Abstract
Numerous studies have shown that biochemical and behavioral effects of cocaine are mediated by dopamine D1 receptor (D1R) and NMDA R1 receptor (NR1)-mediated transmissions. In this study, we investigated the physical interactions between D1R and NR1 in response to acute cocaine administration in a time course of 5 minutes to 60 minutes. In the caudate-putamen (CPu) of male Fischer rats, a single cocaine injection (30 mg/kg) reduced D1R-NR1 protein-protein interactions 30 minutes after treatment. In addition, activation or blockade of the NMDA receptor using NMDA (25 mg/kg) or MK-801 (0.25 mg/kg), respectively, also reduced the D1R-NR1 physical interactions. Acute cocaine administration did not alter total D1R or NR1 protein levels in our time course of study. These results indicate that D1R-NR1 physical interaction rather than total protein levels may regulate the intracellular signaling after acute cocaine administration.
1. Introduction
Cocaine, a psychostimulant, is one of the most widely abused drugs in Western countries. By acting as an indirect dopamine agonist, cocaine increases dopamine as well as glutamate release in the striatum [2,19,23,27,33,35,45]. At the receptor level, pharmacological or genetic manipulation of D1R and NMDA receptors attenuates acute cocaine-induced locomotor activity [18,20,32,41,48], reinforcing and rewarding effects of cocaine [1,17,30,32], and intracellular signaling alteration (e.g., the phosphorylation of extracellular signal-regulated kinase (ERK) and cyclic AMP response element-binding protein) in response to acute cocaine in the dorsal striatum/CPu [18,20,42,49]. Taken together, these findings indicate that both D1R and NMDA receptors are involved in cocaine-induced behavioral and biochemical changes. Although repeated cocaine exposure alters both D1R binding density in the striatum and NR1 mRNA/protein levels in the striatum and ventral tegmental area (VTA), acute cocaine administration does not change D1R binding or NR1 protein levels [4,12,14,21,22,39,40]. Therefore, how acute cocaine administration regulates D1R and NR1 protein levels remains ambiguous.
One mechanism may involve in D1R and NR1 communication via protein-protein interactions at their carboxyl terminal regions [10,24,25,31]. In the primary hippocampal neurons or dopamine D1 and NR1 co-transfected cell lines, the stimulation of D1R results in a dissociation of the receptors [25]. The activation of NMDA receptor enhances D1R cAMP accumulation and recruits D1R membrane surface insertion in a D1R/NR1 physical protein-protein interactions manner [31]. Taken together, it suggests that dopamine and glutamate levels modulate the physical interaction between D1R and NR1 receptors. The present study was designed to evaluate the effects of acute cocaine and NMDA receptor activation and blockade on NR1 and D1R interactions in the dorsal striatum.
2. Methods
Animals
60-day-old male Fischer rats (Charles River, Raleigh, NC) were individually housed in Plexiglas chambers (20 × 20 × 41 cm) and maintained on a 12-hour light/dark cycle (lights on at 9:00 a.m.) with free access to food and water. Animal care and use was in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication 85-23, Bethesda, MD) and approved by the Institutional Animal Care and Use Committee of Hunter College.
Drugs and antibodies
Cocaine hydrochloride, NMDA and MK-801 were purchased from Sigma Chemical Co. (St. Louis, MO). Antibody for D1R was purchased from Chemicon International (Temecula, CA). Mouse monoclonal antibody for NR1 was purchased form BD Pharmingen (San Diego, CA). α-tubulin antibody and protein A/G agarose were from Santa Cruz Technologies (Santa Cruz, CA). Both horseradish peroxidase-conjugated anti-rabbit IgG and anti-mouse IgG were purchased from Amersham Pharmacia (Piscataway, NJ).
Drugs administration
For cocaine time course study, cocaine solutions were prepared by dissolution in physiological saline (0.9%) and injected intra-peritoneally (i.p.). Rats were injected with saline (1 ml/kg) or cocaine (30 mg/kg) and sacrificed 5, 15, 30 or 60 min later. A separate cohort of rats were injected with saline, NMDA (25 mg/kg, i.p.) or MK-801 (0.25 mg/kg, i.p) and sacrificed 30 min later.
Protein preparation and measurement
After decapitation (following a brief 20 s exposure to CO2), rat brains were removed, flash frozen in 2-methylbutane (-40° C), and stored at -80° C until used. The coronal slices (1mm thick) were cut out in a matrix (ASI instruments, Warren, MI). CPu was dissected out on a cold glass plate, and then homogenized using a Polytron handheld homogenizer (Kinematica, Luzern, Switzerland) in a lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 10% Glycerol, 1% Triton X-100, 1% Igepal CA-630, 1% sodium deoxycholic acid) containing phosphatase inhibitors mixture. After 30 min incubation, homogenates were centrifuged at 13,000 rpm for 15 min at 4° C. Supernatants were then collected and stored at -80° C until used. Total protein content was determined using a Bradford kit from Bio-Rad Laboratories (Hercules, CA).
Western blot analysis
Protein samples were analyzed as previously described [18]. Briefly, 40 μg of proteins were boiled in Lammeli buffer containing 1% β-mercaptoethanol for 5 min and separated on 10% SDS-PAGE gels, then transferred to PVDF membranes. Membranes were blocked with 5% nonfat dry milk for 1hr at room temperature and incubated with antibodies for D1R (1:1000) or NR1 (1:1000) overnight at 4° C. After three washes with Tris-Tween-20 Buffer (TBST; pH = 7.4), membranes were incubated with their appropriate secondary antibodies (1:1000) for 1hr at room temperature followed by three more washes with TBST. Antibody binding was detected using an enhanced chemiluminescence kit (ECL; Amersham Pharmacia, Piscataway, NJ). Intensity of protein bands was quantified with a computer densitometer and Image Quant Program (Molecular Dynamics). For normalization of protein levels, all membranes were re-probed with α-tubulin antibody (1:1000).
Immunoprecipitation analysis
For immunoprecipitation experiments, equal amount of protein extracts (500μg) were incubated in lysis buffer containing protease inhibitors mixture with anti-NR1 or anti-D1R primary antibody (1:50-100) overnight at 4° C, followed by the addition of protein A/G agarose (20 μl) for 4hrs. For control samples, protein extracts were incubated in absence of primary antibody. Beads were then washed four times in the lysis buffer. After final centrifugation, Lammeli buffer containing 1% β-mercaptoethanol (50-70 μl) was added and samples were boiled for 5 min. Equal amount of supernatants were loaded on 8% SDS-PAGE gels and the intensity of D1R or NR1 were analyzed via Western blot. Each sample for immunoprecipitation using anti-D1R antibody to pull down was performed three times to confirm the conclusion.
Statistical analysis
Protein levels were expressed as a ratio to α-tubulin levels in Western blot analysis. For immunoprecipitation analysis, NR1 protein levels were expressed as a ratio over optic density of total NR1 levels obtained from Western blot experiments. Data was expressed as mean % ± SEM relative to respective saline controls, which were arbitrarily set at 100%. Student’s t-tests were used to determine differences between cocaine- and saline-treated animals at each time point. To determine effects of NMDA and MK-801 administration on receptors physical protein-protein interactions, a one-way ANOVA was used followed by LSD post hoc analysis when appropriate. Differences were considered significant at 0.05 level.
3. Results
Effects of acute cocaine on NR1 and D1R interactions
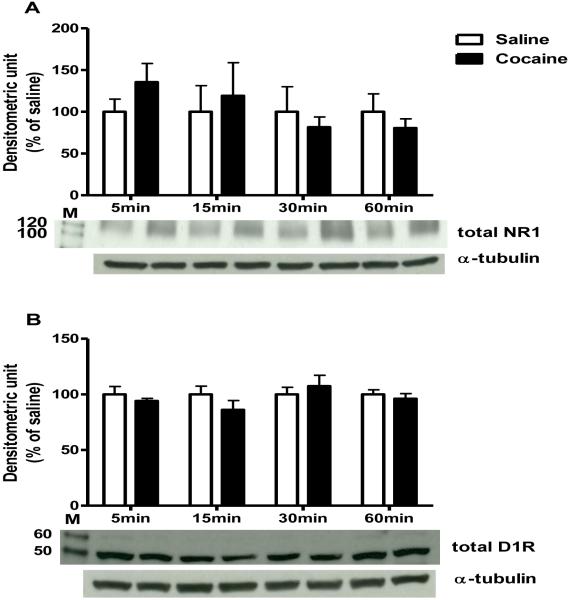
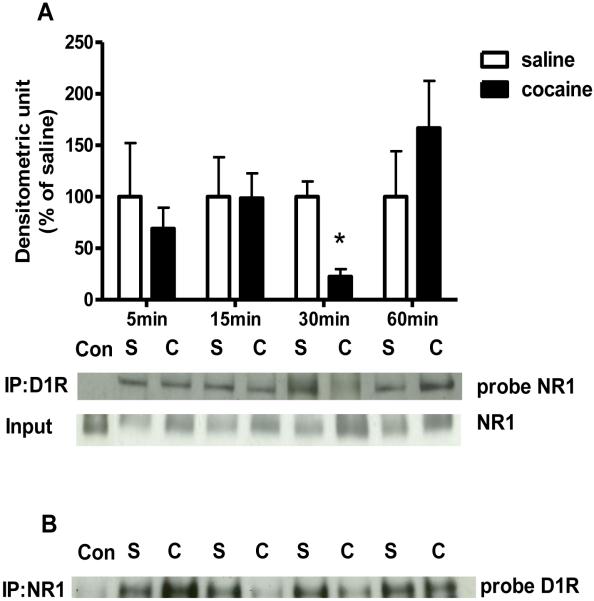
As shown in Figure 1, acute cocaine administration did not alter total D1R or NR1 protein levels in the CPu. Immunoprecipitation analysis however, indicated that 30 min after a single cocaine administration NR1 and D1R interactions were decreased as compared to the respective saline control [t (4) = 4.69, P< 0.01; Fig. 2A]. This data was confirmed using a reciprocal immunoprecipitation approach: immunoprecipitation using NR1 antibody followed by probing with D1R antibody (Fig 2B).
Figure 1. Time course of cocaine effects on total NR1 and D1R in the CPu.
(A) NR1; (B) D1R. Total protein levels after 5, 15, 30, or 60 min of cocaine administration over α-tubulin levels, expressed as percentage of saline control (4-6 animals per group). M is the molecular marker in kDa.
Figure 2. Time course of cocaine effects on NR1 and D1R physical interactions in the CPu.
(A) NR1 protein levels after D1R antibody immunoprecipitation. Data shown is the protein level over total NR1 level, expressed as percentage of saline control after 5, 15, 30, or 60 min of cocaine injections (3-6 animals per group). *p <0.05 as compared with respective saline group. (B) A reciprocal immunoprecipitation showing D1R protein levels after NR1 antibody pull down (Con = Control; S = Saline; C = Cocaine).
Effects of NMDA and MK-801 administration on NR1 and D1R interactions
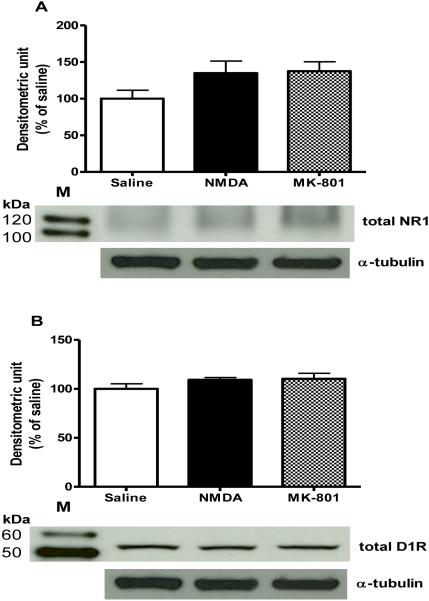
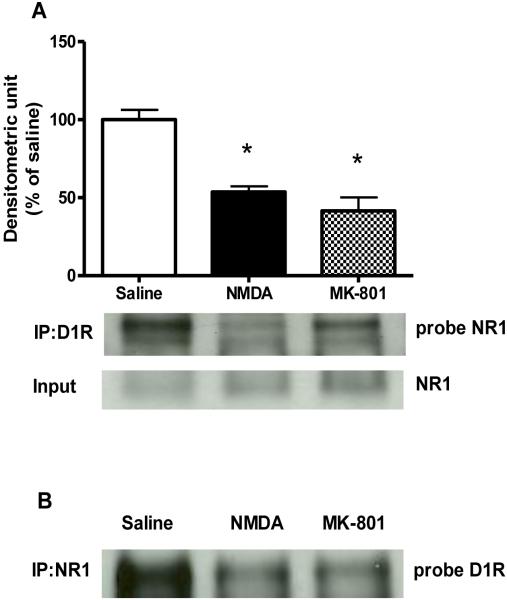
Similar to acute cocaine, a single NMDA or MK-801 injection did not change the total protein levels of NR1 subunit or D1R [Fig. 3A and B, respectively]. However, immunoprecipitation experiment showed that there was a significant treatment effect on receptors interactions [F (2, 6) = 22.23, P<0.05; Fig. 4A]. Further, post hoc test revealed that NMDA and MK-801 treatments significantly reduced NR1 and D1R interactions as compared to saline-treated animals [P<0.01 and P<0.01, respectively]. There were no differences between NMDA- and MK-801-treated groups. This data was also confirmed using a reciprocal immunoprecipitation approach: immunoprecipitation using NR1 antibody followed by probing with D1R antibody (Fig 4B).
Figure 3. Effects of NMDA and MK-801 administration on total NR1 and D1R in the CPu.
(A) NR1; (B) D1R. Results represent total protein levels over α-tubulin levels, expressed as percentage of saline contro after 30 min of saline, NMDA or MK-801 injections (4-6 animals per group). M is the molecular marker in kDa.
Figure 4. Effects of NMDA and MK-801 administration on NR1 and D1R physical interactions in the CPu.
(A) NR1 protein levels in D1R antibody pull down. Results represent protein levels over total NR1 levels, expressed as percentage of saline control, after 30 min of saline, NMDA or MK-801 injections (3 animals per group). *p <0.05 as compared with saline control. (B) A reciprocal immunoprecipitation showing D1a protein levels after NR1 antibody pull down.
4. Discussion
Results presented here demonstrated that acute cocaine administration time-dependently reduced NR1 and D1R protein-protein interactions in the CPu. In line with previous studies we also showed that acute cocaine did not change total protein levels of NR1 or D1R [12,39], suggesting the reduction of physical interactions between theses receptors is not due to total proteins alteration. In addition, after NMDA or MK-801 treatment the association between NR1 and D1R was also decreased. Thus, although acute regulation of D1R or NR1 receptors does not change their total protein levels, it disrupts their physical interactions and thus may represent as an important step in initial cocaine-induced intracellular responses.
Several in vitro studies indicated a complex interaction between NR1and D1R. For example, in hippocampal and striatal cultures, the stimulation of D1R and PKA resulted in the phosphorylation of NR1 subunits, increased in cytosolic calcium and phosphorylation of ERK proteins [3,5,26,38,43,47]. In the cultured striatal neurons or the VTA in vivo, D1R agonists or a single cocaine administration induced a rapid NR1 subunits postsynaptic membrane insertion [8,34]. The membrane insertion of NR1 subunits may contribute to the potentiation of NMDA receptor-mediated current by D1R. However, Lee et al [25] showed that the activation of D1R receptor by dopamine application disrupted the NR1/D1R physical interactions and reduced the NMDA receptor-mediated calcium influx via NMDA-NR2A/D1R carboxyl terminal interactions. Previously, we have demonstrated that, in the CPu, a single cocaine administration (30 mg/kg) transiently induced ERK phosphorylation, which gradually returned to basal levels 30 min after injection [37]. In present study, although the functional significance of cocaine-induced dissociation of NR1/D1R interactions is still unknown, it is tempting to postulate that the dissociation of receptors interactions may act as a time-limited gating mechanism on ERK activation in response to acute cocaine: (1) a rapid ERK phosphorylation due to potentiated NMDA-mediated calcium influx and/or NR1 redistribution through cocaine-elevated extracellular dopamine levels and D1R activation; followed by (2) a deactivation of ERK protein because of the decrease in intracellular calcium via the disruption of NR1 and D1R interactions.
Alternatively, the NR1/D1R physical associations may regulate the D1R surface expression. Recently, NR1/D1R oligomerization was also found in cells co-transfected with D1R and NMDA receptors including NR1/NR2B subunits as well as in the striatal post-synaptic density (PSD) [10,11]. In the presence of the NMDA-NR2B subunit, the NR1/D1R complex was delivered to membrane surface and then prevented the D1R agonist-induced D1R internalization, suggesting these receptors interactions may represent a mechanism to recruit D1R to the striatal PSD [10,29]. However, in the present study, we demonstrated a decreasing of NR1 and D1R receptor physical interactions after acute cocaine. We propose two plausible explanations. First, evidence has shown that D1R stimulation results in NMDA receptor membrane insertion in the striatum [7,8]. Thus, acute cocaine-induced D1R activation, may recruit NMDA receptor to the membrane region. The newly inserted NMDA receptor may not physically associate with D1R immediately but form receptors interactions at later time point. This temporal change in receptors interactions is consistent with present results in that transient reduction of NR1/D1R interactions was only observed 30 min after acute cocaine administration. Second, a previous in vivo study has shown that a single intrastriatal D1R agonist infusion or amphetamine injection (i.p.) rapidly induced D1R internalization in the striatum [6]. The modification of D1R distribution was found in cell bodies and dendrites but not in dendritic spines at the periphery of synaptic clefts, where PSD is located [6]. Thus, after cocaine administration, the dissociation of NR1/D1R physical interactions may indicate D1R segregation and the reduction of receptor availability for extracellular ligands in a region-specific manner as suggested by Fiorentini and Missale [11]. However, further biochemical fractionation procedure and immunohistochemical labeling at the ultrastructural level should be conducted to examine cocaine-induced D1R internalization and the decrease of receptors interactions in different neuronal compartments.
In the CPu, we demonstrated that the NR1/D1R receptors physical interactions were decreased 30 min after MK-801 administration. Similarly, an in vitro study showed that NMDA receptor antagonist, AP-5, pre-application led to the dissociation between NR1 and D1R receptors in cells, suggesting that the NMDA receptor activation is necessary to maintain the protein-protein interactions between theses receptors [31]. In the striatum, a recent study showed that MK-801 (0.1 mg/kg) did not modify NR1 and D1R interactions [11]. However, a higher dose of MK-801 (0.25 mg/kg) was used in the present study. Previous studies have shown that the higher MK-801 (0.2-0.5 mg/kg) doses elevated extracellular dopamine levels in the striatum [28,44,46]. Thus, by increasing synaptic dopamine levels, MK-801 itself may reduce NR1 and D1R interactions (similar to acute cocaine administration). Surprisingly, NMDA treatment also reduced NR1/D1R physical interactions, suggesting that the activation of NMDA receptor also disrupts the receptors interactions. In cortical neurons, a study has demonstrated that chronic neuronal activity promotes the degradation of NR1 through ubiquitin-proteasome system [9]. However, in the present study, total NR1 protein levels were not altered after NMDA administration, indicating that the reduction in NR1/D1R physical interactions is not due to the decrease in NR1 levels. In hippocampal neurons, PKC-induced rapid NMDA receptors dispersal and lateral mobility between the synaptic and extra-synaptic domains have been documented [13,15]. NR2B-containing NMDA receptors exhibit faster mobility than those containing NR2A subunit [16]. In addition, NR2B-containing receptors are prominently expressed in the striatum [36]. Thus, through NMDA receptors, the NMDA treatment (25 mg/kg i.p.) may induce PKC activation and lateral mobility of NMDA receptors and then contribute to the decrease of NR1/D1R physical interactions.
In summary, the present study demonstrated that acute cocaine reduced the NR1 and D1R physical protein-protein interactions in a time-dependent manner. We postulate that the dissociation may regulate cocaine- and/or dopamine-mediated intracellular signaling cascades and D1R distribution underlying the cocaine-induced behavioral changes. In addition, we have also showed that the homeostatic state of NMDA receptor is necessary for the NR1/D1R physical interactions, since both NMDA receptor activation and inhibition reduced receptors associations. However, the functional significance of their receptors interactions on NR1- and D1R-mediated intracellular signaling transduction and addictive behaviors needs further elucidation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
We declare that there is no conflict of interest.
References
- [1].Caine SB, Thomsen M, Gabriel KI, Berkowitz JS, Gold LH, Koob GF, Tonegawa S, Zhang J, Xu M. Lack of self-administration of cocaine in dopamine D1 receptor knock-out mice. J. Neurosci. 2007;27:13140–13150. doi: 10.1523/JNEUROSCI.2284-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Carboni E, Imperato A, Perezzani L, Di Chiara G. Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience. 1989;28:653–661. doi: 10.1016/0306-4522(89)90012-2. [DOI] [PubMed] [Google Scholar]
- [3].Choe ES, McGinty JF. Cyclic AMP and mitogen activated protein kinases are required for glutamate dependent cyclic AMP response element binding protein and Elk-1 phosphorylation in the dorsal striatum in vivo. J. Neurochem. 2001;76:401–412. doi: 10.1046/j.1471-4159.2001.00051.x. [DOI] [PubMed] [Google Scholar]
- [4].Churchill L, Swanson CJ, Urbina M, Kalivas PW. Repeated cocaine alters glutamate receptor subunit levels in the nucleus accumbens and ventral tegmental area of rats that development behavioral sensitization. J. Neurochem. 1999;72:2397–2403. doi: 10.1046/j.1471-4159.1999.0722397.x. [DOI] [PubMed] [Google Scholar]
- [5].Dudman JT, Eaton ME, Rajadhyaksha A, Macias W, Taher M, Barczak A, Kameyama K, Huganir R, Konradi C. Dopamine D1 receptors mediate CREB phosphorylation via phosphorylating the NMDA receptor at Ser897-NR1. J. Neurochem. 2003;87:922–934. doi: 10.1046/j.1471-4159.2003.02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dumartin B, Caillé I, Gonon F, Bloch B. Internalization of D1 dopamine receptor in striatal neurons in vivo as evidence of activation by dopamine agonists. J. Neurosci. 1998;18:1650–1661. doi: 10.1523/JNEUROSCI.18-05-01650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dunah AW, Sirianni AC, Fienberg AA, Bastia E, Schwarzschild MA, Standaert DG. Dopamine D1-dependent trafficking of striatal N-methyl-D-aspartate glutamate receptors requires Fyn protein tyrosine kinase but not DARPP-32. Mol. Pharmacol. 2004;65:121–129. doi: 10.1124/mol.65.1.121. [DOI] [PubMed] [Google Scholar]
- [8].Dunah AW, Standaert DG. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J. Neurosci. 2001;21:5546–5558. doi: 10.1523/JNEUROSCI.21-15-05546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat. Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- [10].Fiorentini C, Gardoni F, Spano P, Di Luca M, Missale C. Regulation of dopamine D1 receptor trafficking and desensitization by oligomerization with glutamate N-methyl-D-aspartate receptors. J. Biol. Chem. 2003;278:20196–20202. doi: 10.1074/jbc.M213140200. [DOI] [PubMed] [Google Scholar]
- [11].Fiorentini C, Missale C. Oligomeric assembly of dopamine D1 and glutamate NMDA receptors: Molecular mechanisms and functional implications. Biochem. Soc. Trans. 2004;32:1025–1028. doi: 10.1042/BST0321025. [DOI] [PubMed] [Google Scholar]
- [12].Fitzgerald LW, Ortiz J, Hamedani AG, Nestler EJ. Drugs of abuse and stress increase the expression of GluR1 and NMDAR1 glutamate receptor subunits in the rat ventral tegmental area: Common adaptation among cross-sensitizing agents. J. Neurosci. 1996;16:274–282. doi: 10.1523/JNEUROSCI.16-01-00274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fong DK, Rao A, Crump FT, Craig AM. Rapid synaptic remodeling by protein kinase C: Reciprocal translocation of NMDA receptors and calcium/calmodulin dependent kinase II. J. Neurosci. 2002;22:2153–2164. doi: 10.1523/JNEUROSCI.22-06-02153.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ghasemzadeh MB, Nelson LC, Lu XY, Kalivas PW. Neuroadaptations in ionotropic and metabotropic glutamate receptor mRNA produced by cocaine treatment. J. Neurochem. 1999;72:157–165. doi: 10.1046/j.1471-4159.1999.0720157.x. [DOI] [PubMed] [Google Scholar]
- [15].Groc L, Heine M, Cognet L, Brickley K, Stephenson FA, Lounis B, Choquet D. Differential activity-dependent requlation of lateral mobilities of AMPA and NMDA receptors. Nat. Neurosci. 2004;7:695–696. doi: 10.1038/nn1270. [DOI] [PubMed] [Google Scholar]
- [16].Groc L, Heine M, Cousins SL, Stephenson FA, Lounis B, Cognet L, Choquet D. NMDA receptor surface mobility depends on NR2A-2B subunits. Proc. Natl. Acad. Sci. USA. 2006;103:18769–18774. doi: 10.1073/pnas.0605238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Heusner CL, Palmiter RD. Expression of mutant NMDA receptors in dopamine D1 receptor-containing cells prevent cocaine sensitization and decreases cocaine preference. J. Neurosci. 2005;25:6651–6657. doi: 10.1523/JNEUROSCI.1474-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jenab S, Festa ED, Nazarian A, Wu HB, Sun WL, Hazim R, Russo SJ, Quinones-Jenab V. Cocaine induction of ERK proteins in dorsal striatum of Fischer rats. Brain Res. Mol. Brain Res. 2005;142:134–138. doi: 10.1016/j.molbrainres.2005.08.015. [DOI] [PubMed] [Google Scholar]
- [19].Kalivas PW, Duffy P. Effects of daily cocaine and morphine treatment on somatodendritic and terminal field dopamine release. J. Neurochem. 1998;50:1498–1504. doi: 10.1111/j.1471-4159.1988.tb03036.x. [DOI] [PubMed] [Google Scholar]
- [20].Karasinska JM, George SR, Cheng R, O’Dowd BF. Deletion of dopamine D1 and D3 receptors differentially affects spontaneous behaviour and cocaine-induced locomotor activity, reward and CREB phosphorylation. Eur. J. Neurosci. 2005;22:1741–1750. doi: 10.1111/j.1460-9568.2005.04353.x. [DOI] [PubMed] [Google Scholar]
- [21].Kleven MS, Perry BD, Woolverton WL, Seiden LS. Effects of repeated injections of cocaine on D1 and D2 dopamine receptors in rat brain. Brain Res. 1990;532:265–270. doi: 10.1016/0006-8993(90)91768-c. [DOI] [PubMed] [Google Scholar]
- [22].Laurier LG, Corrigall WA, George SR. Dopamine receptor density, sensitivity, and mRNA levels are altered following self-administration of cocaine in the rat. Brain Res. 1994;634:31–40. doi: 10.1016/0006-8993(94)90255-0. [DOI] [PubMed] [Google Scholar]
- [23].Lee DK, Bian S, Rahman MA, Shim YB, Shim I, Choe ES. Repeated cocaine administration increases N-methyl-d-asparate NR-1 subunit, extracellular signal-regulated kinase and cyclic AMP response element-binding protein phosphorylation and glutamate release in the rat dorsal striatum. Eur. J. Pharmacol. 2008;590:157–162. doi: 10.1016/j.ejphar.2008.06.048. [DOI] [PubMed] [Google Scholar]
- [24].Lee FJ, Liu F. Direct interactions between NMDA and D1 receptors: A tale of tails. Biochem. Soc. Trans. 2004;32:1032–1036. doi: 10.1042/BST0321032. [DOI] [PubMed] [Google Scholar]
- [25].Lee FJ, Xue S, Pei L, Vukusic B, Nadege C, Wang YS, Wang YT, Niznik HB, Yu XM, Liu F. Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell. 2002;111:219–230. doi: 10.1016/s0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- [26].Leveque JC, Macias W, Rajahyaksha A, Carlson RR, Barczak A, Kang IS, Li XM, Coyle JT, Huganir R, Heckers S, Konradi C. Intracellular modulation of NMDA receptor function by antipsychotic drugs. J. Neurosci. 2000;20:807–820. doi: 10.1523/JNEUROSCI.20-11-04011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Maisonneuve IM, Kreek MJ. Acute tolerance to the dopamine response induced by a binge pattern of cocaine administration in male rats: An in vivo microdialysis study. J. Pharmacol. Exp. Ther. 1994;268:916–921. [PubMed] [Google Scholar]
- [28].Mathé JM, Nomikos GG, Hildebrand BE, Hertel P, Svensson TH. Prazosin inhibits MK-801-induced hyperlocomotion and dopamine release in the nucleus accumbens. Eur. J. Pharmacol. 1996;309:1–11. doi: 10.1016/0014-2999(96)00315-9. [DOI] [PubMed] [Google Scholar]
- [29].Missale C, Fiorentini C, Busi C, Collo G, Spano PF. The NMDA/D1 receptor complex as a new target in drug development. Curr. Top. Med. Chem. 2006;6:801–808. doi: 10.2174/156802606777057562. [DOI] [PubMed] [Google Scholar]
- [30].Nazarian A, Russo SJ, Festa ED, Kraish M, Quinones-Jenab V. The role of D1 and D2 receptors in the cocaine conditioned place preference of male and female rats. Brain Res. Bull. 2004;63:295–299. doi: 10.1016/j.brainresbull.2004.03.004. [DOI] [PubMed] [Google Scholar]
- [31].Pei L, Lee FJS, Moszczynska A, Vukusic B, Liu F. Regulation of dopamine D1 receptor function by physical interaction with NMDA receptors. J. Neurosci. 2004;24:1149–1158. doi: 10.1523/JNEUROSCI.3922-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ramsey AJ, Laakso A, Cyr M, Sotnikova TD, Salahpour A, Medvedev IO, Dykstra LA, Gainetdinov RR, Caron MG. Genetic NMDA receptor deficiency disrupts acute and chronic effects of cocaine but not amphetamine. Neuropsychopharmacology. 2008;33:2701–2714. doi: 10.1038/sj.npp.1301663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Reith ME, Li MY, Yan QS. Extracellular dopamine, norepinephrine and serotonin in the ventral tegmental area and nucleus accumbens of freely moving rats during intracerebral dialysis following systemic administration of cocaine and other uptake blockers. Psychopharmacology. 1997;134:309–317. doi: 10.1007/s002130050454. [DOI] [PubMed] [Google Scholar]
- [34].Schilström B, Yaka R, Argilli E, Suvarna N, Schumann J, Chen BT, Carman M, Singh V, Mailliard WS, Ron D, Bonci A. Cocaine enhances NMDA receptor-mediated currents in ventral tegmental area cells via dopamine D5 receptor-dependent redistribution of NMDA receptors. J. Neurosci. 2006;26:8549–8558. doi: 10.1523/JNEUROSCI.5179-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shin EH, Bain S, Shim YB, Rahman MA, Chung KT, Kim JY, Wang JQ, Choe ES. Cocaine increases endoplasmic reticulum stress protein expression in striatal neurons. Neuroscience. 2007;145:621–630. doi: 10.1016/j.neuroscience.2006.12.013. [DOI] [PubMed] [Google Scholar]
- [36].Standaert DG, Testa CM, Young AB, Penney JB., Jr. Organization of N-methyl-D-aspartate glutamate receptor gene expression in the basal ganglia of the rat. J. Comp. Neurol. 1994;343:1–16. doi: 10.1002/cne.903430102. [DOI] [PubMed] [Google Scholar]
- [37].Sun WL, Zhou L, Hazim R, Quinones-Jenab V, Jenab S. Effects of dopamine and NMDA receptors on cocaine-induced Fos expression in the striatum of Fischer rats. Brain Res. 2008;1243:1–9. doi: 10.1016/j.brainres.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sweatt JD. The neuronal MAP kinase cascade: A biochemical signal integration system subserving synaptic plasticity and memory. J. Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- [39].Tsukada H, Kreuter J, Maggos CE, Unterwald EM, Kakiuchi T, Nishiyama S, Futatsubashi M, Kreek MJ. Effect of binge pattern cocaine administration on dopamine D1 and D2 receptors in the rat brain: An in vivo study using positron emission tomography. J. Neurosci. 1996;16:7670–7677. doi: 10.1523/JNEUROSCI.16-23-07670.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Unterwald EM, Ho A, Rubenfeld JM, Kreek MJ. Time course of the development of behavioral sensitization and dopamine receptor up-regulation during binge cocaine administration. J. Pharmacol. Exp. Ther. 1994;270:1387–1396. [PubMed] [Google Scholar]
- [41].Uzbay IT, Wallis CJ, Lal H, Forster MJ. Effects of NMDA receptor blockers on cocaine-stimulated locomotor activity in mice. Behav. Brain Res. 2000;108:57–61. doi: 10.1016/s0166-4328(99)00129-1. [DOI] [PubMed] [Google Scholar]
- [42].Valjent E, Corvol JC, Pages C, Besson JM, Maldanado R, Caboche J. Involvement of the extracellular signal regulated kinase cascade for cocaine rewarding properties. J. Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Vanhoutte P, Barnier JV, Guibert B, Pages C, Besson ML, Hipskind RA, Caboche J. Glutamate induces phosphorylation of Elk-1 and CREB along with c-fos activation via an extrecellular signal regulated kinase dependent pathway in brain slices. Mol. Cell. Biol. 1999;19:136–146. doi: 10.1128/mcb.19.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wedzony K, Klimek V, Golembiowska K. MK-801 elevates the extracellular concentration of dopamine in the rat prefrontal cortex and increases the density of striatal dopamine D1 receptors. Brain Res. 1993;622:325–329. doi: 10.1016/0006-8993(93)90839-f. [DOI] [PubMed] [Google Scholar]
- [45].Williams JM, Steketee JD. Cocaine increases medical prefrontal cortical glutamate overflow in cocaine-sensitized rats: A time course study. Eru. J. Neurosci. 2004;20:1639–1649. doi: 10.1111/j.1460-9568.2004.03618.x. [DOI] [PubMed] [Google Scholar]
- [46].Wolf ME, White FJ, Hu XT. Behavioral sensitization to MK-801 (dizocilpine): Neurochemical and electrophysiological correlates in the mesoaccumbens dopamine system. Behav. Pharmacol. 1993;4:429–442. [PubMed] [Google Scholar]
- [47].Xia Z, Dudek H, Miranti CK, Greenberg ME. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase ERK dependent mechanism. J. Neurosci. 1996;16:5425–5436. doi: 10.1523/JNEUROSCI.16-17-05425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Xu M, Hu XT, Cooper DC, Moratalla R, Tonegawa S. Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell. 1994;79:945–955. doi: 10.1016/0092-8674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- [49].Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, Zhang J, Xu M. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by dopamine D1 and D3 receptors. J. Neurosci. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]