Abstract
Avian hepatitis E virus (avian HEV) is the primary causative agent of Hepatitis-Splenomegaly (HS) syndrome in chickens. Recently, a genetically unique strain of avian HEV, designated avian HEV-VA, was recovered from healthy chickens in Virginia. The objective of this study was to experimentally compare the pathogenicity of the prototype strain recovered from a chicken with HS syndrome and the avian HEV-VA strain in specific-pathogen-free chickens. An infectious stock of the avian HEV-VA strain was first generated and its infectivity titer determined in chickens. For the comparative pathogenesis study, fifty-four chickens of 6-week-old were assigned to 3 groups of 18 chickens each. The group 1 chickens were each intravenously inoculated with 5×102.5 50% chicken infectious dose of the prototype strain. The group 2 received the same dose of the avian HEV-VA strain, and the group 3 served as negative controls. Six chickens from each group were necropsied at 2, 3 and 4 weeks post-inoculation (wpi). Most chickens in both inoculated groups seroconverted by 3 wpi, and the mean anti-avian HEV antibody titers were higher for the prototype strain group than the avian HEV-VA strain group. There was no significant difference in the patterns of viremia and fecal virus shedding. Blood analyte profiles did not differ between treatment groups except for serum creatine phosphokinase levels which were higher for prototype avian HEV group than avian HEV-VA group. The hepatic lesion score was higher for the prototype strain group than the other two groups. The results indicateded that the avian HEV-VA strain is only slightly attenuated compared to the prototype strain, suggesting that the full-spectrum of HS syndrome is likely associated with other co-factors.
1. Introduction
Hepatitis E is an acute enterically-transmitted hepatic disease in humans (Aggarwal & Krawczynski, 200; Emerson & Purcell, 2003; Harrison, 1999; Jameel, 1999; Purcell & Emerson, 2008). Hepatitis E virus (HEV), the causative agent of hepatitis E, is a positive-sense, non-enveloped RNA virus. The genome of HEV is approximately 7.2 kb in size and contains three open reading frames (ORFs) (Emerson et al., 2004; Schlauder & Mushahwar, 2001; Worm et al., 2002). Hepatitis E is epidemic and endemic in many developing countries of the world due to poor sanitation conditions (Emerson & Purcell, 2003; Purcell & Emerson, 2008). Sporadic cases of acute hepatitis E have also been reported in industrialized countries including the United States (Meng, 2000; Nishizawa et al., 2003; Takahashi et al., 2003a, 2003b; Van der Poel et al., 2001; Wang et al., 2001, 2002; Yazaki et al., 2003). The primary mode of HEV transmission is via the fecal-oral route (Arankalle et al., 1994, 2006; Emerson and Purcell, 2003), although blood-borne (Khuroo et al., 2004; Matsubayashi et al., 2004) and food-borne (Matsuda et al., 2003; Tei et al., 2003; Yazaki et al., 2003) transmissions have also been documented.
The first animal strain of HEV, swine hepatitis E virus (swine HEV), was identified and characterized from commercial swine in the United States (Meng et al., 1997). Since then, many strains of HEV have been isolated from pigs in different geographical regions of the world, and it have been shown that the swine HEV is closely-related to the genotypes 3 and 4 strains of human HEV (Choi et al., 2003; Garkavenko et al., 2001; Meng, 2003, 2006, 2009; Nishizawa et al., 2003; Takahashi et al., 2003a, 2003b; Van der Poel et al., 2001; Wang et al., 2002; Yazaki et al., 2003). More recently, another animal strain of HEV, avian hepatitis E virus (avian HEV), was isolated and characterized from chickens with Hepatitis-Splenomegaly (HS) syndrome in the United States (Haqshenas et al., 2001, 2002). HS syndrome is an emerging disease of commercial egg laying hens of 30–72 weeks of age in North America and is characterized by ovarian regression, enlarged liver and spleen, and red fluid in the abdomen (Meng et al., 2008; Ritchie & Riddell, 1991). The complete sequence of avian HEV was determined and shown to be very similar in genomic organization to that of mammalian HEVs with approximately 50% nucleotide sequence identity (Huang et al., 2002, 2004). Apart from functional and structural similarities to human and swine HEVs, the avian HEV from chickens also shares common antigenic epitopes with the mammalian HEVs in the capsid protein (Guo et al., 2006; Zhou et al., 2008). The avian HEV from chickens in the United States also shares approximately 80% nucleotide sequence identity with the Australian chicken big liver and spleen disease virus (BLSV), and it is believed that the Australian BLSV is a variant strain of avian HEV (Haqshenas et al., 2001; Payne et al., 1999). Sequence analyses of avian HEV strains identified from the United States, Canada and Europe revealed significant sequence variations (Agunos et al., 2006; Billam et al, 2007; Peralta et al., 2008). Phylogenetic analyses of known avian HEV isolates indicate that avian HEV likely belongs to a separate genus within the Hepeviridae family (Billam et al., 2007; Meng et al., 2008). We have demonstrated that 60-week-old specific-pathogen-free (SPF) chickens experimentally inoculated either by oronasal route or by intravenous route with 5 × 104.5 50% chicken infectious doses of the prototype avian HEV strain recovered from a chicken with HS syndrome developed microscopic liver lesions characterized by lymphocytic periphlebitis and phlebitis. Also, approximately 25% of the infected chickens developed subcapsular hemorrhages or enlargement of the right intermediate lobe of the liver. Slight elevations of the plasma liver enzyme lactate dehydrogenase were also observed in infected chickens (Billam et al., 2005). The discovery of avian HEV associated with a hepatic disease provided a useful homologous small animal model system to study HEV replication and pathogenesis (Billam et al., 2005).
Prevalence of antibodies to avian HEV was reported in healthy chicken flocks in the United States (Huang et al., 2002; Sun et al., 2004a), suggesting that chickens in the United States are subclinically infected with HEV. From a prospective study (Sun et al., 2004a), we identified a genetically unique strain of avian HEV, designated avian HEV-VA, from healthy chickens without clinical disease in a Virginia chicken farm (Billam et al., 2007; Sun et al., 2004a). The complete genomic sequence of the avian HEV-VA strain was 90.1% identical to the prototype strain of avian HEV recovered from a chicken with HS syndrome in California (Billam et al., 2007). The objective of this present study is to compare the pathogenicity, in specific-pathogen-free chickens, of the two unique strains of avian HEV recovered from a healthy chicken and a diseased chicken with HS Syndrome, respectively.
2. Materials and Methods
2.1. Virus stock of the prototype strain of avian HEV
The prototype strain of avian HEV used in this study was originally recovered from a bile sample of a 56-week-old chicken with HS syndrome in California, USA. An infectious stock of the prototype strain of avian HEV was generated as 10% suspension in PBS buffer of fecal and bile materials from experimentally-infected chickens, and titrated in young specific-pathogen-free (SPF) chickens (Sun et al., 2004b). This prototype strain of avian HEV stock had an infectious titer of 5×102.5 50% chicken infectious dose (CID50) per ml, and was used as inocula in this study.
2.2. Generation of an infectious stock of avian HEV-VA strain recovered from a healthy chicken
In order to compare the pathogenicity between the prototype strain and the avian HEV-VA strain, an infectious stock of the avian HEV-VA strain with known infectious titer had to be produced. Since avian HEV cannot be propagated in vitro, the virus stock and its infectivity titration must be done in vivo. The original virus material used for the generation of an infectious stock of the avian HEV-VA strain was collected from healthy chickens in Virginia in a prospective study (Sun et al., 2004a). The virus recovered from the healthy chickens in Virginia, designated as the “avian HEV-VA”, was experimentally inoculated into young SPF chickens. The fecal and bile suspension from two of the inoculated chickens that tested positive for avian HEV by RT-PCR were harvested, and subsequently pooled to produce an infectious virus stock. The initial virus stock was biologically amplified by intravenous (I.V.) inoculation of two additional young SPF chickens. The feces collected from the two inoculated SPF chickens on alternate days were tested by RT-PCR for the presence of avian HEV RNA. The chickens were necropsied when fecal virus shedding was detected by RT-PCR. During necropsies, bile and intestinal contents were collected and used to make a 10% fecal suspension in PBS buffer (w/v) as the virus stock for the avian HEV-VA strain. The infectivity titer of the virus stock was subsequently determined in SPF chickens (see below).
2.3. Infectivity titration of the avian HEV-VA strain in SPF chickens
Due to the lack of a cell culture system for HEV, a chicken bioassay was used to determine the infectivity titer of the virus stock of the avian HEV-VA strain. Briefly, twenty-four young SPF chickens (Charles River SPAFAS Inc., Wilmington, MA) were divided into 6 isolators of 4 birds each. The avian HEV-VA infectious stock was serially diluted 10 fold from 10−1 to 10−5 in PBS buffer. Each of four chickens housed in an isolator was I.V.-inoculated with 200 µl of each dilution of the infectious stock, and the last set of 4 control chickens were inoculated with 200 µl of PBS buffer. Weekly blood and fecal swabs were collected from each chicken. ELISA was performed on the weekly sera to detect IgG anti-avian HEV antibodies, and RT-PCR was done on the weekly sera and fecal materials to detect avian HEV RNA. The inoculated chickens were monitored for 10 weeks for evidence of virus infection. The infectivity titer of the virus stock for the avian HEV-VA strain was calculated as 50% chicken infectious dose (CID50) per ml of the virus inoculum.
2.4. Experimental design for the comparative pathogenesis study
Fifty-four, 6-week-old, SPF chickens were divided into 3 groups of 18 chickens each. Chickens in group 1 (n=18) were each I.V.-inoculated with 5×102.5 CID50 of the prototype avian HEV stock. Chickens in group 2 (n=18) were each IV inoculated with 5×102.5 CID50 of the avian HEV-VA virus stock. Eighteen chickens in group 3 served as uninoculated controls. Each group was housed in a separate isolation room, and the chickens were allowed access to feed and drinking water ad libitum.
2.5. Sample collection and processing
Blood and colorectal swabs were collected from each chicken prior to inoculation and weekly thereafter. Weekly blood plasmas were tested for liver enzyme profiles as previously described (Billam et al., 2005). Weekly serum samples were tested by ELISA for IgG anti-avian HEV antibodies (Huang et al., 2002). Weekly serum samples and colorectal swab materials were tested for avian HEV RNA by RT-PCR (Billam et al., 2005; Sun et al., 2004a, 2004b). Six chickens from each group were necropsied at 2, 3 and 4 weeks post-inoculation (wpi), respectively. Tissue samples including liver, thymus, spleen, heart, pancreas, duodenum, jejunum, ileum, cecum and colorectum were collected from each chicken during each necropsy, fixed in formalin and processed for histopathological evaluation.
2.6. Gross pathology and histopathology evaluations
Livers were evaluated for gross pathological lesions during necropsies as previously described (Billam et al., 2005). Tissue samples collected at each necropsy were fixed in 10% neutral buffered formalin and processed for routine histopathological examinations. Histopathological lesions in various tissues were evaluated in a blinded fashion by a board-certified veterinary pathologist (T.L.) and were scored according to the lesion severity based on standard scoring systems reported previously (Billam et al., 2005). Liver lesion scores range from 0 to 4 (0, no lesions; 1, < 5 foci; 2, 5–8 foci; 3, 9–15 foci; 4, >15 foci. The numbers of foci were bond on a single shade). Thymic lesions were given scores from 0 to 4 (0, no foci; 1, 1 to 5 foci; 2, 5–10 foci; 3, 10–20 foci; 4, >20 foci) and spleens were scored from 0 to 3 (0 = normal, 1 = minimal, 2 = moderate, 3 = severe).
2.7. Blood analyte profiles
A total of 18 chickens (6 each from the prototype avian HEV-infected group, the avian HEV-VA-infected group, and the control group) were monitored weekly for biochemical evidence of hepatitis from 2 wpi to the end of the study at 4wpi. Plasma levels of aspartate aminotransferase (AST), lactate dehydrogenase (LDH), creatine phosphokinase (CPK), bile acids (BA), and total protein (TP) were determined by standard methods (Avian and Exotic Animal Clinical Pathology antibodies in chickens as previously described (Billam et al., 2005; Huang et al., 2002; Sun et al., 2004a, 2004b). The capsid gene of the prototype avian HEV strain, which the ELISA antigen is derived from, shared 90.7% nucleotide sequence identity (with six non-silent amino acid mutations) with the avian HEV-VA strain (Billam et al., 2007). Briefly, the purified avian HEV antigen was coated onto 96-well plates (Thermo Labsystems, Franklin, MA). A labs, Wilmington, OH).
2.8. ELISA for IgG anti-avian HEV antibodies
A purified truncated recombinant ORF2 capsid protein of the prototype strain of avian HEV expressed in Escherichia coli was used as the antigen in the ELISA to detect IgG avian HEV horseradish peroxidase-conjugated rabbit anti-chicken IgG (Sigma Chemical Co., St. Louis, Mo.) was used as the secondary antibody. The optical density values were measured at 405 nm. Samples with OD values greater than 0.30 were considered positive (Huang et al., 2002). Convalescent sera from chickens experimentally-infected with avian HEV (Billam et al., 2005) and serum samples from normal SPF chickens were included as positive and negative controls, respectively.
2.9. RT-PCR for detection of avian HEV RNA
To detect avian HEV RNA in feces and sera, RT-PCR was performed as previously described (Huang et al., 2002). Briefly, RNA was extracted with TRI reagent (MRC) from 100 µl of serum or 10% fecal suspension. Total RNA was re-suspended in 12.25 µl of DNase, RNase, and proteinase-free water (Invitrogen). Reverse transcription was performed at 42°C for 60 min with 1µl (10 pm) of reverse primer (prototype avian HEV-specific P2 primer (5’ - ACAGTTTCACCTCAGGCTCG −3’) or the avian HEV-VA-specific YR primer (5’ -CTGCGCAACAGTATCCATTAAG −3’), 0.25 µl [50 U] of superscript II reverse transcriptase (Invitrogen), 1 µl of 0.1M dithiothreitol, 4 µl of 5x RT buffer, 0.5 µl [20 U] of RNasin RNase inhibitor (Promega), and 1 µl of 10 mM dNTPs. Five microliters of the resulting cDNA was subsequently amplified in a 50 µl reaction using Platinum High Fidelity Supermix (Invitrogen). For detection of viral RNA in chickens inoculated with the prototype avian HEV, the first round PCR produced an expected fragment of 595 bp with the forward primer P1 (5’- ACAACATCCACCCCTACAAG-3’) and the reverse primer P2. For the second round PCR, the forward primer P3 (5’-AGAACAATGGTTGGCGGTCC-3’) and the reverse primer P4 (5’-GAGGGCAAGCCACCTAAAAC-3’) amplified an expected fragment of 394 bp. The PCR parameters included an initial incubation at 94°C for 9 min, followed by 39 cycles of denaturation at 94°C for 0.5 min, annealing at 52°C ( 56°C for 2nd rd PCR), 0.5 min, extension at 72°C for 1 min, and a final extension at 72°C for 7 min.
For detection of viral RNA in chickens inoculated with the avian HEV-VA strain, forward primer YF (5’- GCTGCCCTTGGGATGTTTGCAT-3’) and the reverse primer YR were used in the first round PCR to produce an expected fragment of 712 bp. For the second round PCR, the forward primer YF2 (5’-AGTTTTGCGGTCTGTCGTGTTT-3’) and the reverse primer YR2 (5’-AGCGTGTTAATCACCGCAAGGC-3’) amplified an expected fragment of 578 bp. The PCR conditions were essentially the same as described above, except for annealing temperatures of 48°C and 54°C in the 1st and 2nd rounds, respectively.
Due to the sequence variation between the avian HEV-prototype and the avian HEV-VA, we had to use two separate RT-PCR assays to ensure the specificity. However, the sensitivity of the two assays is expected to be similar base dupon earlier studies (Billam et al., 2005, 2007). The PCR products amplified from sera and feces of selected chickens from each of inoculated groups were sequenced to confirm the identity of the virus recovered from the experimentally infected chickens.
2.10. DNA sequencing
The PCR products were excised from 0.8% agarose gel, purified using Geneclean III kit (Qbiogene), and both strands were sequenced at the Virginia Bioinformatics Institute Core Laboratory Facility (Blacksburg, Virginia) using an automated DNA sequencer (Applied Biosystems).
2.11. Statistical analyses
Histopathologic lesions were recorded as lesion scores. The means of lesion scores were compared by weighted least squares analysis of variance using the CATMOD procedure of SAS® (version 9.1.3; SAS Institute, Inc., Cary, N.C.). Antibody titers were compared by repeated measures analysis of covariance using baseline titers as co-variants by the GLIMMIX procedure. A box-cox transformation was applied to antibody titers to obtain approximate Gaussian distribution. The biochemical profiles of liver enzymes, total proteins and bile acids were compared by repeated measures analysis of variance using the GLIMMIX procedure. Models included group (GRP) and WPI and GRP x WPI interaction.
3. Results
3.1. Generation of an infectious stock of the avian HEV-VA strain recovered from a healthy chicken in Virginia
The original pooled feces and bile suspension from a prospective field study (Sun et al., 2004a) were experimentally inoculated into two young SPF chickens to generate an infectious stock. Avian HEV RNA was detected in feces from 3 dpi. The avian HEV RNA-positive bile and fecal suspension obtained from the necropsy of one chicken at 23 dpi and another chicken at 30 dpi was pooled to make a 10% suspension in PBS buffer as the infectious stock of the avian HEV-VA.
3.2. Infectivity titration of an infectious stock of the avian HEV-VA strain in young SPF chickens
All chickens inoculated with 10−1 and 10−2 dilutions of the virus stock of the avian HEV-VA strain seroconverted (Table 1). However, the chickens in the other groups including the control chicken remained seronegative throughout the study. One of four chickens inoculated with the highest infectious dose (10−1 dilution) seroconverted at 5 wpi, and all of the remaining chickens seroconverted by 6 wpi. In the group inoculated with 10−2 dilution of the infectious stock, one chicken was seropositive from 1 wpi, 2 chickens were seropositive by 4 wpi. The other two chickens seroconverted at 6 and 7 wpi, respectively. Viremia and fecal virus shedding were observed in the chickens inoculated with the highest doses, but not in the group inoculated with 10−3 dilution (Table 2). The chickens inoculated with 10−4 and 10−5 dilution and PBS (control) were not tested by RT-PCR as they remained seronegative during the entire duration of the study. Consistent with the production of the infectious stock of the prototype strain of avian HEV (Sun et al., 2004b), seroconversion in the inoculated chickens was used in this study as the end point for the calculation of the infectivity titer of the infectious virus stock. The infectious titer for the virus stock of the avian HEV-VA strain was determined to be 5 × 102.5 CID50 per ml.
Table 1.
Infectivity titration of the infectious stock of the avian HEV-VA strain recovered from a healthy chicken in a Virginia chicken farm: Seroconversion to IgG anti-avian HEV antibodies in young specific-pathogen-free chickens experimentally inoculated with different dilutions of the infectious stock
| Virus stock dilutiona |
No. of chickens seropositive / no. of chickens tested at the indicated wpi: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 10−1 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 1/4 | 4/4 | 4/4 | 4/4 | 3/4 | 3/4 |
| 10−2 | 0/4 | 1/4 | 1/4 | 1/4 | 2/4 | 2/4 | 3/4 | 3/4 | 3/4 | 2/4 | 2/4 |
| 10−3 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| Control | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
data for 10−4 and 10−5 dilutions, which are all negative, are not shown in the Table.
Table 2.
Infectivity titration of an infectious stock of the avian HEV-VA strain recovered from a healthy chicken from a Virginia chicken farm: Viremia and fecal virus shedding in specific-pathogen-free chickens experimentally inoculated with different dilutions of the infectious stock
| Virus stock dilution |
No. of chickens positive for viremia (fecal virus shedding) / no. of chickens tested at the indicated wpi: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| 10−1 | 0(0)/4 | 0(1)/4 | 0(3)/4 | l(4)/4 | 2(4)/4 | 2(4)/4 | 0(2)/4 | 0(0)/4 | 0(0)/4 | 0(0)/4 | NT |
| 10−2 | 0(0)/4 | 0(0)/4 | 0(2)/4 | 0(l)/4 | 0(1)/4 | l(2)/4 | 0(2)/4 | 0(1)/4 | 0(0)/4 | 0(0)/4 | NT |
| 10−3 | 0(0)/4 | 0(0)/4 | 0(0)/4 | 0(0)/4 | 0(0)/4 | 0(0)/4 | 0(0)/4 | 0(0)/4 | 0(0)/4 | 0(0)/4 | NT |
NT: Not tested: The chickens inoculated with lower dilutions (10−4 and 10−5) and the PBS buffer (negative control chickens) were seronegative throughout the entire duration of the study and were thus not tested by RT-PCR.
3.3. Comparison of gross and microscopic lesions in chickens experimentally infected with the prototype strain and the avian HEV-VA strain
Gross lesions were observed in only two inoculated chickens at 2 wpi. One chicken from the prototype avian HEV-infected group had a localized circular blotched area on left liver lobe. Subcapsular hemorrhages were observed in the right lobe of liver from another chicken inoculated with the avian HEV-VA strain. Both chickens had high scores for histopathological lesions as well (4 and 3, respectively). Gross lesions in other chickens from all groups were not evident.
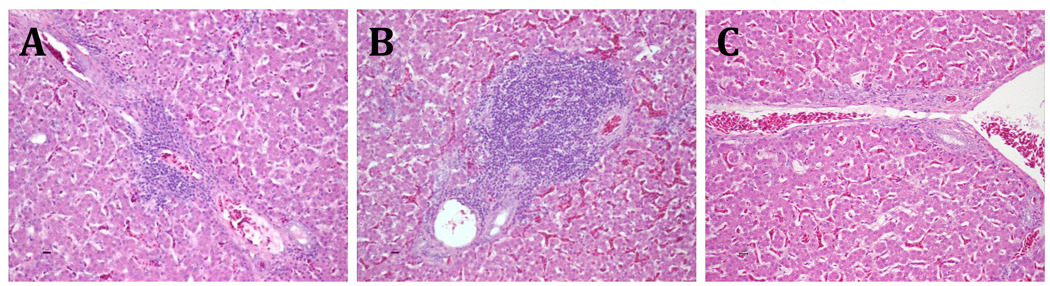
The microscopic lesions in the liver included lymphocytic phlebitis, lymphocytic and heterophilic periphlebitis and fibrinoid necrosis (Fig. 1A and 1B), and were milder in the avian HEV-VA-infected chicken group. Subcapsular hemorrhages, massive necrosis or amyloid-like lakes were not observed. The mean histological lesion scores were higher for the prototype avian HEV-infected group and the avian HEV-VA-infected group than the negative control group at 2 and 3 wpi, but not at 4 wpi (Table 3). The histological hepatic lesion scores for prototype avian HEV group were higher than the scores for avian HEV-VA group at 3 and 4 wpi. Overall, the hepatic lesion scores differed between treatment groups (P = 0.03) and were higher for the prototype avian HEV group than the avian HEV-VA group (least square mean [LSM] for prototype avian HEV group, 2.722; LSM for the avian HEV-VA group, 2.61; LSM for control group, 1.877). However, the hepatic lesion scores did not differ over time (P = 0.48).
Fig. 1.
Microscopic lesions in the liver. (A) Section of liver from a chicken inoculated with the avian HEV-VA strain showing focal, mild lymphocytic and heterophilic portal periphlebitis. (B) Section of liver from a chicken inoculated with the prototype avian HEV strain showing severe lymphocytic portal phlebitis and periplebitis. The tissues were stained with hematoxylin and eosin. (C) Section of liver from a negative control uninfected chicken. Hematoxylin and eosin staining.
Table 3.
. Comparative pathogenesis in specific-pathogen-free chickens experimentally inoculated with avian HEV strains recovered from a chicken with HS syndrome (prototype strain), and from a healthy chicken (avian HEV-VA strain): Microscopic liver lesions (and mean lesion scores) in each group of chickens a.
| Group | No. of chickens with lesions (mean lesion score) / no. of chickens tested at indicated wpi b |
||
|---|---|---|---|
| 2 wpi | 3 wpi | 4 wpi | |
| Prototype strain | 6/6(2.66) | 6/6(3.0) | 6/6(2.5) |
| Avian HEV-VA strain | 6/6(2.83) | 6/6(2.83) | 6/6(2.16) |
| Negative control | 4/6(1.0) | 6/6(1.83) | 5/5c(2.8) |
Six chickens from each group were necropsied at 2, 3 and 4 wpi, respectively.
Microscopic liver lesions included lymphocytic periphlebitis and phlebitis. The hepatic lesion scores differed between treatment groups (P = 0.03) and were higher for the prototype avian HEV group than the avian HEV-VA group (least square mean [LSM] for prototype avian HEV group, 2.722; LSM for the avian HEV-VA group, 2.61; LSM for control group, 1.877). However, the hepatic lesion scores did not differ over time (P = 0.48).
Only 5 chicken samples were available.
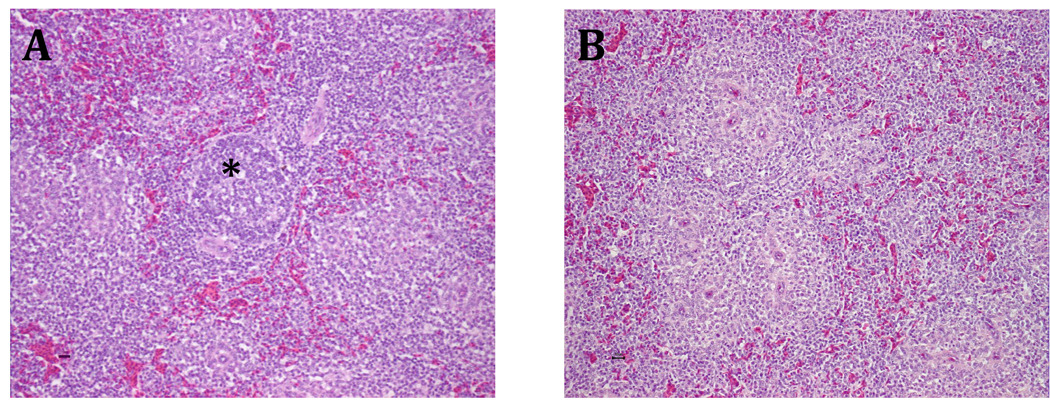
Microscopic lesions were also observed in spleen and thymus. Foci of lymphoid hyperplasia were observed in spleen (Fig. 2). The spleen lesion scores differed between treatment groups (P = 0.0006), but did not differ over time (P = 0.32). Mild cortical hyperplastic lesions were seen in thymus, but the lesion scores did not differ between treatment groups (P = 0.27).
Fig. 2.
Photomicrograph of the spleen. (A) Section of spleen from a chicken inoculated with the prototype avian HEV strain. Note the coalescing foci of lymphoid hyperplasia surrounding arterioles (*). (B) Section of spleen from a negative control uninfected chicken. Hematoxylin and eosin staining.
3.4. Seroconversion to IgG anti-avian HEV antibodies
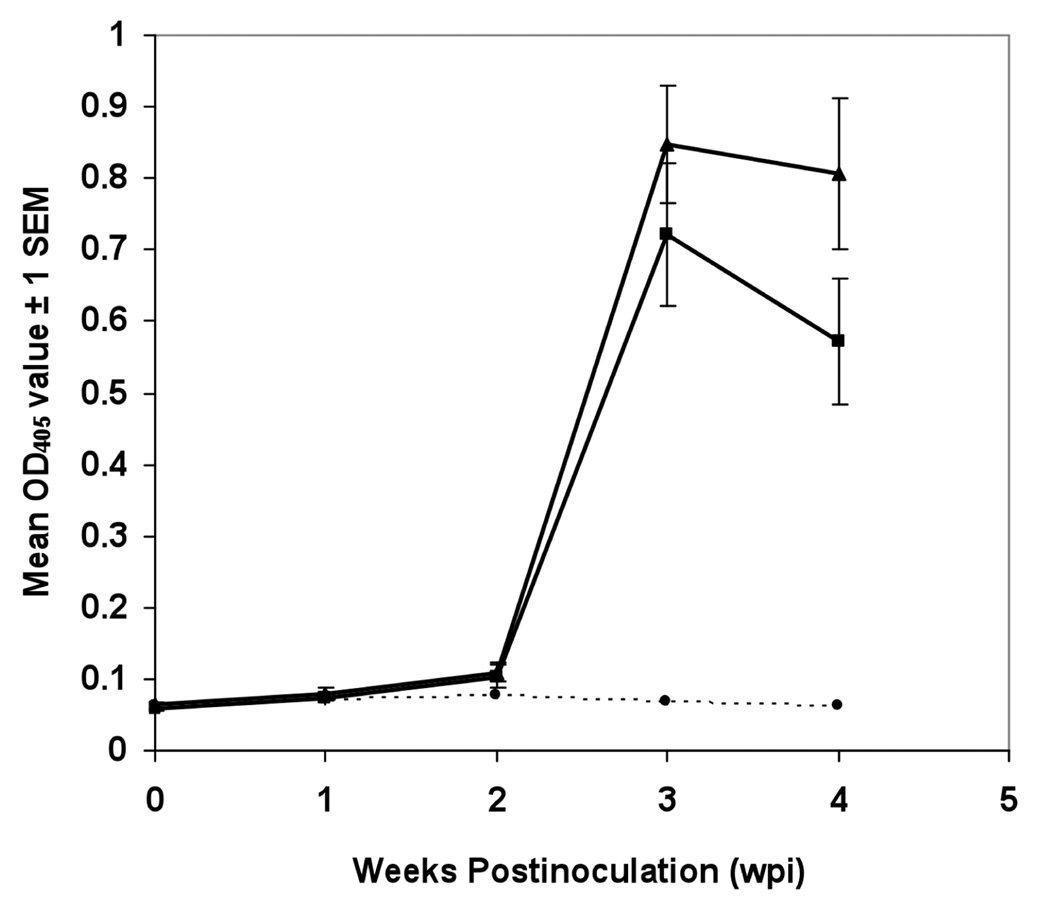
The chickens were seronegative for HEV prior to inoculation. Most of the inoculated chickens seroconverted to IgG anti-avian HEV antibodies by 3 wpi (Table 4 and Table 5). By 3 wpi, IgG anti-avian HEV antibodies were detected in 83% (10/12) chickens in the prototype avian HEV group and 75% (9/12) chickens in the avian HEV-VA group. By 4 wpi, 100% (6/6) chickens in both inoculated groups had seroconverted. The ELISA OD values differed between the treatment groups (P=0.0001) and differed over time (P=0.0001) (for GRP X WPI, P=0.0001). The GRP X WPI effect was significant for both the avian HEV-VA group (P<0.0001) and prototype avian HEV group (P<0.0001), but not for the negative control group (P=0.11). The mean ELISA OD values for both inoculated groups peaked at 3 wpi (Fig. 3), but were higher for prototype avian HEV group (mean OD ± standard error of mean [SEM], 0.847 ± 0.081) compared to the avian HEV-VA group (mean OD ± SEM, 0.721 ± 0.1).
Table 4.
Detection of viral RNA by RT-PCR in feces / sera and seroconversion to IgG avian HEV antibodies in specific-pathogen-free chickens inoculated with the prototype strain of avian HEV
| Chicken ID No. | Detection of viral RNA by RT-PCR in fecal swabs / sera (detection of avian HEV antibodies by ELISA) at the indicated wpi: |
||||
|---|---|---|---|---|---|
| 0 wpi | 1 wpi | 2 wpi | 3 wpi | 4 wpi | |
| 5339 | −/− (−) | +/+(−) | +/+ (−) | +/+(−) | −/−(+)* |
| 5340 | −/− (−) | −/−(−) | +/+ (−) | −/− (+) | −/− (+)* |
| 5341 | −/− (−) | +/− (−) | +/+ (−) | −/− (+) | −/− (+)* |
| 5342 | −/− (−) | +/+(−) | +/+ (−) | +/+(+) | +/+ (+)* |
| 5343 | −/− (−) | +/+(−) | +/+ (−) | +/− (+) | −/− (+)* |
| 5344 | −/− (−) | +/− (−) | +/+ (−) | −/− (+) | −/− (+)* |
| 5348 | −/− (−) | +/−(−) | +/+ (−) | −/− (+)* | |
| 5377 | −/− (−) | +/− (−) | +/+ (−) | +/−(+)* | |
| 5379 | −/− (−) | −/+(−) | +/+(−) | +/+(+)* | |
| 5380 | −/− (−) | +/+(−) | +/+ (−) | +/+ (−)* | |
| 5382 | −/− (−) | +/+(−) | +/+ (−) | +/+ (+)* | |
| 5384 | −/− (−) | +/+(−) | +/+ (−) | +/− (+)* | |
| 5345 | −/− (−) | +/+(−) | +/+(−)* | ||
| 5346 | −/− (−) | −/+(−) | +/− (−)* | ||
| 5347 | −/− (−) | +/+(−) | +/+(−)* | ||
| 5349 | −/− (−) | +/− (−) | +/+(−)* | ||
| 5351 | −/− (−) | +/− (−) | +/+(−)* | ||
| 5376 | −/− (−) | +/+(−) | +/+(−)* | ||
Six chickens from each group were necropsied at 2, 3 and 4 wpi, respectively.
Table 5.
Detection of viral RNA by RT-PCR in feces / sera and seroconversion to IgG avian HEV antibodies in specific-pathogen-free chickens experimentally inoculated with the avian HEV-VA strain
| Chicken ID No. | Detection of viral RNA by RT-PCR in fecal swabs / sera (detection of avian HEV antibodies by ELISA) at the indicated wpi: |
||||
|---|---|---|---|---|---|
| 0 wpi | 1 wpi | 2 wpi | 3 wpi | 4 wpi | |
| 5320 | −/− (−) | +/− (−) | +/+(−) | +/−(+) | −/− (+)* |
| 5321 | −/− (−) | +/+(−) | +/+(−) | +/− (−) | −/− (+)* |
| 5322 | −/− (−) | +/+(−) | +/+(−) | −/−(+) | −/− (+)* |
| 5323 | −/− (−) | +/− (−) | +/− (−) | +/−(+) | +/− (+)* |
| 5324 | −/− (−) | +/− (−) | +/+(−) | +/−(+) | −/− (+)* |
| 5325 | −/− (−) | +/− (−) | +/+(−) | −/− (−) | −/− (+)* |
| 5328 | −/− (−) | +/+(−) | +/+(−) | −/− (+)* | |
| 5330 | −/− (−) | −/−(−) | +/− (−) | +/+ (−)* | |
| 5332 | −/− (−) | +/+(−) | +/− (−) | +/+ (+)* | |
| 5333 | −/− (−) | +/+(−) | +/+ (+) | −/− (+)* | |
| 5337 | −/− (−) | +/+(−) | +/+(−) | +/+(+)* | |
| 5338 | −/− (−) | +/+(−) | +/+(−) | −/− (+)* | |
| 5326 | −/− (−) | +/+(−) | +/+ (−)* | ||
| 5327 | −/− (−) | +/+(−) | −/+(−)* | ||
| 5329 | −/− (−) | +/− (−) | +/+ (−)* | ||
| 5331 | −/− (−) | +/+(−) | +/+ (−)* | ||
| 5334 | −/− (−) | +/+(−) | +/+ (−)* | ||
| 5336 | −/− (−) | +/− (−) | +/+ (−)* | ||
Six chickens from each group were necropsied at 2, 3 and 4 wpi, respectively.
Fig. 3.
Seroconversion to IgG avian HEV antibodies in inoculated SPF chickens. The mean ELISA OD values of all chickens from the prototype avian HEV group (Δ), the avian HEV-VA group (▪), and negative control group (•) at each week postinoculation (wpi) are plotted.
3.5. RT-PCR detection of avian HEV RNA in fecal and serum samples of inoculated chickens
Weekly sera and fecal swabs were tested by RT-PCR with nested sets of primers for the presence of avian HEV RNA. Avian HEV RNA was detected in feces and sera of chickens in both inoculated groups (Table 4 and Table 5). Viremia and fecal virus shedding were observed from 1 wpi. Viral RNA was detected in feces and sera of most inoculated chickens at 2 wpi. In the prototype avian HEV group, fecal virus shedding was observed in 15/18 chickens at 1 wpi, 18/18 chickens at 2 wpi, 8/12 chickens at 3 wpi, and 1/6 chickens at 4 wpi. In the chickens that were inoculated with the avian HEV-VA strain, fecal samples were positive by RT-PCR for avian HEV RNA in 17/18 chickens at 1 wpi, 17/18 chickens at 2 wpi, 7/12 chickens at 3 wpi, and 1/6 chicken at 4 wpi. Viremia was observed in 11/18 chickens at 1 wpi, 17/18 chickens at 2 wpi, 5/12 chickens at 3 wpi and 1/6 chicken at 4 wpi in the group inoculated with the prototype avian HEV. For the avian HEV-VA group, viremia was detectable in 11/18 chickens at 1 wpi, 15/18 chickens at 2 wpi, 3/12 chickens at 3 wpi, and 0/6 chicken at 4 wpi. The control chickens were negative for avian HEV RNA in fecal swabs and serum during the entire course of experiment. The PCR products amplified from sera and fecal swabs of selected chickens from both inoculated groups were sequenced, and sequence analyses confirmed that the viruses recovered from the infected chickens originated from their respective original virus inoculum.
3.6. Blood analyte profiles
AST, BA and TP from 18 chickens (6 from each group) monitored until the end of the study did not differ between treatment groups (data not shown). LDH values did not differ between the Avian HEV-prototype and avian HEV-VA treatment treatment groups (P=0.21), but differed over time (P=0.001). However, the serum CPK responses differed between the avian HEV-protype and avian HEV-VA treatment groups (P=0.049) and over time (P=0.043). The mean CPK levels were higher for the prototype avian HEV group (P<0.0001) than the avian HEV-VA group (P<0.0001) and the negative control group (least square mean [LSM] for prototype avian HEV group, 1686.15; LSM for avian HEV-VA group, 1402.66; LSM for negative control group, 1538.31; SEM, 74.39).
4. Discussion
Avian HEV, recovered from diseased chickens with HS syndrome, produces characteristic hepatic lesions (Billam et al., 2005) and provides a unique homologous small animal model system to study HEV pathogenesis. The identification of a genetically unique avian HEV-VA strain from healthy chickens in Virginia without clinical symptoms prompted us to determine the complete genomic sequence of the avian HEV-VA (Billam et al., 2007). Compared to the prototype strain recovered from a diseased chicken with HS syndrome, the avian HEV-VA strain recovered from a healthy chicken has numerous non-silent amino acid mutations in the capsid gene and other genomic regions (Billam et al., 2007). However, it remains to be determined if the avian HEV-VA strain is attenuated. Therefore, it is important to characterize the pathogenicity of the avian HEV-VA strain under laboratory conditions and compare its pathogenicity with the prototype strain. In this study, we first generated an infectious stock of the avian HEV-VA strain, and subsequently the infectivity titer of this infectious stock was determined in SPF chickens. To compare the pathogenicity of the two unique strains, SPF chickens were experimentally inoculated with the two strains of avian HEV, and the differences in pathogenicity with respect to clinical course and pathological lesions were evaluated and compared in the two groups of inoculated chickens.
Virus replicated in SPF chickens inoculated with both the prototype and the avian HEV-VA strains since the inoculated chickens seroconverted to IgG anti-avian HEV antibodies, and viral RNA was detected in feces and sera of inoculated chickens. There was no difference, however, in the time points for the appearance of seroconversion or virus shedding in chickens inoculated with the two strains, indicating that the avian HEV-VA strain replicated as rapidly as the prototype strain. Gross lesions were not prominent, but microscopic hepatic lesions including periphlebitis and phlebitis, which is the characteristic lesions of avian HEV infection (Billam et al., 2005), were observed in the inoculated chickens. The mean microscopic hepatic lesion scores differed between treatment groups (P=0.03) and were higher for the prototype avian HEV group than the avian HEV-VA group, and thus suggestive of a potential attenuation of the avian HEV-VA strain. However, the numbers of chickens with microscopic lesions among the three groups were not significantly different. It is not surprising to see the presence of nonspecific foci with lymphocytic infiltration in the negative control chickens, since chickens lack lymph nodes and the lymphoid tissues are dispersed in the gastrointestinal tract and liver. Such non-specific background liver lesions were already reported previously in chickens (Billam et al., 2005). The severity (pathological scores) of the liver lesions in chickens (not the numbers of chickens with detectable lesions) measures the virulence potential of the virus, although normal control chickens can also have mild but detectable non-specific histological liver lesions.
It has been previously shown that gross liver lesions (subcapsular hemorrhage and liver enlargement) occurred in only a small proportion of the 60-week-old chickens experimentally infected with avian HEV (Billam et al., 2005). In this study, only two infected chickens had detectable gross liver lesions, which is not very surprising. It has been reported that human HEV infection in non-human primates is dose-dependent with biochemical evidences of hepatitis in primates receiving higher infectious doses of human HEV but only subclinical infection in primates with lower doses (Tsarev et al., 1994). Antibodies to avian HEV were prevalent in healthy chicken flocks in the United States (Huang et al., 2002), suggesting that chickens in the United States are subclinically infected by avian HEV. The fact that the avian HEV-VA strain recovered from a healthy chicken still produces mild but detectable pathological liver lesions suggest that this strain of avian HEV is not “avirulent” as previously thought (Billam et al., 2007). Avian HEV alone may not be solely responsible for the full spectrum of clinical HS syndrome observed in some chickens, and other co-factor(s) may be involved in the induction of the full spectrums of clinical HS syndrome (Meng et al., 2008; Ritchie & Riddell, 1991). It has been reported that commercial layer chicken flocks that were infected with avian HEV and fed with a flax-based diet experienced increased mortality when the birds reached 37 weeks (Agunos et al., 2006), and thus the flax-based diet could be a co-factor in the development of clinical HS syndrome in infected chickens. It is also possible that, like human HEV, the disease-causing potential of avian HEV is dose-dependant: chickens receiving higher infectious dose may develop clinical HS syndrome and severe lesions whereas chickens receiving lower infectious dose may only develop subclinical infection with mild lesions, and thus the infectious dose each chicken receives in the field could be a co-factor in the development of clinical HS syndrome. It will be interesting to see if infection of chickens with a higher infectious stock of avian HEV, when it becomes available, can produce the full spectrum of clinical HS syndrome. In addition, the ages of the SPF chickens (6-week-old) used in the study may contribute to the less prominent gross lesions seen in this study, since HS syndrome is often reported in broiler breeders and laying hens of 30–72 weeks of age in the field (Ritchie & Riddell, 1991; Meng et al., 2008), and thus the age of chickens could also be a co-factor in determining whether or not the infected birds will develop the full spectrum clinical HS syndrome.
The chickens from both inoculated groups seroconverted and the mean antibody titers peaked at 3 wpi, with the chickens inoculated with prototype strain had slightly higher mean antibody titers compared to the chickens inoculated with the avian HEV-VA strain (Fig. 3). The antigen used in the ELISA assay is based upon the recombinant capsid protein of the prototype avian HEV strain, it is unclear if the observed slight difference in antibody titer might be attributable to the use of a heterologous antigen for the avian HEV-VA infected chickens. Viremia and fecal virus shedding were observed in highest numbers of chickens in both groups at 2 wpi, and preceded the appearance of highest antibody titers at 3 wpi. The appearance of antibody response following the detection of virus in feces and sera is consistent with the pathogenesis of HEV infection (Emerson and Purcell, 2003). However, there was no significant difference in the patterns of fecal virus shedding and viremia appearance in the two inoculated groups (Table 4 and Table 5), suggesting that the avian HEV-VA strain has similar replication capacity compared to the prototype strain.
The levels of blood analytes including AST, CLDH, CPK, BA and TP were analyzed. No significant elevations of AST, LDH, BA or TP were detected in either of the inoculated group. However, CPK values were significantly different between groups, being higher for the chickens inoculated with prototype strain than the control group. Elevated CPK levels indicate muscle injury, and elevation of transaminases suggests liver damage (Lumeij, 1997). The importance of the elevated CPK levels in the absence of significant AST elevations is not known but it may be due to non-specific skeletal or cardiac muscle injury.
In summary, young SPF chickens were successfully infected with two unique strains of avian HEV, the prototype strain from a diseased chicken with HS syndrome and the avian HEV-VA strain from a healthy chicken. Overall, the hepatic lesion scores in the liver and the mean anti-avian HEV antibody titers were higher for the chickens inoculated with the prototype strain, suggesting that the avian HEV-VA strain is only slightly attenuated in experimentally inoculated chickens. However, the avian HEV-VA strain still produces, although milder compared to the prototype strain, microscopic liver lesions, and thus is not “avirulent” as originally thought based upon a previous field study (Billam et al., 2007). It is likely that disease-causing potential of avian HEV is multi-factorial including infectious dose, the genetics of virus, and maybe other co-factor(s). Clearly additional studies are warranted to investigate the mechanisms of HEV pathogenesis using chickens and avian HEV as a model system.
Acknowledgements
This study was supported by grants from the National Institutes of Health (AI050611 and AI074667). We thank Dr. Stephen Werre for help with the statistical analysis of this study. We acknowledge the help of Dr. F. F. Huang, Dr. K. Key, Dr. S. Ramamoorthy and D. Guenette in chicken experiments. We thank Dr. Thomas Toth and Dr. Jake Tu for their support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal R, Krawczynski K. Hepatitis E: an overview and recent advances in clinical and laboratory research. J. Gastroenterol. Hepatol. 2000;15:9–20. doi: 10.1046/j.1440-1746.2000.02006.x. [DOI] [PubMed] [Google Scholar]
- Agunos AC, Yoo D, Youssef SA, Ran D, Binnington B, Hunter DB. Avian hepatitis E virus in an outbreak of hepatitis--splenomegaly syndrome and fatty liver haemorrhage syndrome in two flaxseed-fed layer flocks in Ontario. Avian Pathol. 2006;35:404–412. doi: 10.1080/03079450600920976. [DOI] [PubMed] [Google Scholar]
- Arankalle VA, Chadha MS, Tsarev SA, Emerson SU, Risbud AR, Banerjee K, Purcell RH. Seroepidemiology of water-borne hepatitis in India and evidence for a third enterically-transmitted hepatitis agent. Proc. Natl. Acad. Sci. USA. 1994;91:3428–3432. doi: 10.1073/pnas.91.8.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arankalle VA, Chobe LP, Chadha MS. Type-IV Indian swine HEV infects rhesus monkeys. J. Viral. Hepat. 2006;13:742–745. doi: 10.1111/j.1365-2893.2006.00759.x. [DOI] [PubMed] [Google Scholar]
- Billam P, Huang FF, Sun ZF, Pierson FW, Duncan RB, Elvinger F, Guenette DK, Toth TE, Meng XJ. Systematic pathogenesis and replication of avian hepatitis E virus in specific-pathogen-free adult chickens. J. Virol. 2005;79:3429–3437. doi: 10.1128/JVI.79.6.3429-3437.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billam P, Sun ZF, Meng XJ. Analysis of the complete genomic sequence of an apparently avirulent strain of avian hepatitis E virus identified major genetic differences compared to the prototype avian HEV. J. Gen. Virol. 2007;88:1538–1544. doi: 10.1099/vir.0.82754-0. [DOI] [PubMed] [Google Scholar]
- Choi IS, Kwon HJ, Shin NR, Yoo HS. Identification of swine hepatitis E virus (HEV) and prevalence of anti-HEV antibodies in swine and human populations in Korea. J. Clin. Microbiol. 2003;41:3602–3608. doi: 10.1128/JCM.41.8.3602-3608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson SU, Purcell RH. Hepatitis E virus. Rev. Med. Virol. 2003;13:145–154. doi: 10.1002/rmv.384. [DOI] [PubMed] [Google Scholar]
- Emerson SU, Anderson D, Arankalle VA, Meng XJ, Purdy M, Schlauder GG, Tsarev SA. Hepevirus. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, editors. Virus taxonomy. VIIIth report of the ICTV. London, United Kingdom: Elsevier/Academic Press; 2004. pp. 851–855. [Google Scholar]
- Garkavenko O, Obriadina A, Meng J, Anderson DA, Benard HJ, Schroeder BA, Khudyakov YE, Fields HA, Croxson MC. Detection and characterisation of swine hepatitis E virus in New Zealand. J. Med. Virol. 2001;65:525–529. [PubMed] [Google Scholar]
- Guo H, Zhou EM, Sun ZF, Meng XJ, Halbur PG. Identification of B-cell epitopes in the capsid protein of avian hepatitis E virus (avian HEV) that are common to human and swine HEVs or unique to avian HEV. J. Gen. Virol. 2006;87:217–223. doi: 10.1099/vir.0.81393-0. [DOI] [PubMed] [Google Scholar]
- Haqshenas G, Shivaprasad HL, Woolcock PR, Read DH, Meng XJ. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J. Gen. Virol. 2001;82:2449–2462. doi: 10.1099/0022-1317-82-10-2449. [DOI] [PubMed] [Google Scholar]
- Haqshenas G, Huang FF, Fenaux M, Guenette DK, Pierson FW, Larsen CT, Shivaprasad HL, Toth TE, Meng XJ. The putative capsid protein of the newly identified avian hepatitis E virus shares antigenic epitopes with that of swine and human hepatitis E viruses and chicken big liver and spleen disease virus. J. Gen. Virol. 2002;83:2201–2209. doi: 10.1099/0022-1317-83-9-2201. [DOI] [PubMed] [Google Scholar]
- Harrison TJ. Hepatitis E virus -- an update. Liver. 1999;19:171–176. doi: 10.1111/j.1478-3231.1999.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Huang FF, Haqshenas G, Shivaprasad HL, Guenette DK, Woolcock PR, Larsen CT, Pierson FW, Elvinger F, Toth TE, Meng XJ. Heterogeneity and seroprevalence of a newly identified avian hepatitis e virus from chickens in the United States. J. Clin. Microbiol. 2002;40:4197–4202. doi: 10.1128/JCM.40.11.4197-4202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang FF, Sun ZF, Emerson SU, Purcell RH, Shivaprasad HL, Pierson FW, Toth TE, Meng XJ. Determination and analysis of the complete genomic sequence of avian hepatitis E virus (avian HEV) and attempts to infect rhesus monkeys with avian HEV. J. Gen. Virol. 2004;85:1609–1618. doi: 10.1099/vir.0.79841-0. [DOI] [PubMed] [Google Scholar]
- Jameel S. Molecular biology and pathogenesis of hepatitis E virus. Expert Rev Mol. Med. 1999;1999:1–16. doi: 10.1017/S1462399499001271. [DOI] [PubMed] [Google Scholar]
- Khuroo MS, Kamili S, Yattoo GN. Hepatitis E virus infection may be transmitted through blood transfusions in an endemic area. J. Gastroenterol. Hepatol. 2004;19:778–784. doi: 10.1111/j.1440-1746.2004.03437.x. [DOI] [PubMed] [Google Scholar]
- Lumeij JT. Avian clinical biochemistry. In: Kaneko JJ, Harvey JW, Bruss ML, editors. Clinical biochemistry of domestic animals. 5 ed. London, United Kingdom: Academic Press; 1997. pp. 857–883. [Google Scholar]
- Matsubayashi K, Nagaoka Y, Sakata H, Sato S, Fukai K, Kato T, Takahashi K, Mishiro S, Imai M, Takeda N, Ikeda H. Transfusion-transmitted hepatitis E caused by apparently indigenous hepatitis E virus strain in Hokkaido, Japan. Transfusion. 2004;44:934–940. doi: 10.1111/j.1537-2995.2004.03300.x. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Okada K, Takahashi K, Mishiro S. Severe hepatitis E virus infection after ingestion of uncooked liver from a wild boar. J. Infect. Dis. 2003;188:944. doi: 10.1086/378074. [DOI] [PubMed] [Google Scholar]
- Meng XJ, Purcel RH, Halbur PG, Lehman JR, Webb DM, Tsareva TS, Haynes JS, Thacker BJ, Emerson SU. A novel virus in swine is closely related to the human hepatitis E virus. Proc. Natl. Acad. Sci. USA. 1997;94:9860–9865. doi: 10.1073/pnas.94.18.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XJ, Halbur PG, Shapiro MS, Govindarajan S, Bruna JD, Mushahwar IK, Purcell RH, Emerson SU. Genetic and experimental evidence for cross-species infection by swine hepatitis E virus. J. Virol. 1998;72:9714–9721. doi: 10.1128/jvi.72.12.9714-9721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XJ. Novel strains of hepatitis E virus identified from humans and other animal species: is hepatitis E a zoonosis? J. Hepatol. 2000;33:842–845. doi: 10.1016/s0168-8278(00)80319-0. [DOI] [PubMed] [Google Scholar]
- Meng XJ. Swine hepatitis E virus: cross-species infection and risk in xenotransplantation. Curr. Top. Microbiol. Immunol. 2003;278:185–216. doi: 10.1007/978-3-642-55541-1_7. [DOI] [PubMed] [Google Scholar]
- Meng XJ. Hepatitis E as a zoonosis. Viral Hepatitis. In: Thomas H, Zuckermann A, Lemon S, editors. 3rd edition. Oxford, U.K: Blackwell Publishing Ltd; 2006. pp. 611–623. [Google Scholar]
- Meng XJ, Shivaprasad HL, Payne C. Hepatitis E virus infections. In: Saif M, editor. Diseases of Poultry. 12th Edition. Blackwell Publishing Press; 2008. pp. 443–452. [Google Scholar]
- Meng XJ. Hepatitis E virus: Animal reservoirs and zoonotic risk. Vet Microbiol. 2009 Mar 20; doi: 10.1016/j.vetmic.2009.03.017. 2009. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa T, Takahashi M, Mizuo H, Miyajima H, Gotanda Y, Okamoto H. Characterization of Japanese swine and human hepatitis E virus isolates of genotype IV with 99 % identity over the entire genome. J. Gen. Virol. 2003;84:1245–1251. doi: 10.1099/vir.0.19052-0. [DOI] [PubMed] [Google Scholar]
- Payne CJ, Ellis TM, Plant SL, Gregory AR, Wilcox GE. Sequence data suggests big liver and spleen disease virus (BLSV) is genetically related to hepatitis E virus. Vet, Microbiol. 1999;68:119–125. doi: 10.1016/s0378-1135(99)00067-x. [DOI] [PubMed] [Google Scholar]
- Peralta B, Biarnés M, Ordóñez G, Porta R, Martín M, Mateu E, Pina S, Meng XJ. Evidence of widespread infection of avian hepatitis E virus (avian HEV) in chickens from Spain. Vet Microbiol. 2008 Dec 13; doi: 10.1016/j.vetmic.2008.12.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Purcell RH, Emerson SU. Hepatitis E: an emerging awareness of an old disease. J. Hepatol. 2008;48:494–503. doi: 10.1016/j.jhep.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Ritchie SJ, Riddell C. “Hepatitis-splenomegaly” syndrome in commercial egg laying hens. Can. Vet. J. 1991;32:500–501. [PMC free article] [PubMed] [Google Scholar]
- Schlauder GG, Mushahwar IK. Genetic heterogeneity of hepatitis E virus. J. Med. Virol. 2001;65:282–292. doi: 10.1002/jmv.2031. [DOI] [PubMed] [Google Scholar]
- Sun ZF, Larsen CT, Dunlop A, Huang FF, Pierson FW, Toth TE, Meng XJ. Genetic identification of avian hepatitis E virus (HEV) from healthy chicken flocks and characterization of the capsid gene of 14 avian HEV isolates from chickens with hepatitis-splenomegaly syndrome in different geographical regions of the United States. J. Gen. Virol. 2004a;85:693–700. doi: 10.1099/vir.0.19582-0. [DOI] [PubMed] [Google Scholar]
- Sun ZF, Larsen CT, Huang FF, Billam P, Pierson FW, Toth TE, Meng XJ. Generation and infectivity titration of an infectious stock of avian hepatitis E virus (HEV) in chickens and cross-species infection of turkeys with avian HEV. J. Clin. Microbiol. 2004b;42:2658–2662. doi: 10.1128/JCM.42.6.2658-2662.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Nishizawa T, Miyajima H, Gotanda Y, Iita T, Tsuda F, Okamoto H. Swine hepatitis E virus strains in Japan form four phylogenetic clusters comparable with those of Japanese isolates of human hepatitis E virus. J. Gen. Virol. 2003a;84:851–862. doi: 10.1099/vir.0.18918-0. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Nishizawa T, Okamoto H. Identification of a genotype III swine hepatitis E virus that was isolated from a Japanese pig born in 1990 and that is most closely related to Japanese isolates of human hepatitis E virus. J. Clin. Microbiol. 2003b;41:1342–1343. doi: 10.1128/JCM.41.3.1342-1343.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tei S, Kitajima N, Takahashi K, Mishiro S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet. 2003;362:371–373. doi: 10.1016/S0140-6736(03)14025-1. [DOI] [PubMed] [Google Scholar]
- Tsarev SA, Tsareva TS, Emerson SU, Yarbough PO, Legters LJ, Moskal T, Purcell RH. Infectivity titration of a prototype strain of hepatitis E virus in cynomolgus monkeys. J. Med. Virol. 1994;43:135–142. doi: 10.1002/jmv.1890430207. [DOI] [PubMed] [Google Scholar]
- van der Poel WH, Verschoor F, van der Heide R, Herrera MI, Vivo A, Kooreman M, de Roda Husman AM. Hepatitis E virus sequences in swine related to sequences in humans, The Netherlands. Emerg. Infect. Dis. 2001;7:970–976. doi: 10.3201/eid0706.010608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Levine DF, Bendall RP, Teo CG, Harrison TJ. Partial sequence analysis of indigenous hepatitis E virus isolated in the United Kingdom. J. Med. Virol. 2001;65:706–709. doi: 10.1002/jmv.2094. [DOI] [PubMed] [Google Scholar]
- Wang YC, Zhang HY, Xia NS, Peng G, Lan HY, Zhuang H, Zhu YH, Li SW, Tian KG, Gu WJ, Lin JX, Wu X, Li HM, Harrison TJ. Prevalence, isolation, and partial sequence analysis of hepatitis E virus from domestic animals in China. J. Med. Virol. 2002;67:516–521. doi: 10.1002/jmv.10131. [DOI] [PubMed] [Google Scholar]
- Williams TP, Kasorndorkbua C, Halbur PG, Haqshenas G, Guenette DK, Toth TE, Meng XJ. Evidence of extrahepatic sites of replication of the hepatitis E virus in a swine model. J. Clin. Microbiol. 2001;39:3040–3046. doi: 10.1128/JCM.39.9.3040-3046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worm HC, van der Poel WH, Brandstätter G. Hepatitis E: an overview. Microbes Infect. 2002;4:657–666. doi: 10.1016/s1286-4579(02)01584-8. [DOI] [PubMed] [Google Scholar]
- Yazaki Y, Mizuo H, Takahashi M, Nishizawa T, Sasaki N, Gotanda Y, Okamoto H. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J. Gen. Virol. 2003;84:2351–2357. doi: 10.1099/vir.0.19242-0. [DOI] [PubMed] [Google Scholar]
- Zhou EM, Guo H, Huang FF, Sun ZF, Meng XJ. Identification of two neutralization epitopes on the capsid protein of avian hepatitis E virus. J. Gen. Virol. 2008;89:500–508. doi: 10.1099/vir.0.83366-0. [DOI] [PubMed] [Google Scholar]