two smaller clusters. The first cluster (114μl) was located in the right ventral putamen (x= 22, y =3, z= −5) and showed a significant effect of cue (F2, 78 = 3.9, p<0.05), a significant effect of group (F1, 78 = 4.6, p<0.05, Cannabis > Control) but no cue x group interaction (F2, 78 = 0.4, p=0.7). Pairwise comparisons for cue indicated that there was significantly greater BOLD activity during the “loss” cue compared to the “no-outcome” period (p<0.05). Within group post hoc testing showed that in the cannabis group there was significantly more BOLD activity during the “loss” (p<0.05) and “win” (p<0.05) cue periods compared to the “no-outcome” cue period (See Fig 5a & b). The second cluster (66μl), located in the right putamen (x= 19, y =10, z= −1), showed no effect of cue (F2, 78 = 0.4, p=0.7), no effect of group (F1,78 = 1.2, p=0.3), but a significant cue x group interaction (F2, 78 = 3.6, p<0.05). Between group post hoc tests showed that the cannabis group had significantly greater BOLD activity than the control group (p<0.05) during the “win” cue period (See Fig 5a & c). We additionally observed a significant positive correlation between BOLD activity in this putamen cluster during the “win” cue period and the number of reported life-time cannabis joints consumed (r=.7, p<0.01) (See Fig 5d).
Figure 5.
Post hoc analysis showing a) Mean brain activity in the right ventral putamen (x= 22, y =3, z= −5), b) graph showing cannabis users had a greater BOLD response to “loss” and “win” cues compared with “no-outcome” cues (*p<0.05 Paired t-tests) in the right ventral putamen, c) graph showing cannabis users had a greater BOLD response in the right putamen (x= 19, y =10, z= −1) compared to controls during “win” cue presentation (*p<0.05 Independent t-tests) and d) correlation between right putamen BOLD activity during the “win” cue period and the number of reported life-time cannabis joints smoked (r=.7, p<0.01).
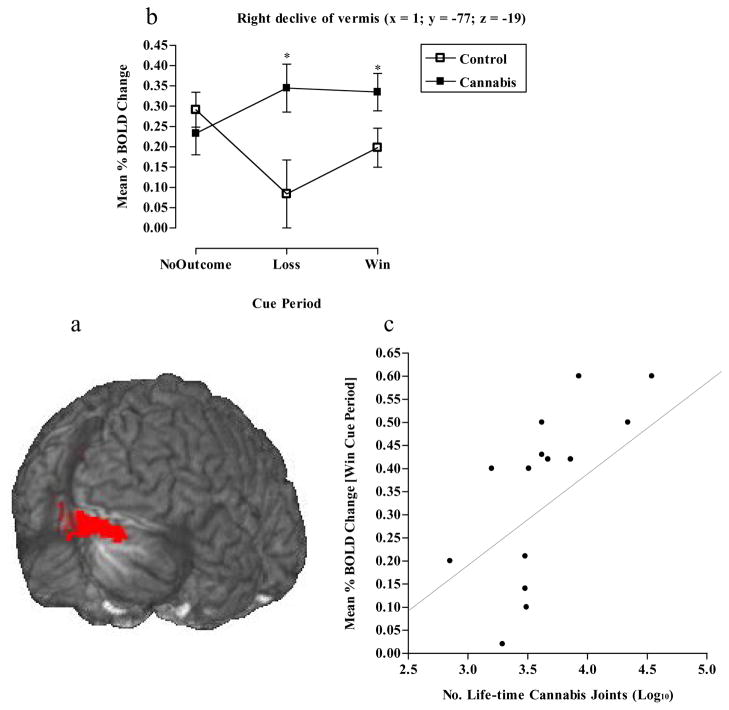
The right cerebellar (declive of vermis) BOLD response showed no effect of cue (F2, 78 = 0.5, p=0.6), a significant effect of group (F1, 78 = 5.7, p<0.05), wherein the cannabis group had significantly more BOLD activity than controls, and a significant cue x group interaction (F2, 78 = 3.9, p<0.05). The interaction was driven by significantly greater BOLD activity during the “loss” (p<0.05) and “win” (p<0.05) cue periods in the cannabis group relative to the controls (See Figs 6a & b). We additionally observed a significant positive correlation between BOLD activity in this region during the “win” cue period and the number of reported life-time cannabis joints consumed (r=.8, p<0.001) (See Fig 6c).
Figure 6.
a) Mean brain activity in the right declive of vermis (x = 1, y = −77, z = −19), b) graph showing cannabis and control groups significantly differed in right declive of vermis BOLD activity during “loss” and “win” cue presentation (*p<0.05 Independent t-tests) and c) correlation between right declive of vermis BOLD activity during the “win” cue period and the number of reported life-time cannabis joints smoked (r=.7, p<0.01).
BOLD activity in the right medial frontal gyrus [BA6] showed no effect of cue (F2, 78 = 0.4, p=0.7), a significant effect of group (F1, 78 = 6.2, p<0.05, Control > Cannabis) but no significant cue x group interaction (F2, 78 = 0.8, p=0.5). In the left fusiform gyrus [BA37], there was a significant effect of cue (F2, 78 = 4.4, p<0.05), a significant effect of group (F1, 78 = 6.8, p<0.05, Control > Cannabis) and a significant cue x group interaction (F2, 78 = 3.9, p<0.05). Pairwise comparisons for cue showed that there was greater BOLD activity during “win” compared to the “loss” (p<0.05) and “no-outcome” (p<0.05) periods. Further post hoc tests of the interaction revealed that it was only the control group that had significantly more BOLD activity during the “win” cue compared to the “loss” (p<0.05) and “no-outcome” (p<0.05) cue periods. Moreover, the control group had significantly more activity than cannabis users (p<0.05) during the “win” cue period. Finally, for the right cuneus [BA18], there was no effect of cue (F2, 78 = 1.0, p=0.4), a significant effect of group (F1, 78 = 4.4, p<0.05, Cannabis > Control) but no cue x group interaction (F2, 78 = 2.1, p=0.1). For clusters in the left MFG and cingulate gyrus, we failed to observe any significant findings involving condition, group or any interaction between these two factors.
MID outcome analyses
Table 3 documents the results of a whole brain, between groups voxel-wise analysis during the 6 outcome delivery periods. There were a number of group differences during these periods. Particularly interesting was the reduced left insula (x = −34, y = 8, z = 7) BOLD activity during the loss outcome delivery periods and similarly in this region (x = −38, y = −1, z = −4) during the loss avoidance (i.e. save 50 cent) notification periods in the cannabis group. Cannabis users demonstrated greater BOLD activity than controls during the neutral lose and neutral win outcome notification periods in a number of regions, including the caudate nucleus, cingulate, inferior frontal and parahippocampal gyri.
Table 3.
Voxel-wise analyses (p≤.005; 276ul cluster criterion) showing whole brain brain activations elicited by the MID delivery outcome periods in the control and cannabis groups. The two groups significantly differed in a number of regions during the different delivery outcome periods.
| Structure | BA | HS | Vol (μl) | x | y | z | Direction of significance |
|---|---|---|---|---|---|---|---|
| Neutral lose outcome | |||||||
| Cingulate gyrus | 32 | L | 771 | −1 | 24 | 27 | THC>Ctrl |
| Superior temporal gyrus | 22 | R | 503 | 48 | −45 | 13 | THC>Ctrl |
| Precentral gyrus | 6 | L | 468 | −21 | −18 | 63 | THC>Ctrl |
| Cuneus | 19 | L | 436 | −20 | −84 | 35 | THC>Ctrl |
| Superior frontal gyrus | 8 | R | 419 | 17 | 47 | 44 | THC>Ctrl |
| Inferior frontal gyrus | 45 | R | 418 | 52 | 24 | 7 | THC>Ctrl |
| Culmen | L | 392 | −37 | −48 | −20 | THC>Ctrl | |
| Middle frontal gyrus | 8 | R | 355 | 42 | 27 | 47 | THC>Ctrl |
| Caudate nucleus | R | 333 | 9 | 8 | 10 | THC>Ctrl | |
| Postcentral gyrus | 3 | L | 317 | −49 | −18 | 47 | THC>Ctrl |
| Middle occipital gyrus | 31 | L | 278 | −28 | −75 | 14 | THC>Ctrl |
| Brainstem | L | 333 | 0 | −19 | −30 | THC>Ctrl | |
| Neutral win outcome | |||||||
| Parahippocampal gyrus | 27 | L | 1072 | −15 | −39 | 2 | THC>Ctrl |
| Declive of vermis | R | 546 | 17 | −82 | −17 | THC>Ctrl | |
| Uncus | 24 | R | 321 | 14 | −6 | −21 | THC>Ctrl |
| Parahippocampal gyrus | 37 | R | 285 | 29 | −46 | −8 | THC>Ctrl |
| Lose 50 cent outcome | |||||||
| Superior parietal lobule | 7 | L | 672 | −38 | −55 | 54 | THC>Ctrl |
| Insula | 13 | L | 368 | −34 | 8 | 7 | Ctrl>THC |
| Precentral gyrus | 6 | R | 323 | 35 | −5 | 34 | Ctrl>THC |
| Save 50 cent outcome | |||||||
| Insula | 13 | L | 428 | −38 | −1 | −4 | Ctrl>THC |
| Miss 50 cent outcome | |||||||
| Superior frontal gyrus | 8 | L | 581 | −19 | 47 | 37 | Ctrl>THC |
| Win 50 cent outcome | |||||||
| Paracentral lobule | 31 | L | 278 | 0 | −24 | 45 | Ctrl>THC |
Nomenclature: Neutral lose and Neutral win following “no-outcome” trials; Lose 50 cent and Save 50 cent following “loss” trials; Miss 50 cent and Win 50 cent delivery following “win” trials. Positive values for x, y and z Talairach coordinates denote, respectively, locations that are left, posterior and inferior relative to the anterior commissure.
Drug-use correlations
Table 4 indicates that there were a number of significant positive correlations between cannabis use demographics and BOLD activity during the “win” cue period of the MID task. There were no correlations between alcohol use or other reported drug use measures and BOLD activity during the “win” cue period of the MID task. We did observe a significant negative correlation between reported cannabis withdrawal and fusiform gyrus BOLD during the “win” cue period (See Table 4 below). We additionally observed a significant negative correlation between superior parietal lobule BOLD activity during loss outcome notifications and life-time cannabis use (r= −.6, p<0.05) and the number of reported life-time cannabis joints consumed (r= −.6, p=0.001). In contrast, there were no significant correlations between self-reported use of cannabis and BOLD activity during either the “loss” and “no-outcome” cue periods. Nor were there any correlations between cue BOLD activity and MID behavioural performance (i.e. accuracy and reaction time) in either the cannabis or control groups. There were no correlations between cannabis craving and withdrawal measures and MID behavioural performance. Finally, there were no other correlations between cannabis withdrawal and cue BOLD activity or between craving and cue or outcome BOLD activity.
Table 4.
Correlations between BOLD activity during the “win” cue period in the cannabis group, and the cannabis use and withdrawal measures.
| Structure | Years of Use | Life-time Joints | Cannabis withdrawal |
|---|---|---|---|
| Left MFG/BA6 | r=.4, p=0.2 | r=.3, p=0.3 | r= −.2, p=0.6 |
| Right MFG/BA6 | r=.5, p<0.05 | r=.7, p<0.01 | r= −.5, p=0.07 |
| Fusiform gyus/BA37 | r=.4, p=0.2 | r=.3, p=0.3 | r=.−7, p<0.01 |
| Left Cingulate gyrus | r= −.4, p=0.2 | r=.7, p<0.01 | r= −.1, p=0.8 |
| Right cuneus/BA18 | r=.6, p<0.05 | r=.7, p<0.01 | r= −.2, p=0.4 |
| Right ventral striatum | r=.6, p<0.05 | r=.6, p<0.05 | r= −.04, p=0.9 |
| Right ventral putamen | r=.3, p=0.3 | r=.4, p=0.1 | r=.02, p=0.9 |
| Right putamen | r=.5, p<0.05 | r=.7, p<0.01 | r= −.1, p=0.7 |
| Right declive of vermis | r=.7, p<0.01 | r=.8, p<0.001 | r= −.4, p=0.1 |
MFG=medial frontal gyrus.
Discussion
The current investigation examined neural activity in chronic cannabis users during the processing of cues predicting non-drug rewards and non-drug losses. The current study, using a cohort of chronic cannabis users, who were demographically well matched to a control group, demonstrated an increased BOLD response in the right ventral striatum (VS) for cues predicting non-drug rewards. The different BOLD responses observed during reward cues occurred in the absence of any behavioural group effect on the MID, enabling us to discount performance-related neural effects from confounding these group comparisons. The observed elevated VS response to cues predictive of non-drug rewards is in contrast to that previously observed in alcoholism and dual alcohol and cocaine dependence, suggesting that the relationship between chronic cannabis use and VS activity may be qualitatively different from that of other drugs during abstinence (Bjork et al, 2008; Wrase et al, 2007).
Animal research suggests that cues for primary reinforcers can activate DA neurons in the ventral tegmental area (VTA) and elicit DA release in the VS (Schultz et al, 1997). Human imaging studies have also demonstrated that cues for non-drug incentives reliably activate the VS BOLD response for goal-objects (Knutson et al, 2001; O’Doherty, 2004), and that reward anticipation increases DA release in this region (Schott et al, 2008). DA D2 receptor down-regulation and reductions in pre-synaptic DA release in some drug-using groups (Heinz et al, 2004; Martinez et al, 2005; Volkow et al, 1997), it is argued, may increase the threshold required for non-drug reinforcers to activate the VS (Martin-Soelch et al, 2001), thereby inducing a reward deficiency syndrome (Blum et al, 2000). The availability of striatal DA D2 receptors, however, has not been shown to significantly differ between cannabis users and drug-naïve controls (Sevy et al, 2008), potentially ruling out a dopaminergic reward deficiency hypothesis. Given that the VTA contains a moderately high density of cannabinoid (CB1) receptors (Herkenham et al, 1991; Tsou et al, 1998), and evidence that cannabinoids increase midbrain DA neuron activity in animals (French et al, 1997; Chen et al, 1990) and humans (Bossong et al, 2009), chronic cannabis use may alter reward processing through sensitizing mesolimbic circuits (under the assumption that the observed effects resulted from, rather than preceded, the cannabis use).
Past research has shown that estimates of life-time cannabis use and life-time “dose” (i.e. cannabis joints) are reliable predictors of behavioural performance and BOLD activity in cannabis users (Solowij et al, 2002; Bolla et al, 2002; Block et al, 1993; Bolla et al, 2005; Pope et al, 2003; Pope and Yurgelun-Todd, 1996; Chang et al, 2006). Correlation analyses revealed that a participant’s reported years of cannabis use and the number of life-time cannabis joints smoked independently predicted their VS BOLD response during cues for non-drug rewards. Importantly, this VS correlation was observed in the absence of any correlations between craving indices or other drug use measures and BOLD activity in this area, potentially ruling out these factors as contributors to the VS response.
We do not believe that the present results were influenced by cannabis abstinence at the time of testing the cannabis-using group, as we found no significant associations between hours of abstinence and task performance or BOLD responses. Cannabis users did demonstrate a reduced BOLD response in the left fusiform gyrus [BA 37], which was significantly negatively correlated with reported cannabis withdrawal. The data, however, do not suggest that the VS findings reflect cannabis withdrawal, as there were no associations between withdrawal scores and BOLD activity in the VS. Furthermore, animal research suggests that there is a decline in mesolimbic DA activity during cannabis withdrawal (Diana et al, 1998), which might predict reductions in the VS BOLD response, rather than increases, as observed herein.
The incentive-sensitization theory of addiction proposes that sensitized mesolimbic neural circuits function to attribute incentive salience to reward-related stimuli, allowing reward cues to trigger excessive “wanting” for the reward (Robinson and Berridge, 1998). In drug addiction, however, the focus of sensitized “wanting” is believed to be primarily towards drug cues and drug rewards, rather than natural rewards (Robinson and Berridge, 2001). Despite this assertion, sensitization has been shown to enhance the pursuit of natural rewards in animals, where pre-treatment with amphetamine, cocaine and morphine has been observed to significantly increase cue-elicited approach behaviour for food, water and sexual contact (Mitchell and Stewart, 1990; Fiorino and Phillips, 1999; Harmer and Phillips, 1999; Taylor and Horger, 1999; Wyvell and Berridge, 2001). This may suggest that chronic pre-exposure to cannabis in humans might sensitize mesolimbic neural circuits, an effect which is manifested by cue-triggered VS responses during the pursuit of non-drug rewards. Therefore, if these results are indeed indicative of sensitization within the VS for non-drug rewards, they may also have significant implications for the future use and misuse of other drugs, as well as other forms of behaviour.
Subjective reports concerning the effects of cannabis suggest that its reinforcing properties are related to its effects on the brain’s “reward circuitry” (Huestis et al, 2007; D’Souza et al, 2008; Hunault et al, 2008; Bossong et al, 2009). There is evidence that Δ9THC exposure in animals affects the developmental plasticity of the reward system (Singh et al, 2006), and that the consumption of cannabis can predict a significantly higher risk for the subsequent use of other more dangerous illicit substances in humans (Fergusson and Horwood, 2000; Lessem et al, 2006). Cannabis users have also been shown to demonstrate more sexual risk (Bon et al, 2001; Castilla et al, 1999; Wingood and DiClemente, 1998; Poulin and Graham, 2001) and pathological gambling behaviour (Kausch, 2003; de Carvalho et al, 2005; Petry and Tawfik, 2001; Toneatto and Brennan, 2002), perhaps indicative of an inability to balance the immediate pursuit of rewards against the long-term negative consequences of actions. Importantly, laboratory-based evidence suggests that cannabis users have a greater level of impulsivity and an increased sensitivity for small, but immediate, rewards (Whitlow et al, 2004; Simons and Arens, 2007), consistent with the notion of mesolimbic reward hyperactivity, together with reductions in frontocortical punishment-avoidance circuitry (Solomon and Corbit, 1973, 1974; Bechara, 2005; Bickel et al, 2007). Therefore, one hypothesis, arising from the current findings, is that chronic cannabis use in humans may induce a VS hypersensitivity to other rewards (e.g., money, sex), thus increasing the likelihood of future reward seeking, risk-taking behaviour, and potentially, the pursuit of more deleterious and illicit drugs of abuse. Despite this evidence, there is the possibility that these VS differences in cannabis users may have preceded cannabis use. Cannabis use may well have arisen from pre-existing differences in VS functioning. Therefore, we cannot unequivocally state that the VS differences observed herein are a direct consequence of chronic cannabis consumption and its effects on DA mesolimbic reward circuitry.
The current study also demonstrated that cannabis users had a significantly greater BOLD response in the right declive of vermis during both “loss” and “win” cue periods. There is evidence that the cerebellum plays a role in cognitive processes required for executing goal-directed behaviours and conditioned response learning (Paradiso et al, 1999; Logan and Grafton, 1995). Moreover, there is evidence for cerebellar vermis connections to DA cell body regions in the VTA, with the VTA shown to project to the cerebellum (Snider et al, 1976; Ikai et al, 1992). Vermis activity has been shown to occur during the provision of non-drug rewards or their anticipation (Rogers et al, 1999; Kunig et al, 2000; Martin-Soelch et al, 2001; Knutson et al, 2001), which may explain activation patterns observed here. Furthermore, the vermis has been shown to respond to drug-related stimuli in cocaine (Volkow et al, 2003) and alcohol (Schneider et al, 2001) dependence, with increased cerebellar activity observed during cognitive tasks in alcoholics (Desmond et al, 2003) and cocaine addicts (Hester and Garavan, 2004). The present study also demonstrated that in the cannabis-using group, there were significant relationships between BOLD activity in the declive of vermis (during the “win” cue period) and cannabis use history (years of use and life-time joints). This group difference in vermis BOLD activity, together with the observed association with cannabis use, may suggest that chronic cannabis use exaggerates cerebellar goal-directed activity in response to cues predictive of non-drug reinforcers.
We additionally observed group differences with respect to outcome-related BOLD activity, most notably detecting left insula cortex hypoactivity in the cannabis group in response to loss notification deliveries. Error-related insula activity has previously been demonstrated in healthy control subjects (Hester, Foxe, Molholm, Shpaner, & Garavan, 2005; Klein, et al., 2007), and is believed to play a crucial role in integrating bodily states and affective value for reward-related adaptive behaviour (Critchley et al, 2001; Craig, 2002; Bechara and Damasio, 2005). Anatomically, the insula is well positioned to integrate a linking of affective value with adaptations in behaviour, possibly through its bidirectional connections with regions implicated in reward and decision making, such as the orbitofrontal cortex, amygdala, anterior cingulate and VS (Reynolds and Zahm, 2005). Research also suggests that the insula and interoceptive awareness are critical to drug craving and addiction (Gray & Critchley, 2007; Naqvi, Rudrauf, Damasio, & Bechara, 2007; Paulus, 2007), whereby the insula monitors interoceptive “urges” for rewarding stimuli such as a drug of abuse. The relative insensitivity of our cannabis group in their error-related insula activity following loss outcome notifications may also be consistent with recent findings in cannabis users demonstrating an absence of insula and anterior cingulate activity during an error awareness task (Hester, Nestor & Garavan, in press), suggesting a potential deficit in error-related monitoring in this population. We also observed insula hypofunctioning in cannabis users following loss avoidance outcome trials. While reward learning is typically associated with the mesolimbic DA system (McClure et al, 2003; O’Doherty et al, 2003), which we surmise may be sensitized by the effects of cannabis for the anticipation of rewards, this result may reflect potential differences in other neurotransmitter systems of cannabis users (e.g., serotonin, noradrenaline and acetylcholine), which are known to modulate insula activation during learning (Doya, 2000; Yu and Dayan, 2003; Berman et al, 2000).
The findings of the current study suggest that in chronic cannabis users, there is an increased VS BOLD response to stimuli which predict potential non-drug rewards, together with a deficit in insula activity following loss and loss avoidance outcomes. Furthermore, the observed VS hyperactivity during reward anticipation was associated with the duration (in years) of cannabis use and the estimated number of life-time cannabis joints consumed. Notwithstanding the possibility that these VS differences may have preceded cannabis use and/or be due to a small control group effect, these findings may suggest a “dose-response” sensitization effect on DA incentive processing within mesolimbic circuitry. One outstanding question with respect to the current findings concerns whether these effects would generalise to cannabis users who show high levels of unmotivated and avolitional behaviour, which may be more characteristic of cannabis-dependence. Furthermore, future studies will be needed to examine factors which determine whether it is drug or non-drug rewards that become excessively “wanted” in chronic cannabis users, and indeed other drug-using populations.
Acknowledgments
This research was supported by USPHS grant from the National Institute on Drug Abuse: DA01865-01. The assistance of Ella McCabe, BSc, M.Sc is gratefully acknowledged.
Footnotes
Financial Disclosures: Drs Nestor, Hester, and Garavan reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bechara A, Damasio H, Tranel D, Damasio AR. The Iowa Gambling Task and the somatic marker hypothesis: some questions and answers. Trends Cogn Sci. 2005;9(4):159–162. doi: 10.1016/j.tics.2005.02.002. discussion 162–154. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8(11):1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39(4):376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G. Beck Depression Inventory—Second Edition manual. The Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Berman DE, Hazvi S, Neduva V, Dudai Y. The role of identified neurotransmitter systems in the response of insular cortex to unfamiliar taste: activation of ERK1–2 and formation of a memory trace. J Neurosci. 2000;20(18):7017–7023. doi: 10.1523/JNEUROSCI.20-18-07017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bickel W, Marsch L. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96(1):73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend. 2007;90(Suppl 1):S85–91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34(2–3):133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Hommer DW. Striatal sensitivity to reward deliveries and omissions in substance dependent patients. Neuroimage. 2008 doi: 10.1016/j.neuroimage.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block R, Ghoneim M. Effects of chronic marijuana use on human cognition. Psychopharmacology (Berl) 1993;110(1–2):219–228. doi: 10.1007/BF02246977. [DOI] [PubMed] [Google Scholar]
- Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, et al. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs. 2000;32(Suppl):i–iv. 1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- Bolla K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 2005;26(2):480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Bon SR, Hittner JB, Lawandales JP. Normative perceptions in relation to substance use and HIV-risky sexual behaviors of college students. J Psychol. 2001;135(2):165–178. doi: 10.1080/00223980109603688. [DOI] [PubMed] [Google Scholar]
- Bossong MG, van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, et al. Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology. 2009;34(3):759–766. doi: 10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- Brower KJ, Maddahian E, Blow FC, Beresford TP. A comparison of self-reported symptoms and DSM-III-R criteria for cocaine withdrawal. Am J Drug Alcohol Abuse. 1988;14(3):347–356. doi: 10.3109/00952998809001556. [DOI] [PubMed] [Google Scholar]
- Castilla J, Barrio G, Belza MJ, de la Fuente L. Drug and alcohol consumption and sexual risk behaviour among young adults: results from a national survey. Drug Alcohol Depend. 1999;56(1):47–53. doi: 10.1016/s0376-8716(99)00008-3. [DOI] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129(Pt 5):1096–1112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29(2):537–545. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, et al. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008;33(10):2505–2516. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho SV, Collakis ST, de Oliveira MP, da Silveira DX. Frequency of pathological gambling among substance abusers under treatment. Rev Saude Publica. 2005;39(2):217–222. doi: 10.1590/s0034-89102005000200012. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage. 2003;19(4):1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ. Does cannabis use encourage other forms of illicit drug use? Addiction. 2000;95(4):505–520. doi: 10.1046/j.1360-0443.2000.9545053.x. [DOI] [PubMed] [Google Scholar]
- Fiorino DF, Phillips AG. Facilitation of sexual behavior and enhanced dopamine efflux in the nucleus accumbens of male rats after D-amphetamine-induced behavioral sensitization. J Neurosci. 1999;19(1):456–463. doi: 10.1523/JNEUROSCI.19-01-00456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French ED. delta9-Tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neurosci Lett. 1997;226(3):159–162. doi: 10.1016/s0304-3940(97)00278-4. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. J Int Neuropsychol Soc. 2003;9(5):679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- Gray MA, Critchley HD. Interoceptive basis to craving. Neuron. 2007;54(2):183–186. doi: 10.1016/j.neuron.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175(3):296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD. Enhanced dopamine efflux in the amygdala by a predictive, but not a non-predictive, stimulus: facilitation by prior repeated D-amphetamine. Neuroscience. 1999;90(1):119–130. doi: 10.1016/s0306-4522(98)00464-3. [DOI] [PubMed] [Google Scholar]
- Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addict Behav. 2006;31(7):1290–1294. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, et al. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161(10):1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Heishman S, Singleton E, Liguori A. Marijuana Craving Questionnaire: development and initial validation of a self-report instrument. Addiction. 2001;96(7):1023–1034. doi: 10.1046/j.1360-0443.2001.967102312.x. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11(2):563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24(49):11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Foxe JJ, Molholm S, Shpaner M, Garavan H. Neural mechanisms involved in error processing: a comparison of errors made with and without awareness. Neuroimage. 2005;27(3):602–608. doi: 10.1016/j.neuroimage.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Hester R, Nestor L, Garavan H. Impaired error awareness and anterior cingulate cortex hypoactivity in chronic cannabis users. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.67. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Boyd SJ, Heishman SJ, Preston KL, Bonnet D, Le Fur G, et al. Single and multiple doses of rimonabant antagonize acute effects of smoked cannabis in male cannabis users. Psychopharmacology (Berl) 2007;194(4):505–515. doi: 10.1007/s00213-007-0861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunault CC, Mensinga TT, de Vries I, Kelholt-Dijkman HH, Hoek J, Kruidenier M, et al. Delta-9-tetrahydrocannabinol (THC) serum concentrations and pharmacological effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg THC. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1260-2. [DOI] [PubMed] [Google Scholar]
- Ikai Y, Takada M, Shinonaga Y, Mizuno N. Dopaminergic and non-dopaminergic neurons in the ventral tegmental area of the rat project, respectively, to the cerebellar cortex and deep cerebellar nuclei. Neuroscience. 1992;51(3):719–728. doi: 10.1016/0306-4522(92)90310-x. [DOI] [PubMed] [Google Scholar]
- Kausch O. Patterns of substance abuse among treatment-seeking pathological gamblers. J Subst Abuse Treat. 2003;25(4):263–270. doi: 10.1016/s0740-5472(03)00117-x. [DOI] [PubMed] [Google Scholar]
- Klein TA, Endrass T, Kathmann N, Neumann J, von Cramon DY, Ullsperger M. Neural correlates of error awareness. Neuroimage. 2007;34(4):1774–1781. doi: 10.1016/j.neuroimage.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18(2):263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, et al. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27(8):739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Kunig G, Leenders KL, Martin-Solch C, Missimer J, Magyar S, Schultz W. Reduced reward processing in the brains of Parkinsonian patients. Neuroreport. 2000;11(17):3681–3687. doi: 10.1097/00001756-200011270-00019. [DOI] [PubMed] [Google Scholar]
- Lessem JM, Hopfer CJ, Haberstick BC, Timberlake D, Ehringer MA, Smolen A, et al. Relationship between adolescent marijuana use and young adult illicit drug use. Behav Genet. 2006;36(4):498–506. doi: 10.1007/s10519-006-9064-9. [DOI] [PubMed] [Google Scholar]
- Logan CG, Grafton ST. Functional anatomy of human eyeblink conditioning determined with regional cerebral glucose metabolism and positron-emission tomography. Proc Natl Acad Sci U S A. 1995;92(16):7500–7504. doi: 10.1073/pnas.92.16.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Bonson KR, Ernst M, Grant S. Brain imaging studies of cocaine abuse: implications for medication development. Crit Rev Neurobiol. 1999;13(3):227–242. doi: 10.1615/critrevneurobiol.v13.i3.10. [DOI] [PubMed] [Google Scholar]
- Martin-Solch C, Leenders KL, Chevalley AF, Missimer J, Kunig G, Magyar S, et al. Reward mechanisms in the brain and their role in dependence: evidence from neurophysiological and neuroimaging studies. Brain Res Brain Res Rev. 2001;36(2–3):139–149. doi: 10.1016/s0165-0173(01)00089-3. [DOI] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58(10):779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- McClure SM, Berns GS, Montague PR. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38(2):339–346. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Stewart J. Facilitation of sexual behaviors in the male rat associated with intra-VTA injections of opiates. Pharmacol Biochem Behav. 1990;35(3):643–650. doi: 10.1016/0091-3057(90)90302-x. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson H, O’Connell A. Dementia: The estimation of pre-morbid intelligence levels using the new adult reading test. Cortex. 1978;14(2):234–244. doi: 10.1016/s0010-9452(78)80049-5. [DOI] [PubMed] [Google Scholar]
- Nestor L, Roberts G, Garavan H, Hester R. Deficits in learning and memory: Parahippocampal hyperactivity and frontocortical hypoactivity in cannabis users. Neuroimage. 2008;40(3):1328–1339. doi: 10.1016/j.neuroimage.2007.12.059. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron. 2003;38(2):329–337. doi: 10.1016/s0896-6273(03)00169-7. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Johnson DL, Andreasen NC, O’Leary DS, Watkins GL, Ponto LL, et al. Cerebral blood flow changes associated with attribution of emotional valence to pleasant, unpleasant, and neutral visual stimuli in a PET study of normal subjects. Am J Psychiatry. 1999;156(10):1618–1629. doi: 10.1176/ajp.156.10.1618. [DOI] [PubMed] [Google Scholar]
- Paulus MP. Decision-making dysfunctions in psychiatry--altered homeostatic processing? Science. 2007;318(5850):602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- Petry NM, Tawfik Z. Comparison of problem-gambling and non-problem-gambling youths seeking treatment for marijuana abuse. J Am Acad Child Adolesc Psychiatry. 2001;40(11):1324–1331. doi: 10.1097/00004583-200111000-00013. [DOI] [PubMed] [Google Scholar]
- Pope H, Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. Journal of the American Medical Association. 1996;275:521–527. [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69(3):303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Poulin C, Graham L. The association between substance use, unplanned sexual intercourse and other sexual behaviours among adolescent students. Addiction. 2001;96(4):607–621. doi: 10.1046/j.1360-0443.2001.9646079.x. [DOI] [PubMed] [Google Scholar]
- Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn Reson Med. 1999;42(5):952–962. [PubMed] [Google Scholar]
- Reynolds SM, Zahm DS. Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci. 2005;25(50):11757–11767. doi: 10.1523/JNEUROSCI.3432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T, Berridge K. Incentive-sensitization and addiction. Addiction. 2001;96(1):103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, et al. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. J Neurosci. 1999;19(20):9029–9038. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Ramnani N, Mackay C, Wilson JL, Jezzard P, Carter CS, et al. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry. 2004;55(6):594–602. doi: 10.1016/j.biopsych.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, et al. Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychiatry. 2001;158(7):1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sevy S, Smith GS, Ma Y, Dhawan V, Chaly T, Kingsley PB, et al. Cerebral glucose metabolism and D(2)/D (3) receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacology (Berl) 2008;197(4):549–556. doi: 10.1007/s00213-008-1075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Arens AM. Moderating effects of sensitivity to punishment and sensitivity to reward on associations between marijuana effect expectancies and use. Psychol Addict Behav. 2007;21(3):409–414. doi: 10.1037/0893-164X.21.3.409. [DOI] [PubMed] [Google Scholar]
- Singh ME, McGregor IS, Mallet PE. Perinatal exposure to delta(9)-tetrahydrocannabinol alters heroin-induced place conditioning and fos-immunoreactivity. Neuropsychopharmacology. 2006;31(1):58–69. doi: 10.1038/sj.npp.1300770. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26(1):25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Snider RS, Maiti A, Snider SR. Cerebellar pathways to ventral midbrain and nigra. Exp Neurol. 1976;53(3):714–728. doi: 10.1016/0014-4886(76)90150-3. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. II. Cigarette addiction. J Abnorm Psychol. 1973;81(2):158–171. doi: 10.1037/h0034534. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens R, Roffman RA, Babor T. Does marijuana use cause long-term cognitive deficits? Jama. 2002;287(20):2653–2654. author reply 2654. [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System - an Approach to Cerebral Imaging. Thieme Medical Publishers; New York, NY: 1988. [Google Scholar]
- Taylor JR, Horger BA. Enhanced responding for conditioned reward produced by intra-accumbens amphetamine is potentiated after cocaine sensitization. Psychopharmacology (Berl) 1999;142(1):31–40. doi: 10.1007/s002130050859. [DOI] [PubMed] [Google Scholar]
- Toneatto T, Brennan J. Pathological gambling in treatment-seeking substance abusers. Addict Behav. 2002;27(3):465–469. doi: 10.1016/s0306-4603(00)00173-8. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83(2):393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Volkow N, Fowler J, Wang G, Goldstein R. Role of dopamine, the frontal cortex and memory circuits in drug addiction: insight from imaging studies. Neurobiol Learn Mem. 2002;78(3):610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386(6627):830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Zhu W, Maynard L, et al. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. J Neurosci. 2003;23(36):11461–11468. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow C, Liguori A, Livengood LB, Hart SL, Mussat-Whitlow BJ, Lamborn CM, Laurienti PJ, Porrino LJ. Long-term heavy marijuana users choose high immediate gains despite higher future losses on a decision-making task. Drug & Alcohol Dependence. 2004;76(1):107–111. doi: 10.1016/j.drugalcdep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Wingood GM, DiClemente RJ. The influence of psychosocial factors, alcohol, drug use on African-American women’s high-risk sexual behavior. Am J Prev Med. 1998;15(1):54–59. doi: 10.1016/s0749-3797(98)00027-0. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, et al. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35(2):787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. J Neurosci. 2001;21(19):7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A, Dayan P. Advances in neural information processing systems. Vol. 15. Cambridge, MA: MIT; 2003. Expected and unexpected uncertainty: ACh and NE in the neocortex; pp. 173–180. [Google Scholar]