Abstract
We used event-related functional magnetic resonance imaging (fMRI) to determine blood-oxygen-level-dependent (BOLD) signal changes following microsaccades, visually-guided saccades, and eyeblinks in retinotopically mapped visual cortical areas V1–V3 and hMT+. A deconvolution analysis revealed a similar pattern of BOLD activation following a microsaccade, 0.16° voluntary saccade, and 0.16° displacement of the image under conditions of fixation. In all areas, an initial increase in BOLD signal peaking at approximately 4.5 seconds after the event was followed by a decline and decrease below baseline. This modulation appears most pronounced for microsaccades and small voluntary saccades in V1, diminishing in strength from V1 to V3. In contrast, 0.16 degree real motion under conditions of fixation yields the same level of BOLD signal increase in V1 through V3. BOLD signal modulates parametrically with the size of voluntary saccades (0.16°, 0.38°, 0.82°, 1.64°, and 3.28°) in V1–V3, but not in hMT+. Eyeblinks generate larger modulation that peaks by 6.5 seconds, and dips below baseline by 10 seconds post-event, and also exhibits diminishing modulation from V1 to V3. Our results are consistent with the occurrence of transient neural excitation driven by changes in input to retinal ganglion cell receptive fields that are induced by microsaccades, visually-guided saccades, or small image shifts. The pattern of results in area hMT+ exhibits no significant modulation by microsaccades, relatively small modulation by eyeblinks, and substantial responses to saccades and background jumps, suggesting that spurious image motion signal arising from microsaccades and eyeblinks is relatively diminished by hMT+.
Introduction
The goal of the present research is to determine the neural/fMRI effects of microsaccades in early cortical visual areas. We compare these responses to those evoked by comparably sized saccades and whole image motions under conditions of fixation. All three event types should generate similar changes at the level of the retinal image. By comparing cortical responses to these three event types, our aim is to determine the degree to which cortical responses to microsaccades are driven by image changes versus other factors, such as, for example, microsaccadic suppression of spurious motion signals.
Microsaccades are involuntary, conjugate, brief (~25ms), very small eye movements that occur during visual fixation, whereas saccades, even if as small as microsaccades, are voluntary. Microsaccades shift the retinal image over several dozen (Moller et al., 2002; Yarbus, 1967; Ditchburn and Ginsbourg, 1953; Lord, 1951; Ratliff and Riggs, 1950) to several hundred (Martinez-Conde et al., 2006) photoreceptors. They exhibit the same linear relationship between peak velocity and amplitude as voluntary and corrective saccades (Martinez-Conde et al., 2006; Zuber and Stark, 1965; Wurtz, 1996). Because voluntary saccades can be as small in magnitude as microsaccades, it is not primarily size that is the defining characteristic of microsaccades, but rather their largely involuntary nature1, which itself may indicate a subcortical origin for microsaccades.
These observations suggest that microsaccades, voluntary, and corrective saccades may be generated by at least partially overlapping circuitry that drives saccade-triggering burst neurons in the superior colliculus (Zuber and Stark, 1965; Wurtz, 1996; Sparks, 2002). Recently, it was shown (Hafed, Goffart, and Krauzlis, 2009) that microsaccades and saccades are both generated by the same mechanism in the superior colliculus, and that microsaccades and saccades lie on the same continuum. This might seem to imply that microsaccades and saccades of equivalent magnitudes should generate the same responses in visual cortical areas. But one cannot infer from the finding that microsaccades and saccades share a partly common generation mechanism in the colliculus, that same-sized saccades and microsaccades must lead to the same type and level of activation in visual areas. For example, different cortical processes might both send commands to the same collicular neurons that then move the eyes.
We used high temporal resolution event-related fMRI to determine the blood oxygen level dependent (BOLD) signal changes in V1–V3, and hMT+ associated with microsaccades, eyeblinks, voluntary saccades to peripheral targets of varying amplitude (0.16, 0.38, 0.82, 1.64, and 3.28 degree), and 0.16 degree background image shifts, in an effort to determine whether these events trigger net neural excitation or inhibition in the regions of interest (ROIs) specified. Because microsaccades, small saccades, and small background image shifts generate comparable changes at the level of the retinal image, any differences in cortical neural activity would presumably result from differences in how these types of stimulus events are processed rather than from differences in the image change per se. This would require that the visual system rely on information other than the information available solely from the sequence of images, such as knowledge that a command to make a saccade or microsaccade has been given.
Materials and Methods
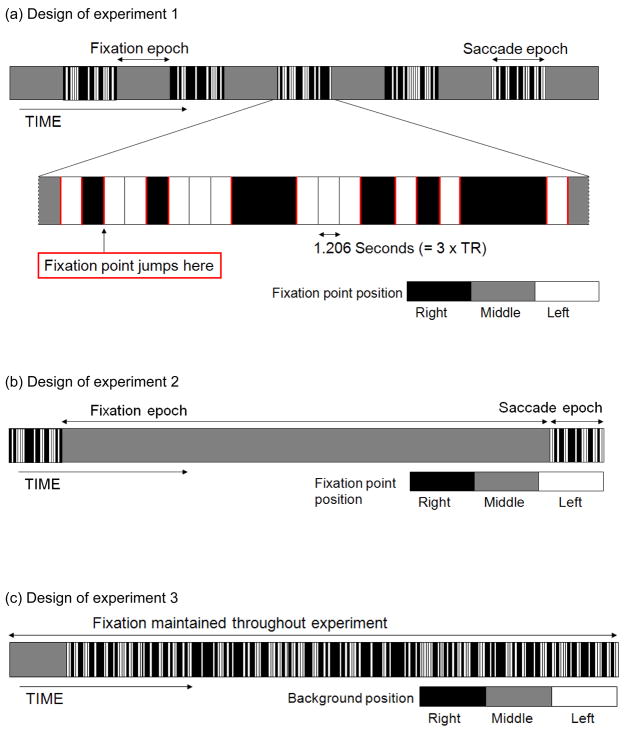
Three fMRI experiments were carried out. Experiment 1 was designed to measure BOLD signal activity as a function of voluntary saccade size. In this experiment the background remained stationary, while voluntary saccades were made by tracking the fixation spot as it changed position instantaneously, leading to jumps of 0.16, 0.38, 0.82, 1.64, and 3.28 visual degrees. Experiment 2 was designed to measure BOLD signal activity as a function of microsaccade occurrence. In this experiment the background remained stationary, and a run began and ended with a 30 second epoch where 0.16 visual degree voluntary saccades were made; However, during seven minutes in the middle of each run, the fixation point remained stationary, and subjects had to maintain fixation in order to facilitate the detection of microsaccades. In experiment 3, designed to measure BOLD signal activity as a function of actual image shifts comparable in size to those caused by microsaccades, the fixation point remained stable, and the background shifted position 0.16 visual degrees. This was done to mimic the image change that would occur upon a microsaccade or a small visually-guided saccade.
Participants
A total of 8 different participants (male n = 5) carried out one or more of the three experiments conducted in the MR-scanner (mean age = 32.3 yrs; SD = 6.8 yrs). All subjects had normal or corrected-to-normal vision. None showed evidence of visual and/or neurological abnormalities.
Functional MRI Acquisition
Experiment 1: Small voluntary saccades
fMRI data were collected in a high temporal resolution mixed event-related block design (3T Siemens Allegra scanner at the University of Regensburg Imaging Center, TR=402 ms, 7 slices along calcarine, TE=30ms, FA=35°, 800 volumes, n=6, 6–8 runs per subject, discarding approximately 10 seconds of scans to guarantee that proton spins were at steady-state). Each run lasted 5 minutes and 45 seconds. In blocks of small voluntary saccades (0.16, 0.38, 0.82, 1.64, and 3.28 visual degrees; 12 pseudorandom events per block; one saccade size was presented per block; 5 saccade blocks per run; 6 fixation blocks per run), while subjects were requested to steadily track a small point that jumped either leftwards or rightwards to locations symmetrical around the center of the screen. A displacement of the fixation point never took place more often than once every 3 TRs. The temporal order of the experimental design is shown in Figure 1a. The background polar grating was present throughout a run.
Figure 1.
Designs of the three experiments. Black/Gray/White indicate that the fixation point was in the Right/Middle/Left position respectively. In (a) and (b) a red vertical line indicates the temporal position of a fixation point jump, and in (c) it indicates the occurrence of a background jump.
Experiment 2: Microsaccades
fMRI data were collected in a high temporal resolution mixed event-related block design (T2*-weighted gradient echo planar imaging, TR=631ms, 11 slices along calcarine, TE=30ms, FA=35°, slice thickness 3mm, 0.3mm interslice gap, 800 volumes as in Exp. 1, n=5, 5–8 runs per subject, discarding approximately 10 seconds of scans to guarantee that proton spins were at steady-state). Each run lasted 8 minutes and 24 seconds. For 30 seconds at the beginning and end of each run, small voluntary saccades were initiated by discrete jumps in the fixation point (0.16°, 12 pseudorandom events per epoch). During voluntary saccade epochs, subjects maintained fixation on a small point that jumped 0.08° to the left and right of the central fixation point that was presented only during the fixation-only period. A displacement of the fixation point never took place more often than once every 3 TRs. The size 0.16° was chosen for voluntary saccades because this falls within the size range of involuntary microsaccades, and was detectable using our eye tracker and detection algorithm. A long fixation-only period of 7 minutes, 25 seconds separated these two saccade epochs, during which the fixation point stayed in the central position. Details of the temporal order of the experimental design are shown in Figure 1b.
Experiment 3: Image jumps under fixation
In this high temporal resolution event-related control experiment, the fixation point remained in the central location throughout the entire run (800 volumes, n=6, 6–8 runs per subject; TR=631ms, 11 slices along calcarine, TE = 30ms, FA=35°), and the polar checkerboard background jumped leftward and rightward 0.16 visual degrees. A background jump never took place more often than once every 3 TRs. The temporal sequence used in this experimental design is shown in Figure 1c.
Stimulus presentation
The stimulus for all three experiments was a 22×16 visual degree polar grating containing a one degree-wide strip, which divided the upper from the lower halves of the radial checkerboard image, as shown in Fig. 2. Subjects were required to fixate a black dot (0.05 visual degrees) centered along the white horizontal strip. In experiments 1 and 2 the background remained stationary and the fixation point changed position instantaneously (leading to 0.16, 0.38, 0.82, 1.64, and 3.28 visual degree horizontal image shifts) or remained stationary (during the middle seven minutes of each run of experiment 2), while in experiment 3 the fixation point remained stationary and the background instantaneously changed position by 0.16 visual degrees at pseudorandom times. Stimuli were presented using a VSG card from Cambridge Research Systems. The high contrast polar grating was chosen as the background in order to maximize the type of input known to drive activity in early visual areas. The white band was used so that the fixation point was always visible against a common background with a constant local contrast.
Figure 2.
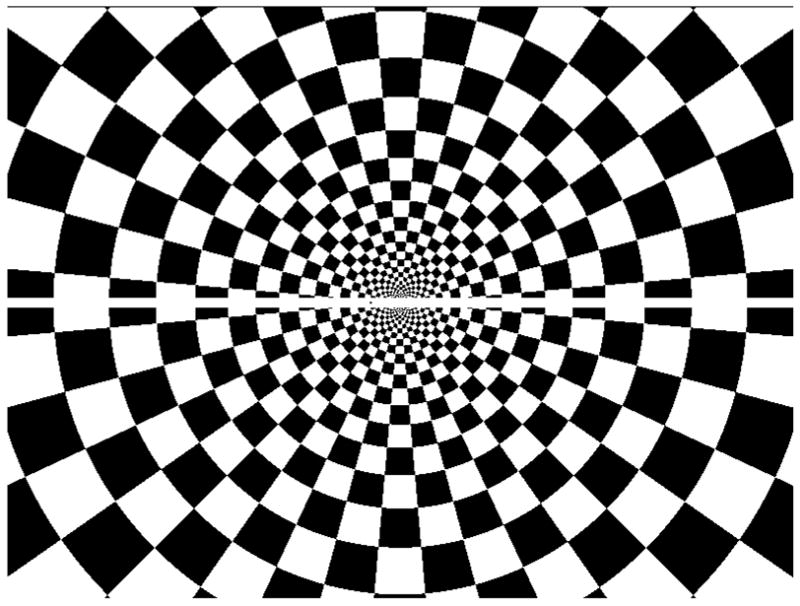
The stimulus was the same polar checkerboard in all three experiments. In experiment 1 the checkerboard was stationary and the fixation point jumped back and forth within the central horizontal white band 0.16, 0.38, 0.82, 1.64, and 3.28 visual degrees in a block design. In experiment 2 the checkerboard was stationary while the fixation point was placed on the central horizontal white band and remained stationary, except during the two 30s epochs at the beginning and end of each run, during which the fixation point, but not the checkerboard, alternated position pseudorandomly between 0.08 visual degrees to the left and 0.08 visual degrees to the right of the center for a 0.16 visual degree jump, in order to induce voluntary visually-guided saccades that were as small as microsaccades. Experiment 3: The fixation point remained stationary at the central location while the checkerboard jumped 0.08 visual degrees to the left and right of the fixation point, for a 0.16 visual degree jump of the background overall. The task was to maintain fixation on the fixation point throughout all three experiments, whether moving or not.
fMRI Data Analysis
Data were analyzed in BrainVoyager 2000 and Matlab. The effect of head motion on the MR-images was corrected for during preprocessing. The general linear model was carried out within regions of interest V1, V2, V3 and hMT+ localized separately for each subject’s brain. Slice scan time correction was carried out to correct for the fact that slices were not collected at the same time but were rather collected in interleaved, ascending order. Functional data were not smoothed in the space domain, but low-frequency temporal fluctuations were removed. This procedure did not introduce any new correlations between a voxel and its neighbors. For each subject, the functional data were co-registered to the high-resolution anatomical image and normalized into the Talairach stereotactic coordinate space, which enabled region-of-interest analysis of the BOLD signal within V1, V2, V3 and hMT+ for each subject.
Region of Interest Specification
Retinotopic mapping of the visual areas was carried out on each participant using the standard phase-encoding cross-correlation technique (T2*-weighted gradient echo planar imaging using the same 3T Siemens Allegra scanner, TR=2000 ms, 30 slices oriented along the AC-PC plane, TE=30ms, FA=90°, slice thickness=3 mm thickness, inter-slice distance 0.3mm, 152 brain volumes per run, 304 seconds/run, interleaved slice acquisition, matrix size=64×64mm) with the modification that two wedges of an 8Hz flicker black and white polar checkerboard grating were bilaterally positioned opposite each other, forming a bowtie. This stimulus arrangement led to an enhanced signal-to-noise ratio, since the total number of stimulations for each location was twice that of the single wedge stimulus (Sereno et al., 1995; Slotnick and Yantis, 2003). Each wedge subtended 20° of 360°. Counterphase flickering checkerboard wedges rotated continuously at an angular velocity of 11.25°/sec, undergoing nineteen 180 degree rotations during each run (304 seconds), beginning and ending at the vertical meridian. A minimum of two runs were collected for both the leftward and rightward directions for each subject and then averaged by common direction to minimize noise. Retinotopic areas (V1, V2d, V2v, V3d, V3v) were defined as masks on the basis of standard criteria (Sereno et al., 1995), assuming a contralateral hemifield representation for V1, and a contralateral quadrant representation for V2d, V2v, V3d, and V3v. The union of leftward and rightward V1 masks was created for each hemisphere of each subject. The corresponding unions were also created for V2d, V2v, V3d, and V3v. Any intersections of these masks (i.e. shared voxels) were removed from each union mask, creating a conservative mask that contained, respectively, only ‘pure’ V1, V2d, V2v, V3d, or V3v voxels. V2d and V2v voxels were then combined into a single V2 mask, and V3d and V3v masks we combined into a single V3 mask for each subject. Corresponding masks were then combined across hemispheres for each subject. Within these masks, separate general linear model (GLM) analyses were carried out using the Brain Voyager 2000 event-related deconvolution procedure with autocorrelation correction implemented to counteract correlation among error of successive measurements. It was not possible to reliably examine BOLD signal in other potential retinotopic ROIs, such as V4v or V3A/B, because the thinness of the volumes (7 or 11 slices along the calcarine sulcus, which permitted short TRs) collected in the three experiments did not provide sufficient overlap with regions too distal from the calcarine sulcus.
Area hMT+ was localized in each subject using the methods of Huk, Dougherty, and Heeger (2002). The parameters of the EPI sequence were identical to those used for retinotopic mapping. Subjects fixated a red fixation spot while their eye movements were monitored to assure fixation. White dots were presented on a black background. In stimulation epochs the dots moved in unison along radial trajectories, alternating between inward and outward motion each second. During rest epochs, the dots remained stationary. Stimulation and rest epochs alternated. Each epoch lasted 18s (9 TRs), and there were a total of eleven epochs per run (200 TRs). Dots were always present on the screen. Areas of activation were specified in each subject using a general linear model contrast between stimulation and rest conditions. Head motion corrections were included as regressors in the GLM to minimize variation linked with head motion. The threshold was set to a point (minimum value t=7) where hMT+ activation was distinguishable from coactivating areas. Anatomical criteria were applied that limited the specification of hMT+ to an upper posterior limb of the inferior temporal sulcus. Voxels that met these criteria were defined as hMT+ ROIs in the left and right hemispheres.
Anatomical data were collected in each subject as a T1-weighted MPRAGE scan (TR=2300ms, 160 sagital slices, TE=2.6ms, FA=9°, 256×256 voxels/slice, 1×1×1mm voxels).
Eye Movement Recordings
Eye position data were collected using a custom-built MR-compatible Limbus tracker (MR-Eyetracker; Cambridge Research Systems; See Supplementary Figure 4 to view an image of the eyetracker) over the right eye, which measured both horizontal and vertical components of the eye movements in the scanner during scan acquisition. Eye traces were acquired using the data acquisition toolbox for Matlab software via an Advantech IO-card Analog signal was converted into a 1000Hz digital signal. The eyetracker contained infrared LEDs (< 0.2 mW at 880 nm) with a potential spatial resolution < 0.1°. This eyetracker proved challenging to use because small head movements could make the difference between being able to detect 0.16 visual degree visually-guided saccades and losing the ability to detect them. Subjects’ heads were therefore restrained with cushions and headphones to a much greater degree than is traditionally required. In addition, subjects were asked to keep their depth of breathing and head motion to a minimum. Thus only subjects who could stay still for several minutes at a time were able to carry out these experiments. Online analysis of collected eye movements revealed whether the 0.16 visual degree visually-guided saccades at the beginning and end of each run had been detected at a level above chance. Recalibration of the eyetracker after each run was possible, and carried out as needed, in order to bring the eyetrace signals back into the zone of peak sensitivity.
We are confident of our ability to detect microsaccades because each run of experiment 2 had a built-in internal check in the 30 second epochs of 0.16 degree visually-guided saccades at the beginning and end of each run. For runs where we could reliably detect these known eye movements at the beginning and end of a run, we could use the threshold for detecting microsaccade-sized visually-guided saccades to define a threshold for detecting microsaccades in the middle of the run when subjects maintained fixation on a stationary fixation spot.
Eyeblinks were defined and removed from eye movement traces and the TR of eyeblink onset was recorded in order to define individual eyeblink events for later event-related deconvolution analysis of the BOLD signal within specified ROIs. Only the horizontal eye movement signal was analyzed because microsaccades are approximately an order of magnitude more likely to occur horizontally than vertically (for a probability density function of microsaccade directions see Tse, Sheinberg, and Logothetis, 2004). Eye traces were converted into a velocity signal by taking the first derivative of the raw data that had been purged of eyeblinks. The velocity signal was then filtered with a 20-point (ms) symmetric Hanning window for zero-phase forward and reverse digital filtering (low pass filter cutoff frequency 55Hz). Zero-crossings were specified in the velocity signal. Time bins were then defined between successive zero-crossings. Time bins shorter than 10 and longer than 100 ms were discarded as outside the domain of microsaccades. Outliers in velocity space were then specified in two ways within each of the two thirty-second saccade epochs at the beginning and end of each run of experiment 2: (1) The maximum absolute magnitude was defined within each time bin. Bins containing the highest 5% magnitudes were defined as outliers in velocity space. (2) The area under the curve was determined for each time bin. The top 5% of areas were defined as outliers in amplitude space. Events were conservatively specified by the intersection of these two sets of velocity-space outliers, comprising typically about the top 3% of velocity or area outliers.
An internal check on the quality of the detected microsaccade data was the ability to detect the 24 known occurrences of 0.16 visual degree visually-guided saccades that occurred during the two saccade epochs, one at the beginning and end of each run. Thus, from within this set, for each saccade epoch, velocity and area outliers were discarded which did not fall within 100ms and 400ms after the known onset of a 0.16 visual degree jump in the fixation point. The number of detected velocity-space outliers that lay within 100ms and 400ms after the known onset of a 0.16 visual degree jump of the fixation point was counted for each run. Simply on the basis of chance we would expect some outliers to fall within this window. This number can be described as falling within a binomial distribution. This number exceeded a threshold specified by meeting or surpassing that number for which 90% of the area under the binomial distribution was passed in seven runs in three of the five subjects shown. On good runs, this typically amounted to eight or more of the twelve known 0.16 visual degree saccades in each of the two saccade epochs. The median velocity and area of these outliers was then defined. Because the first and second saccade epochs occurred for thirty seconds at the beginning and end of a run, a linear interpolation between the median velocities of these epochs was used to define a threshold for the detection of microsaccades over the entire run, in order to account for possible changes in the signal to noise ratio that may have occurred over a run. A corresponding linear interpolation was also defined for the two median areas. For each run, microsaccades were defined as those non-saccade events which surpassed both thresholds. The rate of microsaccades defined in this manner was typically between 0.3 and 0.4/sec, which is less than half the approximately one microsaccade per second reported by other authors (Martinez-Conde et al., 2004). Because of its conservative nature, erring on the side of making incorrect rejections of microsaccades rather than false alarms, events specified as microsaccades using this algorithm contained microsaccades with a conservative level of detection confidence. Although we could not detect all microsaccades because of our conservative criteria, we are confident that the events specified as microsaccades for our event-related deconvolution analysis were indeed microsaccades.
Results
Eye Movement Data
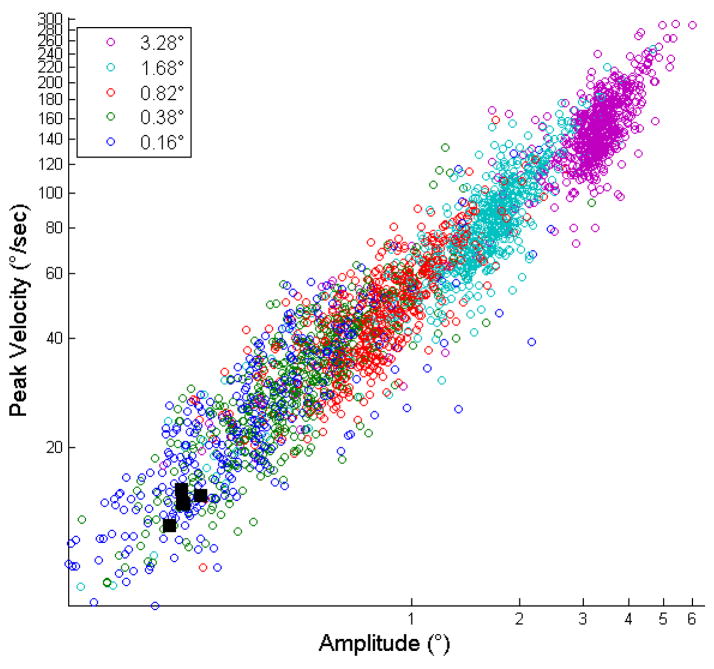
Fig. 3 shows the main sequence for visually-guided saccades detected in experiment 1, plotting saccade amplitude against maximum saccade velocity in llog-log coordinates in order to make apparent the smallest magnitude eye movements. Note that even the smallest visually-guided (0.16 visual degrees) saccades fall on the same regression line, supporting the claim that these small amplitude saccades are truly saccades. In addition, the black squares indicate the mean magnitude of measured microsaccades for five subjects (data shown in Figure 5), indicating that they too fall on the saccade main sequence, consistent with past findings that microsaccade main sequence falls within the saccade main sequence (e.g. Hafed et al., 2009: Engbert, 2006; Zuber, 1965).
Figure 3.
Measured saccade amplitudes from experiment 1 plotted on a log-log scale against their peak velocities, color coded to indicate the actual size of the fixation point jump, revealing that all measured saccades fall on the same main sequence. The five black squares indicate the mean magnitude of microsaccades measured for each subject. These are the means of the data shown in Figure 5, and reveal that microsaccades fall on the same main sequence as saccades.
Figure 5.
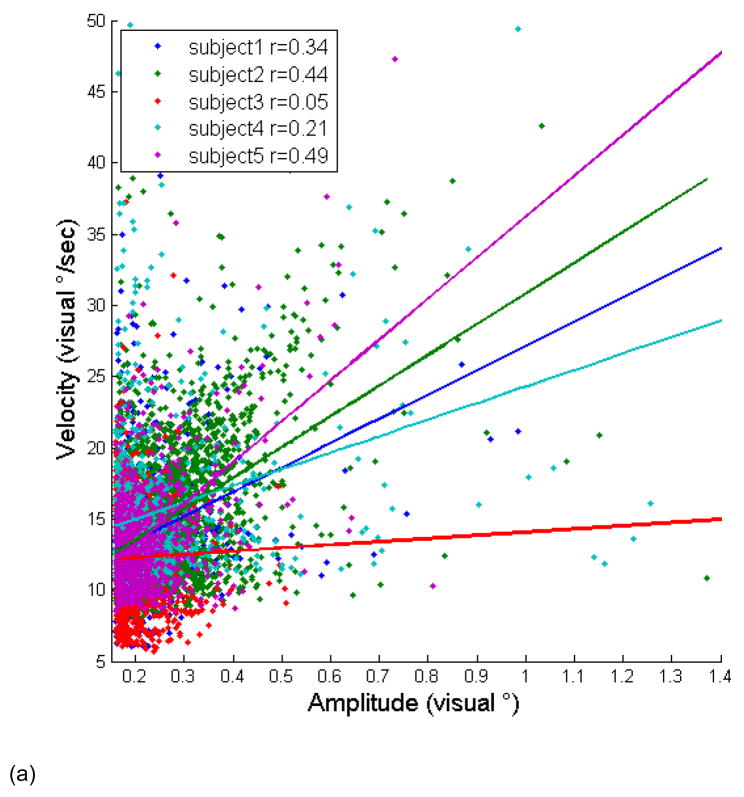
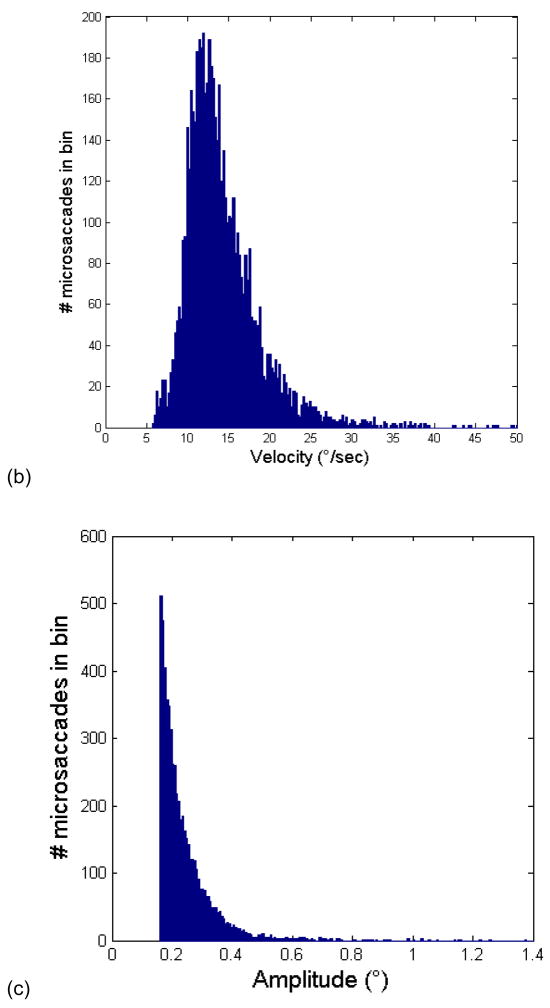
(a) Main sequence of detected microsaccades from experiment 2, plotting microsaccade amplitude against peak velocity. Superimposed in the corresponding color is the regression line that best fits a given subject’s data. Values of the correlation coefficient r are given in the legend. Note that only the three highest correlation coefficient subjects’ data was used in averaging BOLD signal deconvolution timecourses (see Fig. 7). (b) Histogram of the velocities of detected microsaccades. (c) Histogram of the amplitudes of detected microsaccades. Data below x=0.16 are not shown because we did not count as microsaccades visual events whose amplitude was less than 0.16 visual degress.
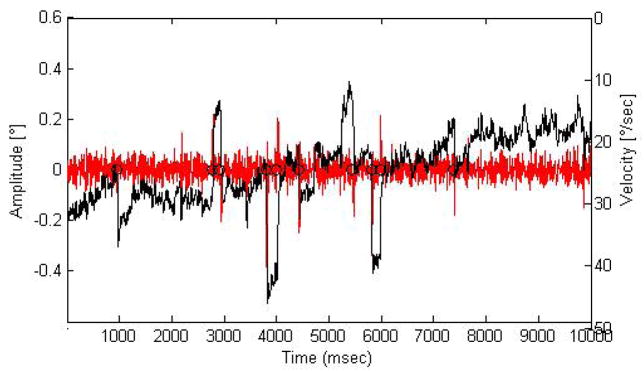
Figure 4 shows ten seconds of eye movement data from the right eye of one subject in black as well as the corresponding velocity data superimposed over this. Circles indicate detected microsaccades.
Figure 4.
An example of 10 seconds of horizontal channel eyetraces (black) from a single subject, superimposed upon the corresponding velocity of the eye movements (red, centered at 0 velocity). Microsaccades were detected as outliers in velocity space subject to other criteria (see text). Detected microsaccades are indicated with black circles. Eyetrace units are z-scores of amplitude in degrees of visual angle, and eye movement velocity units are visual degrees per second.
Figure 5 plots the main sequence of microsaccades that were detected in experiment 2. A regression line for each subject’s data makes apparent that the population of microsaccades falls on the main sequence of saccades shown in the log-log plot in Figure 3 (for a linear-linear version, see Supplementary Figure 3). The r statistic for each subject is shown in the legend. Note that only the three subjects whose r-values exceed 0.3 passed the binomial test described above, meaning that we can only be confident that microsaccades were accurately and significantly detected for these three subjects. The other two subjects are shown for comparison.
Functional MRI Data
Experiment 1
The magnitude of the BOLD signal response was found to vary parametrically with the magnitude of voluntary saccades in areas V1, V2, and V3, but not in area hMT+ (see Figure 6). The steepest slope of the function describing the relationship between the BOLD response and the saccade amplitude was found for the ROI located in V1. Higher visual areas also showed a dependence of the BOLD response amplitude on saccade size. Interestingly BOLD signal in hMT+, although showing a response for all saccade sizes tested, did not exhibit a strong dependence on saccade amplitude. A whole volume GLM for one representative subject can be seen in Supplementary Figure 2a–e.
Figure 6.
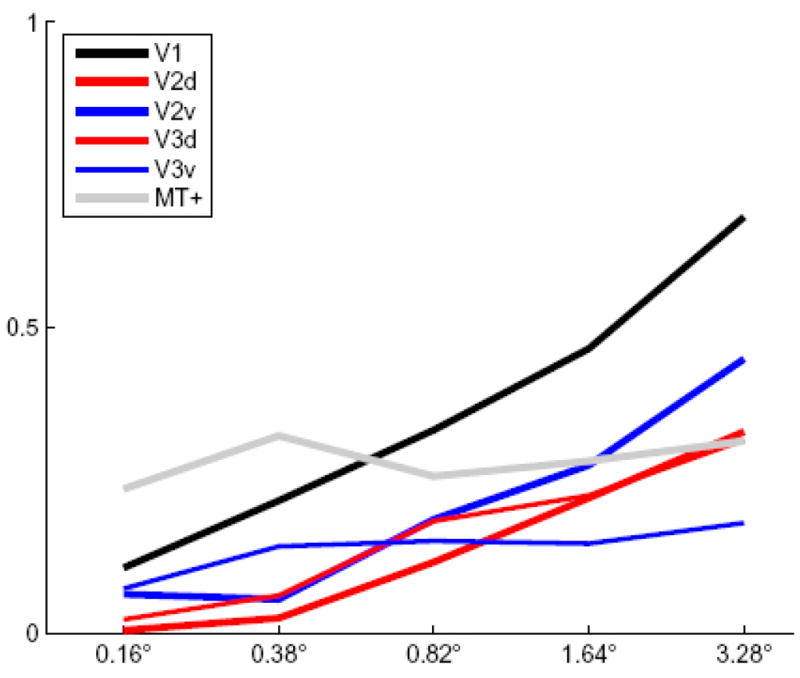
The level of BOLD signal activation increases in V1, V2, and V3 as a function of the size of a voluntary saccadic eye movement, but remains flat for hMT+.
Experiments 2 and 3
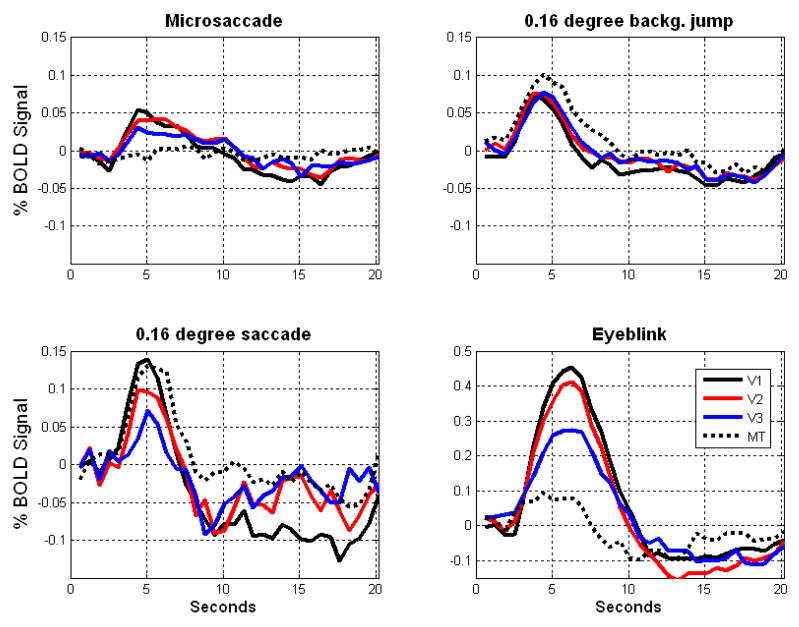
A deconvolution analysis of event-related BOLD data in five subjects2 revealed a similar pattern of activation across subjects following a microsaccade, small saccade, or image background jump in V1, V2, and V3, where an upward modulation in signal, peaking at approximately four seconds post-event, was followed by a longer period of decline and negative activation, as shown in Figure 7. (For a full volume GLM from experiment 2 for a representative subject, see supplementary Figure 2f–i online). The signal modulation to a 0.16 visual degree visually-guided saccade, which was as small as a microsaccade, revealed a similar signature to that of microsaccades across subjects. As shown in Figure 7, BOLD signal increases in V1 for voluntary saccades that have amplitudes comparable to those of microsaccades. The pattern of response to a microsaccade, saccade, and eyeblink all tended to diminish in strength as one progresses from V1 to V2 to V3.
Figure 7.
The curves indicate the average deconvolution functions corrected for autocorrelation following an event (x=0) in V1 (black), V2 (red), V3 (blue), and hMT+ (black dotted) for microsaccades (top left) from experiment 2 (n=5, but only showing the average of the three subjects that passed the conservative criteria for inclusion described in text), background shifts (i.e. image motions) from experiment 3 (n=5, top right), 0.16 visual degree visually-guided saccades from experiment 2 (n=5, bottom left), and (4) eyeblinks from experiment 2 (n=5, bottom right). The data are plotted in all subplots at increments of volume acquisitions (TR=631ms, eleven slices oriented along the calcarine sulcus), but the x-axis has been relabeled as seconds for clarity. 32 volumes equals approximately 20 seconds. Zero on the x-axis indicates the beginning of a volume in which an event was detected. The first datapoint in each deconvolution function is drawn at the end of the first TR. The units of the y axis are % BOLD signal change. Note that the scale of the y-axis is the same for microsaccades, 0.16 visual degree background jumps and 0.16 visual degree visually guided saccades, but different for eyeblinks because of the large difference in response to eyeblinks.
The pattern of BOLD responses in hMT+ was qualitatively different from the responses in areas V1, V2, and V3, as can be seen in Figure 7. In hMT+ there was no evidence of modulation of BOLD signal by microsaccades, but responses to background jumps and saccades were more robust than in areas V1, V2, and V3, while following a similar time course. Similarly, responses to eyeblinks were diminished in hMT+ relative to the strong response to eyeblinks observed especially in V1. This pattern of results suggests that spurious image changes generated by microsaccades or eyeblinks are diminished by the time the signal is processed in hMT+. Our finding that microsaccade response in hMT+ was not significant as measured using the BOLD signal fails to corroborate neurophysiological data (Bair and O’Keefe, 1998) showing that microsaccades lead to neural excitation in macaque MT. Note, however, that Bair & O’Keefe (1998) did not report that there is any kind of systematic modulation of neural activity with fixational saccade amplitude; They only pointed out a modulation of neuronal activity as a function of the preferred direction tuning of MT neurons. As far as we know, to date, there has not been a neurophysiological report of neuronal activity in MT varying with microsaccade amplitude. It is possible that microsaccades do trigger responses in hMT+ neurons, but that our event-related fMRI method lacked the power to detect these changes. Indeed, in one of our subjects (see Figure 8, green), there was significant microsaccadic activation in hMT+, but this was not apparent or significant in the group data, possibly because of an insufficient number of subjects. Thus, we are not prepared to state with confidence that microsaccades do not trigger measurable BOLD signal in hMT+ under any circumstances. Indeed, given the results of Bair & O’Keefe (1998), showing a modulation of firing rate in MT as a function of microsaccade direction (although they did not show modulation with microsaccade amplitude), we believe that it is more likely that our power, while adequate to detect significant signal in V1–V3, was not adequate to detect signal in hMT+. We can, however, state with confidence that the strength of BOLD signal modulation, and correlated neural activity presumably associated with microsaccades, diminishes from V1 to hMT+.
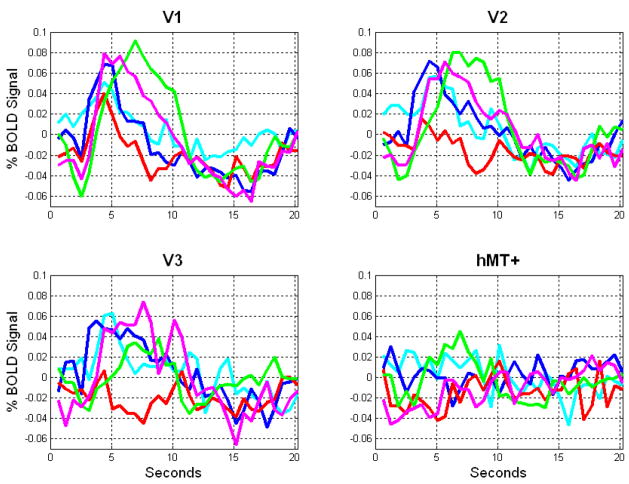
Figure 8.
Individual subject microsaccade event-related BOLD signal timecourses in the four regions of interest studied. Each subject’s timecourse is color-coded to match the color code of Figure 5a. Note that only the data from the green, maroon, and blue subjects were averaged for Figure 7. To see the within-subject error bars for the maroon and green subjects, see Supplementary Figure 1 online.
The BOLD signal timecourse following a microsaccade for each of the five subjects tested is shown in Figure 8. Color coding of timecourses mirrors the color-coding of subject data in Figure 5a. Note that only the data from the the green, maroon, and blue subjects were averaged in making the microsaccade subplot in Figure 7, because only these three subjects passed the conservative criteria described above based on successful detection of the 0.16 degree saccades at the beginning and end of a run.
General Discussion
The present findings support the claim that microsaccades are associated with a significant increase in BOLD signal response, and concomitant neural activity, in striate and extrastriate visual cortex. The responses in V1 associated with spontaneously occurring microsaccades are qualitatively similar to those evoked during visually-guided saccades and image motions of comparable amplitude (Fig. 7), although not quite as high in amplitude. These fMRI findings are broadly consistent with recent single unit data in the macaque showing that V1 saccade neurons burst equivalently upon microsaccades, small voluntary saccades, and abrupt image changes (Kagan, Gur, and Snodderly, 2008). Unlike Kagan et al (2008), we also examined extrastriate cortex, and find a diminishing of BOLD signal following a microsaccade from V1 through V3, with no significant modulation in hMT+. While there is a similar diminishment of BOLD signal following a comparably sized visually-guided saccade from V1 through V3, there is no comparable diminishment of BOLD signal response in hMT+ following a saccade. Indeed, we find that the size of hMT+ response does not appear to modulate as a function of the size of the voluntary saccade. Thus hMT+ responses are very different for microsaccades versus saccades that create the same change in the image as a microsaccade. In this regard, the BOLD signal response to a microsaccade resembles the pattern of BOLD signal response to an eyeblink, albeit it much reduced in magnitude, in that the magnitude of BOLD signal response to an eyeblink decreases from V1 to V3, and is most substantially reduced in hMT+.
The results summarized in Fig. 7 indicate that the BOLD response amplitude exhibits a similar trend for the microsaccade, visually guided saccade and eyeblink conditions with the greatest response in V1, an intermediate response in V2 and the lowest response in V3. These three conditions all involve a retinal image shift evoked by rotation of the eye. An image shift caused by stimulus motion on the display during steady fixation evokes a BOLD response that is indistinguishable in V1, V2 and V3. This pattern of results could represent a signature of extraretinal signals in the visual cortex during eye movements. However, we need to be careful when making comparisons between these conditions and the condition of stimulus motion. As pointed out by Bristow et al 2005 for eyeblinks and Sylvester et al (2005 and Sylvester et al (2006) for saccades, differences in retinal image movements for these different conditions, and not extraretinal signal processing per se, could be at least partially responsible for this pattern of results. We took care to assure that the voxels used in the data analysis represented the retinotopic coordinates of the stimulus display and not the peripheral visual field. Furthermore, at least for the comparison between the microsaccade, visually guided saccade (amplitude 0.16 deg) and stimulus display shift, the image displacement was small in amplitude. Large saccades surpassing 1 deg or greater would have led to considerable displacements on the retina of off-display image components (like the edge of the screen or inside housing of the magnet). Such large saccades were avoided in the present comparison.
Constant input to retinal ganglion cells can lead to neural adaptation and, as a consequence, perceptual fading (Clarke and Belcher, 1962; Kotulak and Schor, 1986; Livingstone and Hubel, 1987; Martinez-Conde, Macknik, and Hubel, 2004; Millodot, 1967, Ramachandran, 1992). Steady illumination leads to diminished neural responses, whereas abrupt spatial or temporal changes in stimulus input evoke stronger neural responses (Hartline, 1940; Kuffler, 1952; Hubel and Wiesel, 1965). Fixational microsaccades are thought to play a central role in counteracting neural adaptation and perceptual fading by providing the necessary change in the image needed to bring novel stimuli into adapted ganglion cell receptive fields, thereby triggering new transient responses (Riggs and Ratliff, 1953; Skavenski et al., 1979; Martinez-Conde et al., 2004, 2006; Engbert and Kliegl, 2004; Livingstone et al., 1996; Macknik and Livingstone, 1998). If effects of microsaccades are counteracted by image stabilization, the visual scene quickly fades, presumably because of the effects of neural adaptation at the level of retinal ganglion cells. The image ‘refresh’ signal following a microsaccade is thought to trigger an increase in neural activity in ganglion cells and subsequent neurons in the visual system. In support of this view, microsaccades have been found to increase the probability of subsequent bursting in the lateral geniculate nucleus of the thalamus (LGN) and V1 neurons in macaques (Martinez-Conde et al., 2000, 2002, 2004). However, there is as yet no evidence that the onset of fading triggers microsaccades. Indeed, there may be no such fading-induced trigger, if the system makes a microsaccade at some baseline rate as an ‘automatic refresh’ (Kagan et al., 2008).
The neural basis of microsaccades is still a matter of debate. Barlow (1952) proposed that microsaccades occur upon a shift of visual attention, and it has since been shown that microsaccade rate is indeed modulated by shifts of attention (Engbert and Kliegl, 2003; Hafed and Clark, 2002; Horwitz and Albright, 2003; Rolfs et al., 2004; Tse et al, 2002, 2004; Turatto et al., 2007; Valsecchi and Turatto, 2009) and perceptual state (Hsieh and Tse, 2009; Martinez-Conde et al., 2006), although microsaccade occurrence is not limited to the time of attentional shifts or perceptual shifts (Tse, Caplovitz and Hsieh, 2009). However, there has been a debate about whether microsaccade directionality is also influenced by the directionality of attentional shifts, with some arguing for an influence of attention (Engbert, 2006; Engbert and Kliegl, 2003; Hafed and Clark, 2002; Laubrock et al., 2007; Rolfs et al., 2004; Turatto et al., 2007; Valsecchi and Turatto, 2009), and others finding that attention plays no significant role in the distribution of microsaccade directions (Horwitz and Albright, 2003; Horowitz et al., 2007a, b, Tse et al., 2002, 2004). Whether microsaccade directionality is correlated with attentional directionality or not, the fact that microsaccade rate is modulated by attentional shifts suggests that eye-shifting circuitry is not independent of attention-shifting circuitry. This does not prove that microsaccades are generated cortically; they might be generated subcortically, yet be inhibited or released by cortical commands.
The attentional system is thought to have at least two subsystems, one involved in automatic and rapid shifts of “exogenous” attention to abrupt onsets (Irwin et al., 2000; Jonides & Yantis, 1988; Remington, Johnston, & Yantis, 1992; Theeuwes, 1994; Yantis & Hillstrom, 1994; Yantis & Jonides, 1984; Yantis & Jonides, 1990), and the other subsystem involved in volitional shifts of “endogenous” attention. The bottom-up subsystem is thought to involve circuitry in the superior colliculus (Valsecchi and Turatto, 2007), and the top-down subsystem is thought to involve circuitry in the frontal lobe (Mesulam, 1981; Posner & Petersen, 1990).
Similarly, saccade generation involves at least two parallel subsystems. A sub-cortical pathway involving the superior colliculus generates reflexive, orienting saccades, and a cortical pathway involving the frontal eye fields generates voluntary saccades via top-down input into the superior colliculus (e.g. Everling & Munoz, 2000; Hanes, Patterson, & Schall, 1998; Schall, 1995). Both the abrupt attentional shift system and abrupt eye movement system appear to recruit some of the same circuitry in the superior colliculus, one to move the direction of gaze and the other to move the focus of processing without necessarily moving the eyes (Corbetta et al., 1998; Kustov & Robinson, 1996; Rizzolatti, 1994; Robinson & Kertzman, 1995). Moreover, the superior colliculus cells thought to trigger saccades may inhibit the superior colliculus cells that maintain eye fixation, and vice versa (e.g. Munoz & Wurtz, 1993). If an exogenous or endogenous attentional shift activates saccade cells in the superior colliculus, their activation might influence the behavior of fixation cells through their mutual inhibition, perhaps accounting for the influence of attention on microsaccade rate. Thus, while attentional shifts modulate the rate of microsaccade occurrence, microsaccades are not fundamentally attentional in nature. They appear to comprise a built-in reset mechanism to counteract visual fading. As such, it appears that microsaccades are involuntary and automatic under normal circumstances (Martinez-Conde et al., 2004).
Microsaccades are associated with neuronal responses in all visual areas that have been considered to date (Martinez-Conde et al., 2004, Greschner et al., 2002; Martinez-Conde et al., 2002; Gur and Snodderly, 1997; Bair and O’Keefe, 1998; Leopold and Logothetis, 1998; Snodderly and Kagan, 2001; Reppas et al., 2002), and may account for neuronal response variability in awake monkey V1 (Gur et al., 1997). Microsaccades have an excitatory effect in the retina (Greschner et al., 2002), LGN (Martinez-Conde et al., 2002, Reppas et al., 2002), V1 (Martinez-Conde et al., 2000, 2002, Leopold and Logothetis, 1998; Snodderly and Kagan, 2001) extrastriate cortex (Leopold and Logothetis, 1998), and MT (Bair and O’Keefe, 1998) in monkeys. This is due to changes in the visual input induced by microsaccades rather than motor signals or top-down feedback, at least in the LGN and area V1, because microsaccades only lead to an increase in neural activity when a microsaccade actually brings a new stimulus into a neuron’s receptive field; In the absence of a stimulus other than the fixation point, microsaccades would not be expected to increase neural activity, because no new stimulus input would be brought into the adapted cells’ receptive fields (Martinez-Conde et al., 2000, 2002).
Leopold and Logothetis (1998), however, found that microsaccades are associated with transient inhibition of neural activity in V1. Note that they nonetheless found that cells in V2 and V4 showed strong excitation that coincided in time with the striate depression of activity following a microsaccade. Because we do not notice changes in the relative position of the retinal image induced by microsaccades, it has been suggested that there is inhibition of neural firing associated with the occurrence of microsaccades which might carry information that would be perceived as spurious image motion if not suppressed. Although we now have ample evidence for the existence of saccadic suppression from electrophysiology (Wurtz, 1968; 1969; Macknik et al., 1991), psychophysics (Bridgeman and Macknik, 1995; Burr et al., 1994; Ross et al., 2001) and functional MRI (Kleiser et al., 2004; Sylvester et al 2005, 2006; Vallines & Greenlee, 2007) the existence of microsaccade suppression remains controversal (compare Ditchburn, 1955; Beeler; 1967 and Krauskopf, 1966; Sperling, 1990). Collorary discharges from the brainstem oculomotor nuclei (Zuber et al, 1964; Zuber and Stark, 1966) and/or superior colliculus (Lee et al., 2007) could inhibit the processing of retinal input at the level of the LGN (Sylvester et al 2005) or V1 (Vallines & Greenlee, 2007). The present findings suggest that V1, V2 and V3 are significantly activated after microsaccades, but the limited number of subjects used in this analysis requires further confirmation.
Alternatively, microsaccadic suppression need not involve a collateral discharge mechanism like that presumed to underlie saccadic suppression. Murakami and Cavanagh (1998), for example, suggested that suppression of the effects of retinal image shifts elicited by fixational eye movements occurs by continually subtracting the smallest instantaneous velocity motion vector in a scene from all other motion vectors, because this smallest vector could arise as a spurious motion signal during fixational eye movements. Such a computation might take place cortically rather than subcortically, such as in area hMT+ (Murakami and Cavanagh, 2001; Sasaki et al., 2002).
Although microsaccades have been reported to induce excitatory responses in macaque hMT+ (Bair and O’Keefe, 1998), it could be that this activity induces suppression of neural activity elsewhere. The existence of microsaccadic suppression would be indicated by inhibition of neural activity or by cancellation of neural activity related to retinal image shifts during or following a microsaccade at some level in the visual system.
Thus, neural activity would be expected to increase following release from the neuronal adaptation in retinal ganglion cells that is believed to lead to perceptual fading (i.e. BOLD signal should increase from the baseline that exists just prior to the occurrence of a microsaccade), but would be expected to decrease as a function of microsaccadic suppression (i.e. decrease from the baseline that exists just prior to the occurrence of a microsaccade) at some location in the visual pathway, if such a mechanism indeed exists.3
The exact nature of the activation in visual cortex as a consequence of microsaccades remains to be determined. One important aspect is related to the retinal image shift elicited by microsaccades. Although the size of image shifts caused by microsaccades are clearly above the threshold for motion detection, we do not consciously perceive these retinal image shifts. Because the magnocellular/parvocellular geniculostriate pathway inputs that first trigger hMT+ responses emerge from V1, and because V1 exhibits microsaccade modulation but hMT+ does not, at least as measured by us using BOLD signal, one might want to conclude that the computation that minimizes the spurious motion signals arising from microsaccades occurs in hMT+ itself. However, our data cannot rule out the possibility that there are other pathways to hMT+ that do this. For example, it is possible that direct koniocellular LGN to hMT+ inputs (Sincich et al, 2004) modulate spurious motion signals that arise from microsaccades or other sources. Although there is direct tectopulvinar input to hMT+ that bypasses V1, it is not likely that microsaccadic suppression is driven by this bottom-up pathway, because the motion and other visual tuning properties of pulvinar nucleus cells appear to be driven cortically rather than via bottom-up input from superior colliculus cells (Bender, 1983); Indeed, tectal cells are not orientation or direction selective (Cynader and Berman, 1972), making it unlikely that a bottom-up motion signal reaches hMT+ via the tectopulvinar pathway at all. A collateral discharge signal that could accomplish microsaccadic suppression of spurious image changes has been hypothesized to arise in the brainstem ocular-motor nuclei (Zuber et al., 1964; Zuber and Stark, 1966). If this hypothesis is correct, then our data suggest that the discounting of spurious image motion signals that arise from microsaccades is not accomplished via collateral discharge, or that such collateral discharge, if it exists, does not operate on V1, but may operate on hMT+. While it is important to place our data in the context of what is known, the present data alone cannot establish whether microsaccadic suppression occurs, and if so, by what mechanism it occurs.
Interestingly, the level of response to a voluntary, visually-guided saccade is at least as strong in hMT+ as it is in area V1. This would appear to suggest that the presumed discounting of spurious image motions generated by saccades is not operative in either V1 or hMT+ during visually-guided saccades. On the other hand, our experimental design was conceived to determine the effects of microsaccades and small voluntary visually-guided saccades with continual high-contrast checkerboard presentation. Vallines and Greenlee (2006) found that the BOLD signal decreased only for Gabor stimuli flashed immediately around the time of the saccade onset. The present stimulus was always present, so that saccadic suppression may not be easily detected here, because we are not just probing the brief duration when saccadic suppression occurs. Thus, the presence or absence of saccadic suppression cannot be readily determined under our current paradigm. Nonetheless, even though the stimulus was always present, we do find relative dimunition of BOLD signal response to microsaccades in hMT+ compared to responses in V1–V3.
While our data do not support or rule out any particular collateral discharge model of microsaccadic suppression, they also do not support the hypothesis (Murakami and Cavanagh, 1998) that hMT+ discounts spurious image motion generated as a result of microsaccades by subtracting the smallest image motion vector from all motion vectors. In our study microsaccades are not associated with significant BOLD signal modulation in area hMT+, whereas small visually-guided saccades are. Thus the signals associated with the retinal image shifts evoked by microsaccades appear to be suppressed prior to the hMT+ processing stage. Interestingly, retinal image shifts associated with stimulus motion and/or visually-guided saccades are associated with a significant increase in BOLD signal in all visual areas investigated, including hMT+. It follows that the lack of BOLD signal increase in area hMT+ that we report here is not a consequence of poor signal-to-noise in our T2*-weighted MR image series. Rather the observed pattern of results appears to reflect a genuine difference between encoding processes related to stimulus motion, retinal slip due to visually-guided saccades and that evoked by microsaccades. Whether a collateral discharge, vector subtraction, or other model is correct, our data constrain future models of the mechanism whereby spurious image motions are discounted by the visual system.
Microsaccades, comparably sized visually-guided saccades, and comparably sized image shifts under conditions of fixation, all lead to qualitatively similar time courses of BOLD signal change in striate and extrastriate cortex, suggesting comparable changes in neural activity correlated with their occurrence. While microsaccades would be expected to lead to increased activity because of changed input to retinal ganglion cell receptive fields, they might be expected to lead to decreased output because of potential mechanisms associated with suppression of spurious motion signals that arise from microsaccades. Our data establish the existence of net neuronal excitation in V1, V2, and V3 in association with the occurrence of microsaccades, to our knowledge, for the first time in the humans, presumably associated with the ‘refresh’ in the image following small image shifts. Because the pattern of activation that is due to a microsaccade-sized (0.16 visual degrees) visually-guided saccade and to a microsaccade-sized (0.16 visual degrees) jump in the background under steady fixation is similar to that produced by a microsaccade, it is reasonable to assume that the change in the BOLD signal that arises with microsaccades is due to the shift in the image across the retina that microsaccades, small saccades, or small image changes induce. As far as V1 cells are concerned, it does not appear to matter whether the change in ganglion and successive cell input is due to a microsaccade, a voluntary saccade, or a comparable change of the image, supporting recent single unit work in macaque V1 that reports the same basic pattern of results (Kagan et al., 2008). Note, however, that the magnitude of the BOLD signal to microsaccades is less than half of that observed in V1 to a visually-guided saccade. This difference may occur because an image motion (i.e. the motion of the fixation point to a new location) is the cue to make a voluntary saccade to that new location. As such, the BOLD signal measured to a visually-guided saccade under our paradigm might involve the superposition of responses to both this small image change, and the response to the saccade itself. Going beyond the findings of Kagan et al. (2008), we find that responses in V2 and V3 do differ depending on whether the image change was self-generated or not, diminishing for self-generated image changes, but not diminishing for non-self-generated image changes. Reponses in area hMT+ do, however, differ depending on whether retinal image shifts are induced by stimulus motion, visually-guided saccades (i.e., conscious self motion) or by involuntary micromovements of the eyes (Fig. 3).
An initial negative dip in BOLD signal is evident in most timecourse plots in Figures 7 and 8. This negative dip is thought to arise from a short-term increase in deoxyhemoglobin as oxygen metabolism increases with neuronal activity before increased blood flow and/or volume brings in fresh, oxygenated blood to flush out deoxygenated blood (Fox and Raichle, 1986; Frahm et al., 1996; Magistretti & Pellerin, 1999; Frostig et al., 1990; Röther, et al., 2001). Recent modeling of the BOLD signal (Sotero & Trujillo-Barreto, 2007) finds that the magnitudes of the peak in BOLD signal, initial negative dip, and post-stimulus undershoot all increase proportionally with an increase in the magnitude of neuronal excitation. However, whereas increased magnitude of inhibition also increases magnitude of the initial dip and post-stimulus undershoot, it decreases the magnitude of the peak. Moreover, increased inhibition of neuronal activity in the absence of excitation would lead only to a decrease of BOLD signal below baseline. To the extent that this model of BOLD signal dynamics is correct, our data imply that microsaccades, saccades, image motion, and eyeblinks all generate net excitatory neuronal activity in the regions of interest examined. Our data, however, do not rule out the existence of simultaneous effects of both neuronal excitation and inhibition on the BOLD signal timecourses observed.
A further finding in our study is the robust increase in BOLD signals associated with eyeblinks (Fig. 3). In a block design Bristow et al (2005a,b) compared periods where subjects voluntarily blinked to those in which the subjects maintained fixation. This was conducted with and without the presence of diffuse flickering visual stimulation delivered by an optic fiber guide inside the subjects’ mouths (see Supplementary Material in Bristow et al. 2005a). In the absence of visual stimulation, LGN, V1, V2 and V3 are not active during fixation, whereas significant increases in BOLD response were evident during blinking. In the presence of diffuse flickering light, voluntary blinking has an inhibitory effect on visual cortex responses and this effect is most pronounced in V3 (see Fig. 1 in Bristow et al 2005). Based on the event-related approach with combined eye tracking that we took here, we could determine the effect of spontaneously occurring blinks on the time course of activation in V1, V2, V3 and hMT+. Our results indicate that spontaneous blinks in the presence of visual stimulation are associated with a significant increase in the BOLD response in these areas. The amplitude of this response is greatest for V1 and declines as the signal progresses to higher stages of visual processing in V2, V3 and hMT+ (Fig. 3). Although we did not explicitly investigate the effect of blinking in the absence of visual stimulation, our results suggest that spontaneous involuntary blinks have a robust excitatory effect on visual cortex4.
In conclusion, we have described the BOLD signal correlates of microsaccades and microsaccade-sized visually-guided saccades and image motions, as well as eyeblinks in human visual cortical areas V1, V2, V3, and hMT+. A deconvolution analysis of BOLD signal revealed a similar pattern of activation following a microsaccade, a 0.16 visual degree voluntary saccade, and a background jump of 0.16 visual degrees under conditions of fixation. In all cases, an initial increase in BOLD signal peaking at approximately four seconds after the event was followed by a decline and decrease below baseline in V1, V2, and V3. This modulation appears most pronounced for microsaccades and small voluntary saccades in V1, diminishing in strength in V2 and V3. In contrast, 0.16 degree image motions under conditions of fixation yield the same level of modulation in V1, V2, and V3. BOLD signal, moreover, modulates parametrically with the size of small voluntary saccades (0.16, 0.38, 0.82, 1.64, and 3.28 visual degrees) in V1, V2, and V3, but not in hMT+. Eyeblinks generate a much larger modulation that peaks approximately seven seconds after an event, and dips below baseline by approximately ten seconds post-event, and also exhibits diminishing modulation across V1, V2, and V3. Our results are consistent with the occurrence of transient net neural excitation driven by changes in input to retinal ganglion cell receptive fields that are induced by microsaccades, visually-guided saccades, or small image shifts. The pattern of results in area hMT+ exhibits no significant modulation by microsaccades, relatively small modulation by eyeblinks, and substantial responses to saccades and background jumps, suggesting that spurious image motion arising from microsaccades and eyeblinks is diminished by this stage of processing.
Supplementary Material
Supplementary Figure 1. Individual data corresponding to (a) the maroon subject and (b) the green subject of Figure 8, showing error bars across runs.
Supplementary Figure 2. Bonferroni corrected p<0.05 GLM whole volume results for a single representative subject for Experiment 1: saccade size (a) 0.16°, (b) 0.38°, (c) 0.82°, (d) 1.68°, (e) 3.28°; Experiment 2: (f) microsaccades, (g) 0.16° saccades, (h) eyeblinks; Experiment 3: (i) 0.16° background shifts. Note that the whole volume subsumed 7 slices in experiment 1, and 11 slices in experiments 2 and 3, centered along the orientation of the calcarine sulcus and passing through hMT+, the thalamus, the superior temporal sulcus and frontal lobes. Because these GLM results are not from a whole brain functional data set, a lack of activation means either non-significant activation inside the volume, or unknown activation outside the volume.
Supplementary Figure 3. This is a linear-linear version of Figure 3, showing the main sequence for visually-guided saccades.
Supplementary Figure 4. Images of the limbus eyetracker used.
Note: If we are permitted to publish online supplementary figures, we would also be willing to publish as supplementary material our matlab code used for all analyses.
Acknowledgments
This research was supported by an Alexander von Humboldt Foundation Wilhelm Bessel Award to PUT in 2006 and European Commission grant IST 027198 ‘Decisions in Motion’ and the Harris Distinguished Visiting Professor program of Dartmouth College to MWG in 2008. We thank Markus Raabe and Roland Rutschmann for help in collecting data.
Footnotes
Even though some individuals can apparently lower their microsaccade rate voluntarily (Steinman et al., 1973; Fiorentini and Ercoles, 1966; Steinman et al., 1973), or when carrying out a difficult task (Winterson and Collewijn, 1976; Kowler and Steinman, 1977; Kowler and Steinman, 1979; Bridgeman and Palca, 1980), without suffering from perceptual fading, microsaccades cannot be generated voluntarily (Martinez-Conde et al., 2004). For a model that can account for the discrepant findings concerning voluntary and involuntary microsaccade generation, see Rolfs et al. (2008).
Note that the average timecourses shown for the microsaccade case are for the three subjects who passed the conservative criteria for inclusion described in the text. Timecourses for all other conditions (saccade, image motion, eyeblinks) are the average of five subjects’ timecourses.
Of course, it is possible that an inhibitory mechanism, such as the firing of inhibitory interneurons, could lead to an increase in BOLD signal in an area where those interneurons existed, but areas downstream from this area would presumably receive less input following such inhibition, and these areas would presumably show decreased BOLD signal relative to preceding baseline upon the occurrence of a microsaccade.
Note that the majority of researchers do not monitor eye movements while carrying out fMRI experiments. Our results demonstrate that this could be problematic for fMRI research where stimuli are presented visually. To the extent that microsaccades, saccades, or eyeblinks may be correlated with experimental conditions, our data demonstrate that the results of fMRI studies to date could in principle arise because of these potential confounds, and not the experimental variables under consideration.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bair W, O’Keefe LP. The influence of fixational eye movements on the response of neurons in area hMT+ of the macaque. Vis Neurosci. 1998;15:779–86. doi: 10.1017/s0952523898154160. [DOI] [PubMed] [Google Scholar]
- Barlow HB. Eye movements during fixation. JPhysiol Lond. 1952;116:290–306. doi: 10.1113/jphysiol.1952.sp004706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler GW. Visual threshold changes resulting from spontaneous saccadic eye movements. Vision Res. 1967;7:769–775. doi: 10.1016/0042-6989(67)90039-9. [DOI] [PubMed] [Google Scholar]
- Bender DB. Visual activation of neurons in the primate pulvinar depends on cortex but not colliculus. Brain Research. 1983;279:258–261. doi: 10.1016/0006-8993(83)90188-9. [DOI] [PubMed] [Google Scholar]
- Bridgeman B, Palca J. The role of microsaccades in high acuity observational tasks. Vision Res h. 1980;20:813–7. doi: 10.1016/0042-6989(80)90013-9. [DOI] [PubMed] [Google Scholar]
- Bridgeman BB, Macknik SL. Saccadic suppression relies on luminance information. Psychological Research. 1995;58:163–168. doi: 10.1007/BF00419631. [DOI] [PubMed] [Google Scholar]
- Burr DC, Morrone MC, Ross J. Selective suppression of the magnocellular visual pathway during saccadic eye movements. Nature. 1994;371(6497):511–3. doi: 10.1038/371511a0. [DOI] [PubMed] [Google Scholar]
- Clarke FJ, Belcher SJ. On the localization of Troxler’s effect in the visual pathway. Vision Research. 1962;2:53–68. [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21(4):761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Cynader M, Berman N. Receptive-field organization of monkey superior colliculus. Journal of Neurophysiology. 1972;35:187–201. doi: 10.1152/jn.1972.35.2.187. [DOI] [PubMed] [Google Scholar]
- Ditchburn RW. Eye-movements in relation to retinal action. Opt Acta Lond. 1955;1:171–176. [Google Scholar]
- Ditchburn RW, Ginsborg BL. Involuntary eye movements during fixation. J Physiol. 1953;119:1–17. doi: 10.1113/jphysiol.1953.sp004824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engbert R. Microsaccades: A microcosm for research on oculomotor control attention and visual perception. Prog Brain Res. 2006;154:177–92. doi: 10.1016/S0079-6123(06)54009-9. [DOI] [PubMed] [Google Scholar]
- Engbert R, Kliegl R. Microsaccades uncover the orientation of covert attention. Vision Res. 2003;43:1035–1045. doi: 10.1016/s0042-6989(03)00084-1. [DOI] [PubMed] [Google Scholar]
- Engbert R, Kliegl R. Microsaccades keep the eyes’ balance during fixation. Psychological Science. 2004;6:431–6. doi: 10.1111/j.0956-7976.2004.00697.x. [DOI] [PubMed] [Google Scholar]
- Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. Journal of Neuroscience. 2000;20(1):387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Goffart L, Krauzlis RJ. A neural mechanism for microsaccade generation in the primate superior colliculus. Science. 2009;13(323):940–3. doi: 10.1126/science.1166112. 5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes DP, Patterson WF, Schall JD. Role of frontal eye fields in countermanding saccades: visual movement, and fixation activity. Journal of Neurophysiology. 1998;79(2):817–834. doi: 10.1152/jn.1998.79.2.817. [DOI] [PubMed] [Google Scholar]
- Fiorentini A, Ercoles AM. Involuntary eye movements during attempted monocular fixation. Atti FondGiorgio Ronchi. 1966;21:199–217. [Google Scholar]
- Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm J, Krüger G, Merboldt KD, Kleinschmidt A. Dynamic uncoupling and recoupling of perfusion and oxidative metabolism during focal brain activation in man. Magn Reson Med. 1996;35:143–148. doi: 10.1002/mrm.1910350202. [DOI] [PubMed] [Google Scholar]
- Frostig RD, Lieke EE, Tso DY, Grinvald A. Cortical functional architecture ad local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci. 1990;87:6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greschner M, Bongard M, Rujan P, Ammermuller J. Retinal ganglion cell synchronization by fixational eye movements improves feature stimation. Nature. 2002;5:341–347. doi: 10.1038/nn821. [DOI] [PubMed] [Google Scholar]
- Gur M, Snodderly DM. Visual receptive fields of neurons in primary visual cortex. V1. move in space with the eye movements of fixation . Vision Res. 1997;37:257–65. doi: 10.1016/s0042-6989(96)00182-4. [DOI] [PubMed] [Google Scholar]
- Gur M, Beylin A, Snodderly DM. Response Variability of Neurons in Primary Visual Cortex. V1. of Alert Monkeys. Journal of Neuroscience. 1997;17:2914–2920. doi: 10.1523/JNEUROSCI.17-08-02914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafed ZM, Clark JJ. Microsaccades as an overt measure of covert attention shifts. Vision Res. 2002;42:2533–2545. doi: 10.1016/s0042-6989(02)00263-8. [DOI] [PubMed] [Google Scholar]
- Hartline HK. The nerve messages in the fibers of the visual pathway. JOSA. 1940;30:239–247. [Google Scholar]
- Horowitz TS, Fine EM, Fencsik DE, Yurgenson S, Wolfe JM. Fixational eye movements are not an index of covert attention. Psychological Science. 2007a;18:356–363. doi: 10.1111/j.1467-9280.2007.01903.x. [DOI] [PubMed] [Google Scholar]
- Horowitz TS, Fine EM, Fencsik DE, Yurgenson S, Wolfe JM. Microsaccades and Attention: Does a Weak Correlation Make an Index? Reply to Laubrock Engbert Rolfs and Kliegl. Psychological Science. 2007b;18(4):367–368.2. doi: 10.1111/j.1467-9280.2007.01904.x. [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Albright TD. Short-latency fixational saccades induced by luminance increments. Journal of Neurophysiology. 2003;90:1333–1339. doi: 10.1152/jn.00146.2003. [DOI] [PubMed] [Google Scholar]
- Hsieh P-J, Tse PU. Microsaccade rate varies with subjective visibility during motion-induced blindness. PLOS One. 2009;4(4):e5163. doi: 10.1371/journal.pone.0005163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture in two non-striate visual areas. 18 and 19. of the cat. JNeurophysiol. 1965;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- Huk AC, Dougherty RF, Heeger DJ. Retinotopy and functional subdivision of human areas hMT+ and MST. J Neurosci. 2002;22(16):7195–205. doi: 10.1523/JNEUROSCI.22-16-07195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DE, Colcombe AM, Kramer AF, Hahn S. Attentional and oculomotor capture by onset luminance and color singletons. Vision Research. 2000;40:1443–1458. doi: 10.1016/s0042-6989(00)00030-4. [DOI] [PubMed] [Google Scholar]
- Jonides J, Yantis S. Uniqueness of abrupt visual onset in capturing attention. Perception Psychophysics. 1988;43:346–354. doi: 10.3758/bf03208805. [DOI] [PubMed] [Google Scholar]
- Kagan I, Gur M, Snodderly DM. Saccades and drifts differentially modulate neuronal activity in V1: Effects of retinal image motion position and extraretinal influences. Journal of Vision. 2008;8(14):1–25. doi: 10.1167/8.14.19. 19. [DOI] [PubMed] [Google Scholar]
- Kleiser R, Seitz RJ, Krekelberg B. Neural correlates of saccadic suppression in humans. Curr Biol. 2004;14(5):386–90. doi: 10.1016/j.cub.2004.02.036. [DOI] [PubMed] [Google Scholar]
- Kotulak JC, Schor CN. The accommodative response to subthreshold blur and to perceptual fading during the Troxler phenomenon. Perception. 1986;15:7–15. doi: 10.1068/p150007. [DOI] [PubMed] [Google Scholar]
- Kowler E, Steinman RM. The role of small saccades in counting. Vision Res. 1977;17:141–6. doi: 10.1016/0042-6989(77)90212-7. [DOI] [PubMed] [Google Scholar]
- Kowler E, Steinman RM. Miniature saccades: eye movements that do not count. Vision Res. 1979;19:105–8. doi: 10.1016/0042-6989(79)90129-9. [DOI] [PubMed] [Google Scholar]
- Krauskopf J. Lack of inhibition during involuntary saccades. Am J Psychol. 1966;79:73–81. [Google Scholar]
- Kuffler SW. Neurons in the retina: organization inhibition and excitation problems. Cold Spring Harbor Symposia on Quantitative Biology. 1952;17:281–292. doi: 10.1101/sqb.1952.017.01.026. [DOI] [PubMed] [Google Scholar]
- Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature. 1996;384(7):74–77. doi: 10.1038/384074a0. [DOI] [PubMed] [Google Scholar]
- Laubrock J, Engbert R, Rolfs M, Kliegl R. Microsaccades are an index of covert attention: Commentary on Horowitz Fine Fencsik Yurgenson and Wolfe. 2007. Psychological Science. 2007;18:364–366. doi: 10.1111/j.1467-9280.2007.01904.x. [DOI] [PubMed] [Google Scholar]
- Lee PH, Sooksawate T, Yanagawa Y, Isa K, Isa T, Hall WC. Identity of a pathway for saccadic suppression. Proc Natl Acad Sci USA. 2007;104(16):6824–7. doi: 10.1073/pnas.0701934104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold DA, Logothetis NK. Microsaccades differentially modulate neural activity in the striate and extrastriate visual cortex. Experimental Brain Research. 1998;123:341–345. doi: 10.1007/s002210050577. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Psychophysical evidence for separate channels for the perception of form color movement and depth. Journal of Neuroscience. 1987;7:3416–3418. doi: 10.1523/JNEUROSCI.07-11-03416.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Freeman DC, Hubel DH. Visual Responses in V1 of Freely Viewing Monkeys. Cold Spring Harbor Symposia on Quantitative Biology. 1996;LXI:27–37. [PubMed] [Google Scholar]
- Lord MP. Measurement of binocular eye movements of subjects in the sitting position. Brit J Ophthal. 1951;35:21–30. doi: 10.1136/bjo.35.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknik SL, Fisher BD, Bridgeman B. Flicker distorts visual space constancy. Vision Res. 1991;31:2057–64. doi: 10.1016/0042-6989(91)90163-y. [DOI] [PubMed] [Google Scholar]
- Macknik SL, Livingstone MS. Neuronal correlates of visibility and invisibility in the primate visual system. Nat Neurosci. 1998;1:144–9. doi: 10.1038/393. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond B Biol Sci. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Hubel DH. The role of fixational eye movements in visual perception. Nature Reviews Neuroscience. 2004;5(3):229–40. doi: 10.1038/nrn1348. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Hubel DH. The function of bursts of spikes during visual fixation in the awake primate lateral geniculate nucleus and primary visual cortex . Proc Natl Acad Sci USA. 2002;99:13920–5. doi: 10.1073/pnas.212500599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Hubel DH. Microsaccadic eye movements and firing of single cells in the striate cortex of macaque monkeys. Nature Neuroscience. 2000;3:251–8. doi: 10.1038/72961. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Hubel DH. The role of fixational eye movements in visual perception. Nat Rev Neurosci. 2004;5(3):229–40. doi: 10.1038/nrn1348. [DOI] [PubMed] [Google Scholar]
- Martinez-Conde S, Macknik SL, Troncoso XG, Dyar TA. Microsaccades counteract visual fading during fixation. Neuron. 2006;49(2):297–305. doi: 10.1016/j.neuron.2005.11.033. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Annals of Neurology. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Millodot M. Extra foveal variations of the phenomenon of Troxler. Psychologie Francaise. 1967;12:190–196. [Google Scholar]
- Moller F, Laursen ML, Tygesen J, Sjolie AK. Binocular quantification and characterization of microsaccades. Graefes Arch Clin Exp Ophthalmol. 2002;240:765–70. doi: 10.1007/s00417-002-0519-2. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculusIIReversible activation and deactivation. Journal of Neurophysiology. 1993;70(2):576–589. doi: 10.1152/jn.1993.70.2.576. [DOI] [PubMed] [Google Scholar]
- Murakami I, Cavanagh PA. A jitter after-effect reveals motion-based stabilization of vision. Nature. 1998;395:798–801. doi: 10.1038/27435. [DOI] [PubMed] [Google Scholar]
- Murakami I, Cavanagh P. Visual jitter: evidence for visual-motion-based compensation of retinal slip due to small eye movements. Vision Research. 2001;41:173–86. doi: 10.1016/s0042-6989(00)00237-6. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Ramachandran V. Blindspot. Scientific American. 1992;266:4–49. [Google Scholar]
- Ratliff F, Riggs LA. Involuntary motions of the eye during monocular fixation. Journal of Experimental Psychology. 1950;40:687–701. doi: 10.1037/h0057754. [DOI] [PubMed] [Google Scholar]
- Remington RW, Johnston JC, Yantis S. Involuntary attentional capture by abrupt onsets. Perception Psychophysics. 1992;51:279–290. doi: 10.3758/bf03212254. [DOI] [PubMed] [Google Scholar]
- Reppas JB, Usrey WM, Reid RC. Saccadic eye movements modulate visual responses in the lateral geniculate nucleus. Neuron. 2002;35:961–74. doi: 10.1016/s0896-6273(02)00823-1. [DOI] [PubMed] [Google Scholar]
- Riggs LA, Ratliff F, Cornsweet JC, Cornsweet TN. The disappearance of steadily fixated visual test objects. JOptSocAm. 1953;43:495–501. doi: 10.1364/josa.43.000495. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G. In: Attention and performance XV. Umilta C, Moscovitch EM, editors. Cambridge: MIT; 1994. pp. 231–265. [Google Scholar]
- Robinson DL, Kertzman C. Covert orienting of attention in macaquesIIIContributions of the superior colliculus. Journal of Neurophysiology. 1995;74(2):713–721. doi: 10.1152/jn.1995.74.2.713. [DOI] [PubMed] [Google Scholar]
- Rolfs M, Engbert R, Kliegl R. Microsaccade orientation supports attentional enhancement opposite to a peripheral cue: commentary on Tse Sheinberg Logothetis. PsycholSci. 2004;10:705–707. doi: 10.1111/j.0956-7976.2004.00744.x. [DOI] [PubMed] [Google Scholar]
- Rolfs M, Kliegl R, Engbert R. Toward a model of microsaccade generation: The case of microsaccadic inhibition. Journal of Vision. 2008;8(11):5, 1–23. doi: 10.1167/8.11.5. [DOI] [PubMed] [Google Scholar]
- Ross J, Morrone MC, Goldberg ME, Burr DC. Changes in visual perception at the time of saccades. Trends in Neurosciences. 2001;24:113–21. doi: 10.1016/s0166-2236(00)01685-4. [DOI] [PubMed] [Google Scholar]
- Röther J, Knab R, Hamzei F, Fiehler J, Reichenbach JR, Büchel C, Weiller C. Negative dip in BOLD fMRI is caused by blood flow--oxygen consumption uncoupling in humans. Neuroimage. 2002;15(1):98–102. doi: 10.1006/nimg.2001.0965. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Murakami I, Cavanagh P, Tootell RH. Human brain activity during illusory visual jitter as revealed by functional magnetic resonance imaging. Neuron. 2002;35:1147–56. doi: 10.1016/s0896-6273(02)00899-1. [DOI] [PubMed] [Google Scholar]
- Schall JD. Neural basis of saccade target selection. Reviews in the Neurosciences. 1995;6(1):63–85. doi: 10.1515/revneuro.1995.6.1.63. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Sincich LC, Park KF, Wohlgemuth MJ, Horton JC. Bypassing V1: a direct geniculate input to area MT. Nat Neurosci. 2004;7(10):1123–8. doi: 10.1038/nn1318. [DOI] [PubMed] [Google Scholar]
- Skavenski AA, Hansen RM, Steinman RM, Winterson BJ. Quality of retinal image stabilization during small natural and artificial body rotations in man. Vision Res. 1979;19:675–83. doi: 10.1016/0042-6989(79)90243-8. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Yantis S. Efficient acquisition of human retinotopic maps. Human Brain Mapping. 2003;18(1):22–9. doi: 10.1002/hbm.10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodderly DM, Kagan I, Gur M. Selective activation of visual cortex neurons by fixational eye movements: implications for neural coding. Visual Neuroscience. 2001;18:259–77. doi: 10.1017/s0952523801182118. [DOI] [PubMed] [Google Scholar]
- Sotero RC, Trujillo-Barreto NJ. Modelling the role of excitatory and inhibitory neuronal activity in the generation of the BOLD signal. Neuroimage. 2007;35(1):149–65. doi: 10.1016/j.neuroimage.2006.10.027. [DOI] [PubMed] [Google Scholar]
- Sparks DL. The brainstem control of saccadic eye movements. Nat Rev Neurosci. 2002;3:952–64. doi: 10.1038/nrn986. [DOI] [PubMed] [Google Scholar]
- Sperling G. In: Eye movements and their role in visual and cognitive processes. Kowler E, editor. Elsevier; 1990. pp. 307–351. [Google Scholar]
- Steinman RM, Cunitz RJ, Timberlake GT, Herman M. Voluntary control of microsaccades during maintained monocular fixation. Science. 1967;155:1577–9. doi: 10.1126/science.155.3769.1577. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Haddad GM, Skavenski AA, Wyman D. Miniature eye movement. Science. 1973;181:810–9. doi: 10.1126/science.181.4102.810. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Stimulus-driven capture and attentional set: selective search for color and visual abrupt onsets . Journal of Experimental Psychology: Human Perception Performance. 1994;20(4):799–806. doi: 10.1037//0096-1523.20.4.799. [DOI] [PubMed] [Google Scholar]
- Tse PU, Caplovitz GP, Hsieh P-J. Corrigendum to ‘Microsaccade directions do not predict directionality of illusory brightness changes of overlapping transparent surfaces’ [Vision Research 46 (2006) 3823–3830] Vision Research. 2009;49(790):e1–e7. doi: 10.1016/j.visres.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Tse PU, Sheinberg DL, Logothetis NK. Fixational eye movements are not affected by abrupt onsets that capture attention. Vision Res. 2002;42:1663–1669. doi: 10.1016/s0042-6989(02)00076-7. [DOI] [PubMed] [Google Scholar]
- Tse PU, Sheinberg DS, Logothetis NK. The distribution of microsaccade directions need not reveal the location of attention. Psychol Science. 2004;15(10):708–10. [Google Scholar]
- Turatto M, Valsecchi M, Tamè L, Betta E. Microsaccades distinguish between global and local visual processing. Neuroreport. 2007;18(10):1015–1018. doi: 10.1097/WNR.0b013e32815b615b. [DOI] [PubMed] [Google Scholar]
- Vallines I, Greenlee MW. Saccadic suppression of retinotopically localized blood oxygen level-dependent responses in human primary visual area V1. Journal of Neuroscience. 2006;26(22):5965–9. doi: 10.1523/JNEUROSCI.0817-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsecchi M, Turatto M. Microsaccadic response to visual events that are invisible to the superior colliculus. Behav Neurosci. 2007;121(4):786–93. doi: 10.1037/0735-7044.121.4.786. [DOI] [PubMed] [Google Scholar]
- Valsecchi M, Turatto M. Microsaccadic responses in a bimodal oddball task. Psychol Res. 2009;73(1):23–33. doi: 10.1007/s00426-008-0142-x. [DOI] [PubMed] [Google Scholar]
- Winterson BJ, Collewijn H. Microsaccades during finely guided visuomotor tasks. Vision Research. 1976;16:1387–90. doi: 10.1016/0042-6989(76)90156-5. [DOI] [PubMed] [Google Scholar]
- Wurtz RH. Visual cortex neurons: response to stimuli during rapid eye movements. Science. 1968;162:1148–1150. doi: 10.1126/science.162.3858.1148. [DOI] [PubMed] [Google Scholar]
- Wurtz RH. Vision for the control of movement The Friedenwald Lecture. Investigative Ophthalmology Visual Science. 1996;37:2130–45. [PubMed] [Google Scholar]
- Wurtz RH. Comparison of effects of eye movements and stimulus movements on striate cortex neurons of the monkey. J Neurophysiol. 1969;32:987–994. doi: 10.1152/jn.1969.32.6.987. [DOI] [PubMed] [Google Scholar]
- Yantis S, Hillstrom AP. Stimulus-driven attentional capture: evidence from equiluminant visual objects. Journal of Experimental Psychology: Human Perception Performance. 1994;20(1):95–107. doi: 10.1037//0096-1523.20.1.95. [DOI] [PubMed] [Google Scholar]
- Yantis S, Jonides J. Abrupt visual onsets and selective attention: evidence from visual search. Journal of Experimental Psychology: Human Perception Performance. 1984;10(5):601–621. doi: 10.1037//0096-1523.10.5.601. [DOI] [PubMed] [Google Scholar]
- Yantis S, Jonides J. Abrupt visual onsets and selective attention: voluntary versus automatic allocation. Journal of Experimental Psychology: Human Perception Performance. 1990;16(1):121–134. doi: 10.1037//0096-1523.16.1.121. [DOI] [PubMed] [Google Scholar]
- Yarbus AL. Eye Movements and Vision. Plenum Press; New York: 1967. [Google Scholar]
- Zuber BL, Crider A, Stark L. Saccadic suppression associated with microsaccades. QPR. 1964;74:244–249. [Google Scholar]
- Zuber BL, Stark L. Saccadic suppression: elevation of visual threshold associated with saccadic eye movements. Exp Neurol. 1966;16:65–79. doi: 10.1016/0014-4886(66)90087-2. [DOI] [PubMed] [Google Scholar]
- Zuber BL, Stark L. Microsaccades and the velocity-amplitude relationship for saccadic eye movements. Science. 1965;150:1459–60. doi: 10.1126/science.150.3702.1459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Individual data corresponding to (a) the maroon subject and (b) the green subject of Figure 8, showing error bars across runs.
Supplementary Figure 2. Bonferroni corrected p<0.05 GLM whole volume results for a single representative subject for Experiment 1: saccade size (a) 0.16°, (b) 0.38°, (c) 0.82°, (d) 1.68°, (e) 3.28°; Experiment 2: (f) microsaccades, (g) 0.16° saccades, (h) eyeblinks; Experiment 3: (i) 0.16° background shifts. Note that the whole volume subsumed 7 slices in experiment 1, and 11 slices in experiments 2 and 3, centered along the orientation of the calcarine sulcus and passing through hMT+, the thalamus, the superior temporal sulcus and frontal lobes. Because these GLM results are not from a whole brain functional data set, a lack of activation means either non-significant activation inside the volume, or unknown activation outside the volume.
Supplementary Figure 3. This is a linear-linear version of Figure 3, showing the main sequence for visually-guided saccades.
Supplementary Figure 4. Images of the limbus eyetracker used.
Note: If we are permitted to publish online supplementary figures, we would also be willing to publish as supplementary material our matlab code used for all analyses.