Abstract
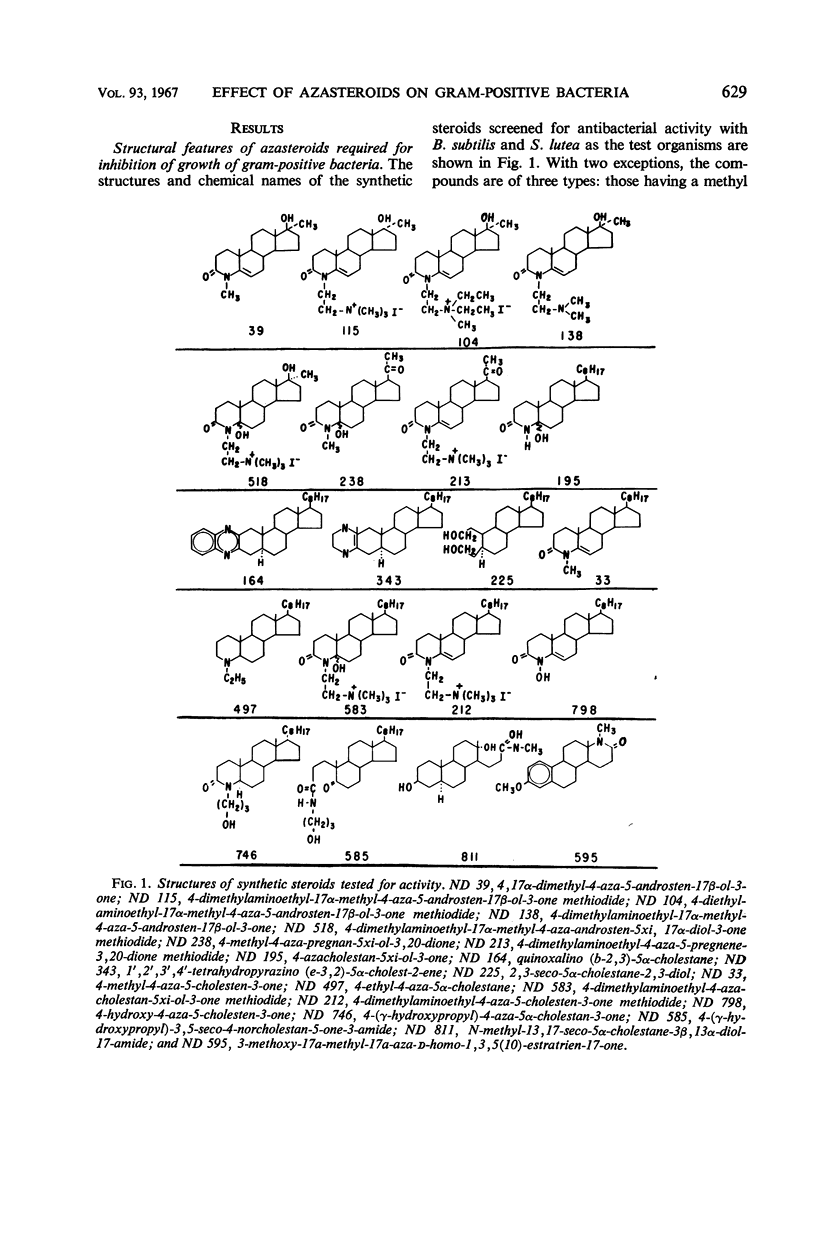
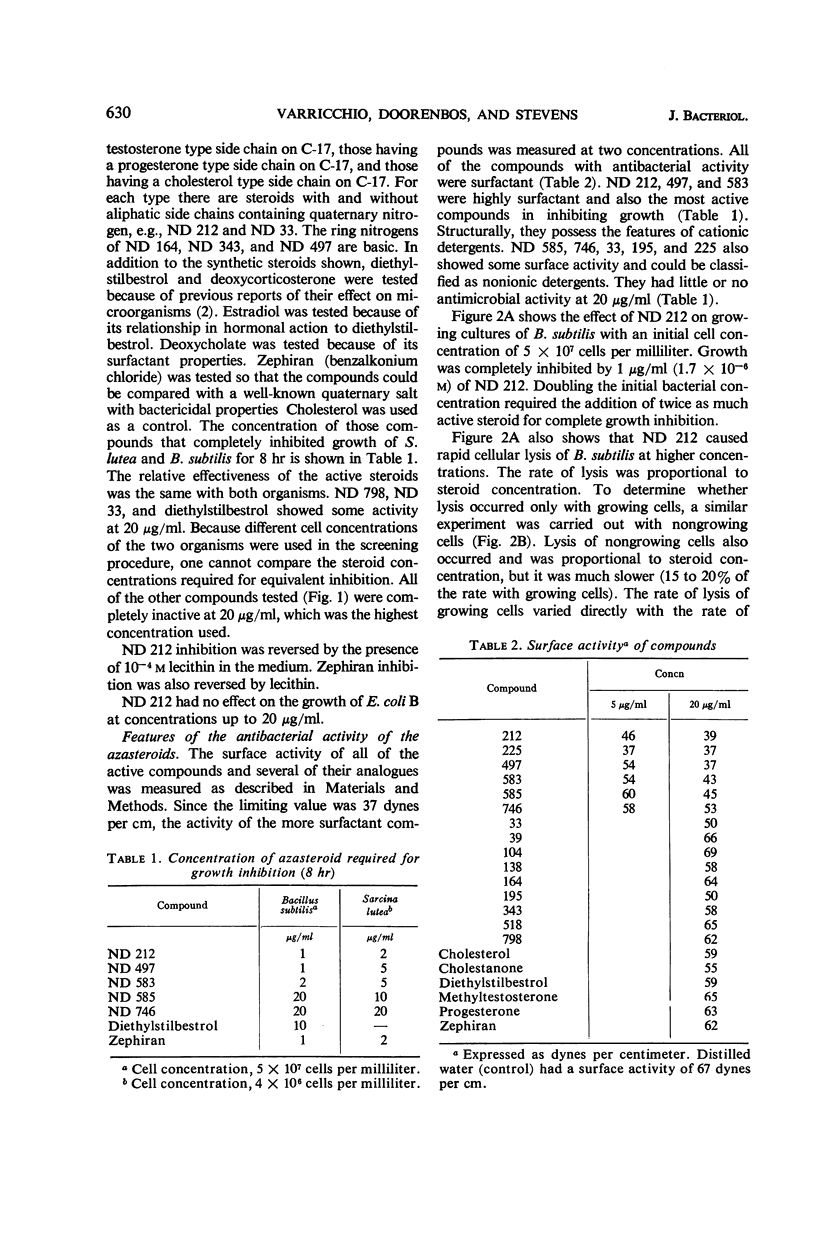
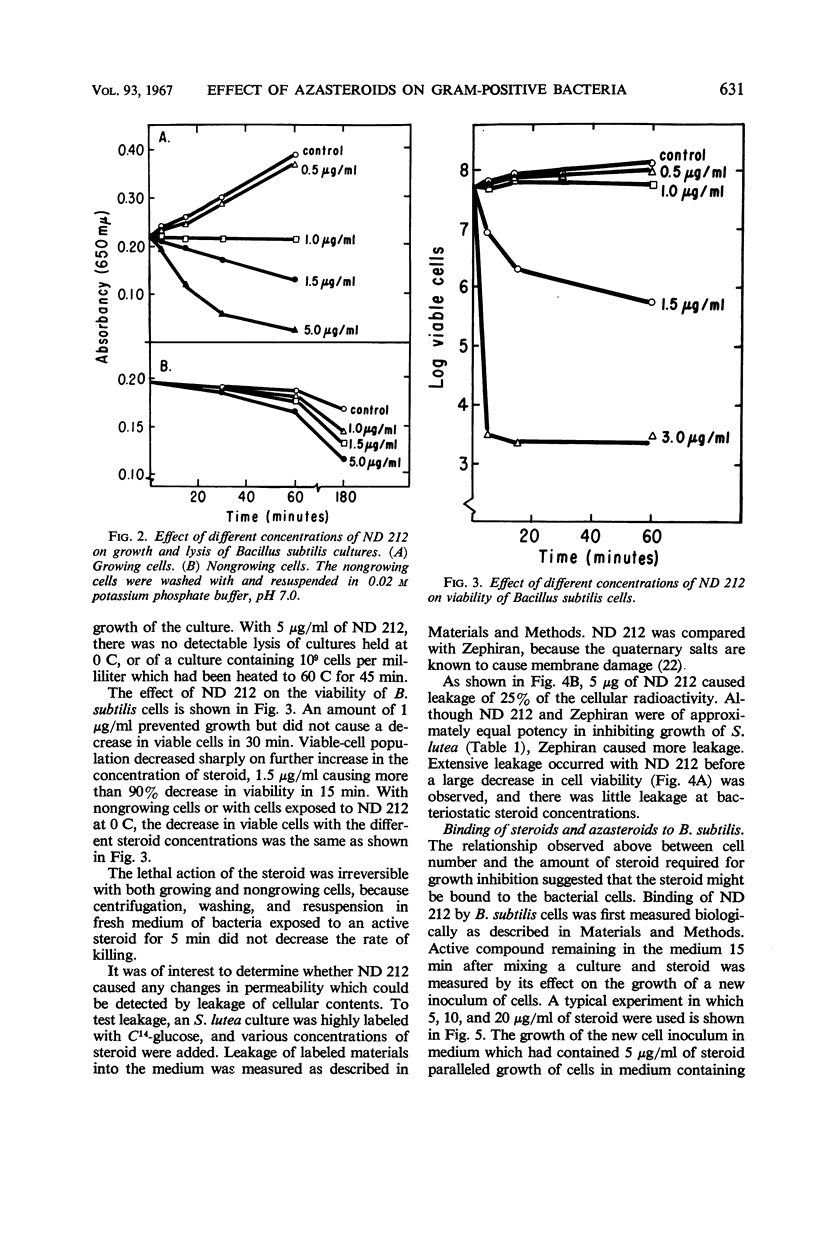
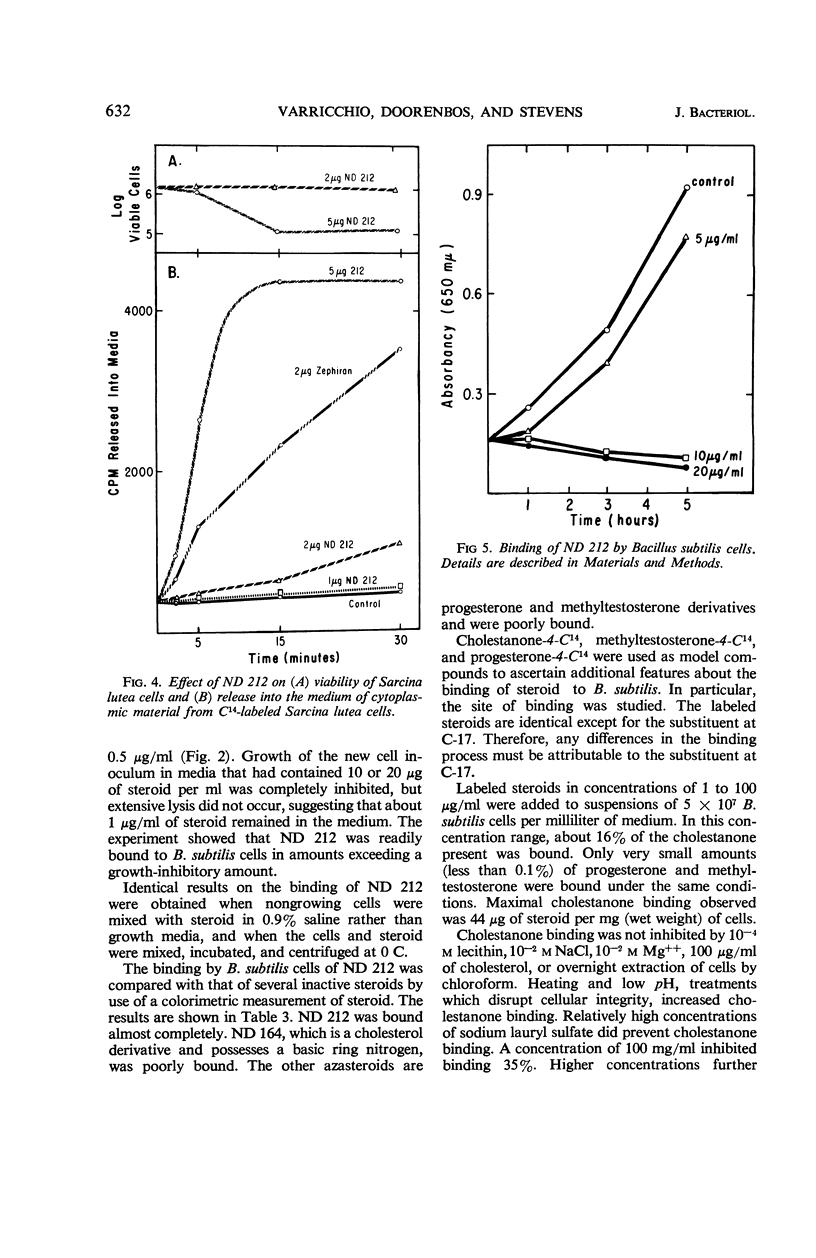
A group of nitrogen-containing steroids closely related in structure was screened for antibacterial activity, by use of Bacillus subtilis and Sarcina lutea as the test organisms. The most active compounds were cholesterol derivatives containing a tertiary or quaternary nitrogen in, or attached to, the A ring. Similar methyltestosterone or progesterone derivatives were inactive. All of the cholesterol derivatives that inhibited growth were surfactant, and, structurally, they would be classified as cationic detergents. Some of the inactive compounds were surfactant, but, structurally, they would be classified as nonionic detergents. Certain features of the antibacterial activity of one of the active steroids—ND 212 (4-dimethylaminoethyl-4-aza-5-cholesten-3-one methiodide)—were studied. Growth of a culture of B. subtilis containing 5 × 107 cells per milliliter was inhibited by 1 μg/ml (1.7 × 10−6m) of ND 212. The amount of growth inhibition was directly related to both cell and steroid concentration. Loss of viability was rapid and irreversible. With B. subtilis, cell lysis was observed. With S. lutea grown in C14-glucose, ND 212 caused release into the media of up to 25% of the cellular radioactivity. Extensive leakage occurred before loss of viability was observed. At bacteriostatic azasteroid concentrations, there was little leakage. ND 212 was readily bound in large amounts to B. subtilis cells. Inactive azasteroids were bound poorly. C14-cholestanone was also bound, whereas C14-methyltestosterone and C14-progesterone were not bound in significant amounts. At least 50% of the bound C14-cholestanone was associated with the membrane fraction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVIGAN J. A method for incorporating cholesterol and other lipides into serum lipoproteins in vitro. J Biol Chem. 1959 Apr;234(4):787–790. [PubMed] [Google Scholar]
- BUETOW D. E., LEVEDAHL B. H. RESPONSES OF MICROORGANISMS TO STEROLS AND STEROIDS. Annu Rev Microbiol. 1964;18:167–194. doi: 10.1146/annurev.mi.18.100164.001123. [DOI] [PubMed] [Google Scholar]
- Doorenbos N. J., Dorn C. P., Jr Steroids 23. Synthesis fo some ring A-fused heterocyclic steroids. J Pharm Sci. 1965 Aug;54(8):1219–1221. doi: 10.1002/jps.2600540832. [DOI] [PubMed] [Google Scholar]
- Doorenbos N. J., Havranek R. E. Steroids XXII. 4-Dialkylaminoethyl-4-aza-cholestanes and androstanes. J Pharm Sci. 1965 Aug;54(8):1163–1166. doi: 10.1002/jps.2600540814. [DOI] [PubMed] [Google Scholar]
- Doorenbos N. J., Wu M. T. Use of N,N'-dicyclohexylcarbodiimide in the synthesis of heterocyclic steroids. Chem Ind. 1965 Apr 10;15:648–648. [PubMed] [Google Scholar]
- EIK-NES K., SCHELLMAN J. A., LUMRY R., SAMUELS L. T. The binding of steroids to protein. I. Solubility determinations. J Biol Chem. 1954 Jan;206(1):411–419. [PubMed] [Google Scholar]
- EPHRATI-ELIZUR E., SRINIVASAN P. R., ZAMENHOF S. Genetic analysis, by means of transformation, of histidine linkage groups in Bacillus subtilis. Proc Natl Acad Sci U S A. 1961 Jan 15;47:56–63. doi: 10.1073/pnas.47.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIED J., KRAKOWER G. W., ROSENTHAL D., BASCH H. STRUCTURE-ACTIVITY RELATIONSHIPS IN THE FIELD OF ANTIBACTERIAL STEROID ACIDS. J Med Chem. 1965 May;8:279–282. doi: 10.1021/jm00327a001. [DOI] [PubMed] [Google Scholar]
- GALE E. F. MECHANISMS OF ANTIBIOTIC ACTION. Pharmacol Rev. 1963 Sep;15:481–530. [PubMed] [Google Scholar]
- GODTFREDSEN W. O., JAHNSEN S., LORCK H., ROHOLT K., TYBRING L. Fusidic acid: a new antibiotic. Nature. 1962 Mar 10;193:987–987. doi: 10.1038/193987a0. [DOI] [PubMed] [Google Scholar]
- HARTMAN R. E., HOLMLUND C. E. Binding of steroids by microorganisms. J Bacteriol. 1962 Dec;84:1254–1259. doi: 10.1128/jb.84.6.1254-1259.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- SMITH R. F., SHAY D. E., DOORENBOS N. J. ANTIMICROBIAL ACTION OF NITROGEN-CONTAINING STEROIDS. J Bacteriol. 1963 Jun;85:1295–1299. doi: 10.1128/jb.85.6.1295-1299.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH R. F., SHAY D. E., DOORENBOS N. J. RELATIONSHIP OF SURFACTANT PROPERTIES OF SOME SYNTHETIC STEROIDS TO BACTERICIDAL ACTION. J Pharm Sci. 1964 Oct;53:1214–1216. doi: 10.1002/jps.2600531019. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Freer J. H. Composition of the membranes isolated from several Gram-positive bacteria. Biochim Biophys Acta. 1965 Oct 18;107(3):531–538. doi: 10.1016/0304-4165(65)90197-2. [DOI] [PubMed] [Google Scholar]
- Smith R. F., Shay D. E. Steroid lysis of protoplasts and effects of stabilizers and steroid antagonists. Appl Microbiol. 1965 Sep;13(5):706–712. doi: 10.1128/am.13.5.706-712.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varricchio F. Inhibition by azasteroids of reduced nicotinamide adenine dinucleotide oxidation with membrane fragments from Bacillus subtilis. Appl Microbiol. 1967 Jan;15(1):206–207. doi: 10.1128/am.15.1.206-207.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



