Abstract
Natriuretic peptides (NPs) are a family of cardiac- and vascular-derived hormones that are well known for regulating blood pressure, but their expression in the brain poses an intriguing yet unanswered question concerning their roles in the nervous system. Here, we report several unique activities of these hormones in regulating axonal development of dorsal root ganglion (DRG) neurons in the spinal cord. First, the C-type NP (CNP) is expressed in a restricted area of the dorsal spinal cord and provides a cue that is necessary for bifurcation of central sensory afferents. Second, in the culture of embryonic DRG neurons, CNP stimulates branch formation, induces axon outgrowth, and attracts growth cones. Furthermore, these activities are mediated by cyclic guanosine-3′,5′-monophosphate (cGMP) signaling and can be elicited by other members of NP hormones. Thus, NPs represent a new class of extracellular factors that regulate key axonal processes during development. Because their receptors are present in many regions of the embryonic and adult brain, we propose that these hormones have wide influence on the development and function of the nervous system.
Keywords: axon branching, guidance, outgrowth, sensory neurons, cGMP
Atrial natriuretic peptide (ANP), brain NP (BNP), and C-type NP (CNP) are 3 structurally related peptide hormones (22- to 32-aa residues) that are involved in homeostatic control of body water and salt (1). They are predominantly produced by the heart and blood vessels in response to increased blood pressure. They are synthesized as peptide precursors (or preprohormones) and cleaved by proteases before or after secretion. The mature hormones stimulate natriuresis and blood vessel relaxation by binding to 2 natriuretic peptide receptors (NPRs), Npr1 and Npr2 (also known as NPR-A and NPR-B), that catalyze the production of cyclic guanosine-3′,5′-monophosphate (cGMP).
Since the first discovery of NPs in the heart nearly 30 years ago, it has been found that both NPs and Npr receptors are also expressed in the brain, with CNP and Npr2 being the most abundant (1). Cell culture studies have implicated NPs in regulating transmitter release, synaptic transmission, and neuronal survival (2), but their physiological roles in the nervous system remain to be established. In addition, the transcripts encoding the precursor of these hormones are found in the developing nervous system (3), and in vitro studies have suggested their potential involvement in neural development (4, 5), but their precise functions in vivo are not clear (2).
Recent studies of cGMP signaling provided some hints on the possible functions of NPs in axonal development. Activation of cGMP signaling, either by pharmacological reagents or by overexpression of the downstream cGMP-dependent protein kinase I (PrkG1), promotes branch formation of cultured embryonic dorsal root ganglion (DRG) neurons (6). Genetic deletion of PrkG1 leads to the disruption of sensory axon bifurcation, a branching process that creates 2 axons from 1, in the developing spinal cord (6, 7). An identical defect is also present in a spontaneous mutant mouse (cn) with a mutation mapped to the Npr2 gene (7), suggesting that CNP, the ligand that preferentially binds to Npr2, could be the environmental cue responsible for generating bifurcated axonal branches. These results suggest an intriguing hypothesis that NPs provide a general extracellular mechanism to regulate different processes, such as branching and guidance, during axonal development via the regulation of cGMP signaling.
To test this hypothesis, we first examined a spontaneous mouse mutant with a mutation in the Nppc gene encoding the CNP precursor and found a similar bifurcation defect during sensory afferent development. In addition, we conducted a systematic analysis of the functions of NPs in axonal development by using the primary culture of DRG neurons. We found that NPs positively regulate axon branching, outgrowth and guidance, and these activities are mediated by their cognate receptors and cGMP signaling. Thus, our study provides direct genetic and biochemical evidence to demonstrate several unique functions of NPs in axonal development and suggests broader roles of these hormones in the development and function of the nervous system.
Results
CNP Precursor Is Expressed in the Developing Spinal Cord.
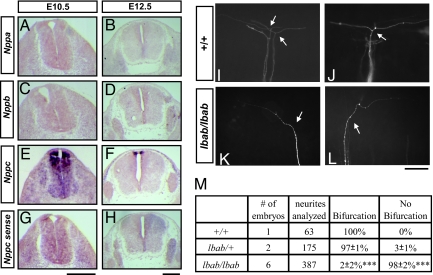
To understand the potential in vivo function of NPs, we first used RNA in situ hybridization to examine the transcripts encoding different NP precursors and their receptors in the developing spinal cord [Fig. 1 A–H and supporting information (SI) Fig. S1]. As shown by the previous report (4), neither precursor for ANP or BNP is present in the embryonic spinal cord (Fig. 1 A–D), but Nppc, the precursor for CNP, is present in specific regions during early development (Fig. 1 E and F). Its transcript appears in the dorsal part of the spinal cord as early as embryonic day 10.5 (E10.5) (Fig. 1B), the time when nascent axons from sensory neurons just start to bifurcate after reaching the dorsal root entry zone (DREZ); and by E12.5, it becomes more restricted to the cells surrounding the roof plate (Fig. 1C).
Fig. 1.
CNP is expressed in the dorsal spinal cord and required for sensory axon bifurcation. (A–H) Expression of NPs in the spinal cord and DRG revealed by digoxigenin-labeled RNA probes for NP precursors Nppa (A and B), Nppb (C and D), and Nppc (E and F) in the cross sections of E10.5 (A, C, E, and G) and E12.5 (B, D, F, and H) mouse embryos. A sense probe for Nppc was used as a control (G and H). (Scale bar: 500 μm.) (I–L) Disruption of DRG afferent bifurcation in the lbab mutant embryos is revealed by DiI labeling at the single-cell resolution. Images were taken from the lateral side of E13.5 spinal cords. Normally in the wild-type (+/+) spinal cord, sensory axons bifurcate (arrows) at the DREZ, resulting in 2 daughter branches that extend in opposite directions (I and J). However, in the lbab mutant spinal cords (K and L), 1 of the branches is missing, whereas the other appears to grow normally by extending in either the rostral or the caudal direction after turning (arrows). (Scale bar: 100 μm.) (M) Quantification of the DiI analysis in I–L. The percentage of bifurcated axons is listed for comparison between different genotypes (mean ± SD from 3 litters). Almost none of the afferents bifurcate in the lbab mutants as compared to the wild-type or heterozygote littermates (***, P < 0.001, t test). The number of embryos and the number of afferents analyzed are also included.
We also examined the expression of NP receptors, including Npr1, Npr2, and the cyclase-deficient receptor Npr3. Consistent with the previous report (7), only Npr2, the high-affinity receptor for CNP, is expressed in the DRG (Fig. S1). Like PrkG1 (Fig. S1 G and H) (6, 8), Npr2 expression in the DRG starts at E10.5 and remains at a similar level at E12.5 (Fig. S1 C and D). Thus, CNP is present in the developing spinal cord at the time when its receptor and downstream signaling target are made in the DRG, and this expression pattern correlates well with its role in regulating sensory afferent bifurcation.
Loss of CNP Leads to Impaired Sensory Axon Bifurcation.
To establish the developmental function for CNP, we next examined sensory afferents in the long bone abnormality (lbab) mouse, a spontaneous autosomal recessive mutant that was isolated in the Jackson laboratory on the basis of its reduced body size and long bone morphology during postnatal development (Mouse genome informatics MGI Ref. ID J:79175; http://mousemutant.jax.org/articles/lbab.html). These phenotypes were attributed to abnormal bone development in the absence of CNP-Npr2 signaling, as similar defects were found in mice with targeted deletion of CNP or Npr2 (9, 10) and in the spontaneous Npr2 mutant (cn) (11). Furthermore, a missense mutation was recently mapped to the Nppc gene that results Arg to Gly substitution in the receptor binding and hence the loss of CNP activity (12). Because the general embryonic development is not affected in this mutant, the lbab mouse provides a useful loss-of-function model to investigate CNP functions in the developing spinal cord.
To examine sensory bifurcation, we used the lipophilic dye Dil to label and visualize individual DRG afferents in E13.5 embryos. As described in refs. 6 and 13, almost all of the sensory axons in the wild-type (n = 63) or the heterozygous (n = 175) embryos bifurcate at the DREZ and have 2 branches that extend in opposite directions along the rostrocaudal axis (Fig. 1 I, J, and M). However, 98% of the afferents analyzed in the lbab mutant embryos (n = 387) turn randomly into the longitudinal track without making the second branch (Fig. 1 K–M). In contrast, collateral branches from the proprioceptive neurons that normally just start to sprout at this age can be still found and have normal length and projection inside the spinal cord as revealed by bulk labeling (Fig. S2). This phenotype is identical to the bifurcation defect found in the Npr2 and PrkG1 mutant embryos (6, 7) and demonstrates the requirement of CNP for sensory axon bifurcation in the developing spinal cord.
CNP Promotes Axon Branching of Dissociated DRG Neurons in Culture.
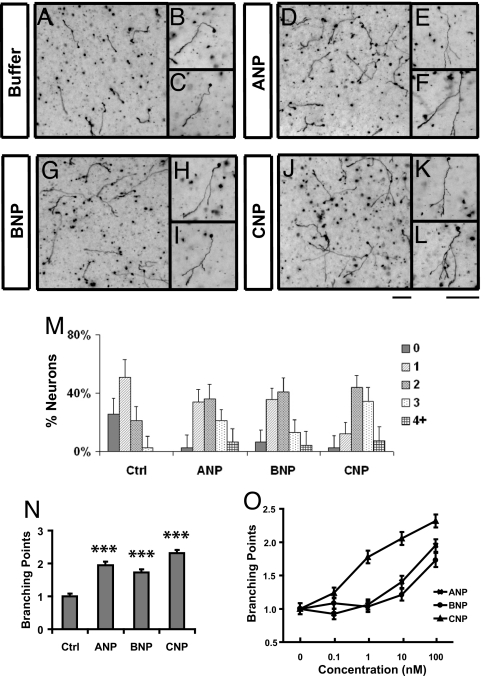
Following the in vivo study, we next asked whether CNP can indeed promote axon branching in an in vitro assay that was previously used to identify and characterize the cGMP pathway (6). In the assay, E14 rat DRG neurons that are equivalent to E12.5 mouse cells were dissociated and cultured in collagen gels. They normally have simple morphology after 2 days in culture, growing only 1 axon and on average having less than one branch (Fig. 2 A–C). However, addition of 100 nM CNP to the culture on the second day led to enhanced branch formation. Only 24 h after the CNP treatment, a higher percentage of neurons generated more branches (Fig. 2 J–L) and the number of branching points reached 2.3 ± 0.1 (mean ± SEM), an ≈2.4-fold increase over the control culture. This effect is similar to direct activation of PrkG1 by a cGMP analog, Br-cGMP (6). In addition, it was nearly abolished by KT5823, a PrkG1 inhibitor (Fig. S3). Thus, CNP can indeed regulate axon branching of sensory neurons by activating cGMP signaling in culture.
Fig. 2.
NPs promote axon branching of dissociated DRG neurons in culture. (A–L) Dissociated E14 DRG neurons were cultured in collagen gels in the presence of NGF (25 ng/mL) for 24 h and then treated with buffer (control, A–C) or 100 nM ANP (D–F), BNP (G–I), or CNP (J–L). After being cultured for another day, they were fixed and stained with neurofilament antibodies. Regions of the cultures are shown at low magnification in A, D, G, and J, whereas individual cells are shown at high magnification in B, C, E, F, H, I, K, and L. Neurons treated with all NPs showed a significant increase in branch formation. (Scale bars: 100 μm.) (M and N) Distribution of neurons with different numbers of branches (M) and the average number of branching points (N) (***, P < 0.001, ANOVA test) measured from the above cultures treated with different NPs (100 nM). (O) Comparison of the average number of branching points in the cultures treated with different doses of NPs. More than 80 neurons were analyzed for each condition, and the results were plotted as mean ± SD in M and mean ± SEM in N and O.
CNP Stimulates Axon Outgrowth from DRG Explants.
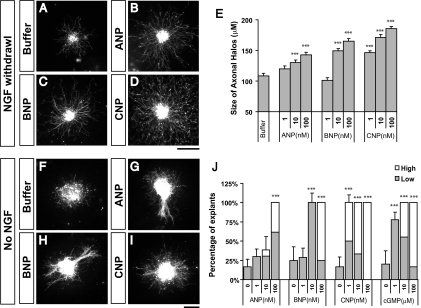
Extracellular cues, such as netrins, neurotrophins, and semaphorins, can regulate multiple aspects of axonal development, including outgrowth, guidance, and branching (14–17). Therefore, we next asked whether CNP, a secreted peptide, has the ability to regulate DRG axon outgrowth. To avoid the dominant effect of neurotrophins, we cultured DRG explants in the presence of NGF (5 ng/mL) for a day and then removed it on the second day. Under this condition, axons grew radially from the explants but did not form dense axonal halos that were often seen in the culture with the continuous presence of NGF. Interestingly, however, addition of CNP (1–100 nM) after the removal of NGF allowed the explants to grow not only longer but also denser axons around them (Fig. 3D). This can be best illustrated by the halo size, which increased by nearly 2-fold in the presence of CNP (Fig. 3 D and E).
Fig. 3.
NPs induce axon outgrowth from DRG explants. (A–D) E14 DRG explants were cultured in collagen gels for 1 day in the presence of NGF (5 ng/mL) and then replaced with the growth medium without NGF and treated with buffer (A) or 100 nM ANP (B), BNP (C), or CNP (D). They were cultured for another day before fluorescent staining by using neurofilament antibodies. Explants treated with all 3 NPs have increased halo size (B–D) as compared to the buffer control (A). (Scale bar: 100 μm.) (E) Quantification of the outgrowth activity by measuring the halo size (mean ± SEM) treated with NPs at different concentrations. ANP or BNP at 10–100 ng/mL and CNP at 1–100 ng/mL significantly increase in halo size as compared to the buffer control (***, P < 0.001, ANOVA test). (F–I) E14 DRG explants were cultured in collagen gels in the absence of NGF and treated with buffer (F) or 100 nM ANP (G), BNP (H), or CNP (I) for 2 days before immunostaining. Axonal bundles and halos can be often seen associated with induced outgrowth by NPs. (J) Quantification of the outgrowth activity by counting the percentage of explants that grew axons (mean ± SEM) in the culture treated with NPs or Br-cGMP at different concentrations (F–I). Significant numbers of explants grew either 1–5 axons (low, shaded box) or >5 axons (high, open box) in the cultures with ANP or BNP at 10–100 ng/mL and with CNP at 1–100 ng/mL (***, P < 0.001, ANOVA test). (Scale bar: 100 μm.)
To further test whether CNP alone could stimulate axonal outgrowth, we next tested the culture in the absence of neurotrophins. Only 20% of explants were able to grow axons after 48 h and none of them had >5 axons that are longer than 50 μm (Fig. 3 F and J). However, addition of CNP elicited extensive axonal outgrowth from the explants. At the lowest CNP concentration tested (1 nM), all of the explants grew axons with half having 1–5 axons (Fig. 3J, shaded bar) and half having >5 axons (Fig. 3J, open bar). At the highest concentration tested (100 nM, Fig. 3 I and J), all explants (n = 12) have >5 axons and some even formed axonal halos (Fig. 3I).
These 2 results indicate that CNP can mimic the outgrowth activity of extracellular factors like NGF for DRG neurons. In addition, this activity can be replicated by using Br-cGMP (Fig. 3J) and blocked by KT-5823 (Fig. S4), again indicating the involvement of cGMP signaling. Finally, the activity is not because of the increase in cell survival that might be promoted by CNP, as TUNEL staining did not reveal any decrease in the number of apoptotic cells (Fig. S5). Thus, CNP can serve as an extracellular cue to stimulate axon outgrowth.
Point Source of CNP Attracts the Growth Cones of DRG Neurons.
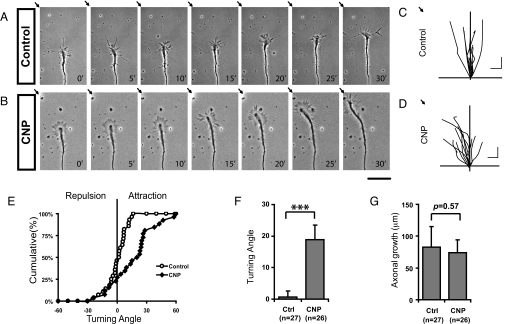
Following the study of outgrowth, we next tested whether CNP could guide axons by presenting the rapidly migrating DRG growth cones with a point source of CNP produced from a pipette (18). As shown in Fig. 4A, growth cones from DRG neurons normally followed a linear trajectory. Occasionally, they changed growth directions (Fig. 4C), but the overall turning angle is ≈0.7° ± 1.9° (mean ± SEM, Fig. 4F). However, significant turning responses were observed for the growth cones that were presented with a CNP gradient, as illustrated by the reorientation of the growth cones (Fig. 4B). At the end of 30-min recording, the majority of the growth cones migrated toward the tip of the pipette (Fig. 4 D and E) with an average turning angle of 18.9° ± 4.5° (mean ± SEM, Fig. 4F) but no significant change in growth rates (Fig. 4 C, D, and G). This attractive response is best illustrated by the time-lapse movie (Movie S1), in which the growth cone first reoriented itself toward the pipette, but after the pipette was repositioned to a new location, the growth cone was able to follow it and change its growth direction. Thus, CNP can serve as a guidance cue to positively control the growth direction of sensory axons.
Fig. 4.
Attractive turning responses of DRG growth cones in the CNP gradient. (A and B) Growth cone turning assays were carried out in the overnight culture of dissociated E14 DRG neurons. Representative phase-contrast images with 5-min intervals demonstrate the nearly linear growth of a control axon (A) and the turning response of a growth cone (B) in the CNP gradient created from a pipette tip (indicated by the arrows at the upper left corner). (Scale bar: 50 μm.) (C and D) Composite drawings of the growth cone trajectory for a period of 30 min of a population of neurons in the control condition (C) or in the CNP gradient (D). (Scale bar: 20 μm.) (E) A cumulative plot of the turning angles of all growth cones at the end of 30 min in the absence (○) or presence (♦) of CNP gradients. (F and G) The average turning angles (F, mean ± SEM; ***, P < 0.003; Mann–Whitney test) and the average distance of axonal extension (G, mean ± SEM, P = 0.57, Mann–Whitney test) are compared during the 30-min period calculated for the above growth cones in the control condition (Ctrl, n = 27) or in the CNP gradient (n = 26).
Specificity of NPs in Axonal Development.
CNP is structurally related to ANP and BNP, which contain a disulfide-linked ring structure with conserved 17-aa residues (1). Because NPs are found in the brain and the circulation, we asked whether the positive activities described above could be also elicited by the other 2 NPs. By using the in vitro assays, we found that addition of either ANP or BNP to dissociated DRG neurons stimulated branch formation (Fig. 2 D–I) and the presence of either NP stimulated axonal outgrowth in the explant assay (Fig. 3 G and H). Interestingly, however, the doses needed for both NPs are much higher (10- to 100-fold) than that needed for CNP. In the branching assay, CNP was active as low as 1 nM whereas ANP and BNP required at least 100 nM to show a comparable activity (Fig. 2O). The same trend holds for axon outgrowth, as CNP promoted outgrowth at 1 nM, whereas ANP and BNP were not active at the same concentration (Fig. 3J). When increased to 100 nM, both NPs elicited outgrowth, with some explants making 1–5 axons and others making more, but these responses are comparable only to that of CNP at 1–10 nM (Fig. 3J). These results are consistent with Npr2 being the only NP receptor expressed in the neurons used in these assays (Fig. S1 and Fig. S6) (7) and correlate well with the binding affinity of NPs to their cognate receptors, as ANP and BNP preferably bind to Npr1 whereas CNP binds to Npr2 (19). Thus, these NP activities depend on the presence of specific Npr receptors.
Discussion
NPs are known for their unique function in regulating vascular physiology, but here we report several unique activities of these hormones in axonal development. First, we provide both biochemical and genetic evidence to show the involvement of CNP in axon branching. In culture, CNP stimulates the formation of axonal branches whereas in the CNP-deficient mice none of the sensory afferents generated bifurcated branches. These 2 lines of evidence strongly support the notion that CNP promotes branching, despite the fact that the branches formed in culture do not completely replicate the bifurcation morphology in vivo. One explanation for such difference is that bifurcation is a special case of branching as previously proposed (6) and may require other environmental cues like Slit to generate the exact branching pattern (13). To fully understand this simple yet faithful process, further study of the cooperation of these cues as well as reconstituting it in culture will be needed. Nonetheless, given the identical defect found in the mice lacking the functional Npr2 receptor and the downstream target PrkG1 (Fig. 1) (6, 7), and the restricted expression pattern of CNP, Npr2, and PrkG1 at a critical time of bifurcation (Fig. 1) (7), our study has clearly established a pathway consisting of CNP-Npr2-cGMP-PrkG1 in shaping such a simple branched structure.
In addition, we provide evidence that CNP can stimulate axon outgrowth and promote growth cone turning. Along with branching, these in vitro activities indicate that NPs can provide a general mechanism in regulating critical axonal processes during development. This conclusion is strengthened by the expression of CNP, which is found in many regions of the developing nervous system (3). Furthermore, these activities are all related to the positive regulation of axonal processes and consistent with the modulatory activities of cyclic nucleotides on guidance molecules (20). Although it remains to be determined how cGMP signaling achieves different morphological regulation, our result provides previously undescribed evidence to show that cGMP production activated directly by an extracellular cue at the cell surface can guide growth cones, stimulate axon outgrowth, and promote branch formation.
Furthermore, our study points to broader roles of NPs in the nervous system, as both ANP and BNP can stimulate axon outgrowth and branching of DRG sensory neurons. Because of the lack of the high-affinity Npr1 receptor in the DRG neurons, they have much lower activities as compared to CNP, indicating that the specificity of the regulation can be achieved at the receptor level. As both Npr1 and Npr2 receptors are expressed dynamically in various regions of the embryonic and adult brain (3, 21), our study of sensory neurons suggests that all 3 NPs can directly regulate axonal development in neurons with the appropriate receptors. Furthermore, high concentrations of NPs present under abnormal conditions may activate the low-affinity receptors to cause unwanted cGMP activation and disrupt normal physiology.
In conclusion, on the basis of the biochemical and genetic evidence presented in this study, we propose that NPs represent a unique class of extracellular cues that control axon branching, outgrowth, and guidance during neural development. Like endothelins, another group of vascular derived peptides that were recently shown to guide sympathetic axon development (22), these hormones offer another molecular link between axonal development and vascular regulation. The identification of their neuronal functions raises an intriguing possibility that hormones like NPs that are produced chronically in response to vascular diseases such as hypertension (1) may contribute to changes in brain development and cognitive functions under certain medical conditions (23, 24).
Materials and Methods
Reagents and Animals.
Purified ANP, BNP, and CNP and 8-Br-cGMP were from Sigma and KT5823 was from Calbiochem. All animal work followed the protocols approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Southern California (Los Angeles, CA) under the National Institutes of Health guidelines. Rat embryos were collected from Sprague–Dawley females (Charles River Laboratories) with the plug day designated as E0. The lbab mouse was obtained from the Jackson Laboratory (stock no. 004521), and mutant animals were generated by crossing heterozygous animals with the plug day as E0.5. The genotype was determined by AvaII digestion of the PCR product, amplified from primers 5′-CTCTTGGGTGCAGAGCTAGG-3′ and 5′-AGCTGGTGGCAATCAGAAAA-3′ (12).
In Situ Hybridization.
Digoxigenin-labeled RNA probes were used to detect mRNA transcripts in cryosections (16 μm) (6). The entire coding region was used for labeling mouse Nppa, Nppb, and Nppc. Probes for Npr1 (1,891–2,769 bp), Npr2 (493–1,342 bp), and Npr3 (707–1,305 bp) were adapted from the Allen Brain Atlas (21).
Analysis of Sensory Axonal Projections in Mice.
To visualize single sensory afferent projections in the spinal cord, a small DiI crystal was deposited by iontophoresis in the DRG attached to the spinal cord of E13.5 mouse embryos (13). The labeled axons were visualized from the lateral side of the dorsal spinal cord in an open book preparation.
Branching Assay.
E14 rat DRG neurons were dissociated and cultured in collagen gel as described in ref. 25. After 1 day in culture, they were treated with different NPs or other reagents and cultured for another 24 h. Neurofilament immunostaining and quantification of branching were done as described in ref. 6.
Outgrowth Assay.
DRGs were cut into small pieces and cultured in collagen gels in the presence or absence of NGF (5 ng/mL) and with different doses of NPs or 8-Br-cGMP. They were fixed and stained by using neurofilament antibodies and Cy3- or HRP-labeled secondary antibodies (13). To quantify the size of axonal halos in the presence of NGF, the radius of the entire halo was measured at every 45° and then subtracted the radius of the explant. To quantify outgrowth in the absence of NGF, explants with axons longer than 50 μm were classified into 2 groups: low for those making 1–5 axons and high for those growing >5 axons or axonal halos. All experiments were repeated twice and 5–6 explants from each condition were analyzed.
Growth Cone Turning Assay.
The turning assay was performed following the method described in refs. 18 and 26 with modifications. Dissociated DRG neurons were grown on poly-D-lysine (PDL)/Laminin-coated glass coverslips. After being cultured overnight, they were transferred to a heat-controlled (37 °C) open chamber on an Axiovert microscope (Zeiss). Microscopic CNP gradients were produced from a capillary glass pipette (1-μm opening) loaded with a 10-μM CNP solution by repetitive application (2 Hz and 20 ms) of a positive pressure of 3 psi (18). The pipette tip was positioned 50 μm away from the growth cone and ≈45° from the initial direction of extension (defined by the 50-μm segment from the growth cone tip). Images were captured by an Axiocam (Zeiss) and analyzed. The turning angle was defined by the angle between the initial direction of neurite extension and a straight line connecting the positions of the growth cone at the onset and the end of the 30-min period. Only growth cones with a net extension >10 μm were included in the analysis.
Supplementary Material
Acknowledgments.
We thank members of the Ma laboratory for helpful discussion, Gage Crump and Zhigang He for critical comments on the manuscript, and Fritz Rathjen and Hannes Schimdt for communicating their work before submission. This work was funded by Whitehall Foundation Research Grant 2007-08-104 (to L.M.) and National Institutes of Health Grant NS062047 (to L.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906880106/DCSupplemental.
References
- 1.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 2.Cao LH, Yang XL. Natriuretic peptides and their receptors in the central nervous system. Prog Neurobiol. 2008;84:234–248. doi: 10.1016/j.pneurobio.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Ryan MC, Gundlach AL. Ontogenic expression of natriuretic peptide mRNAs in postnatal rat brain: Implications for development? Brain Res Dev Brain Res. 1998;105:251–268. doi: 10.1016/s0165-3806(97)00183-1. [DOI] [PubMed] [Google Scholar]
- 4.DiCicco-Bloom E, et al. Embryonic expression and multifunctional actions of the natriuretic peptides and receptors in the developing nervous system. Dev Biol. 2004;271:161–175. doi: 10.1016/j.ydbio.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 5.Kishimoto I, et al. C-type natriuretic peptide is a Schwann cell-derived factor for development and function of sensory neurones. J Neuroendocrinol. 2008;20:1213–1223. doi: 10.1111/j.1365-2826.2008.01778.x. [DOI] [PubMed] [Google Scholar]
- 6.Zhao Z, et al. Regulate axon branching by the cyclic GMP pathway via inhibition of glycogen synthase kinase 3 in dorsal root ganglion sensory neurons. J Neurosci. 2009;29:1350–1360. doi: 10.1523/JNEUROSCI.3770-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt H, et al. The receptor guanylyl cyclase Npr2 is essential for sensory axon bifurcation within the spinal cord. J Cell Biol. 2007;179:331–340. doi: 10.1083/jcb.200707176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt H, et al. cGMP-mediated signaling via cGKIalpha is required for the guidance and connectivity of sensory axons. J Cell Biol. 2002;159:489–498. doi: 10.1083/jcb.200207058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chusho H, et al. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc Natl Acad Sci USA. 2001;98:4016–4021. doi: 10.1073/pnas.071389098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tamura N, et al. Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc Natl Acad Sci USA. 2004;101:17300–17305. doi: 10.1073/pnas.0407894101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuji T, Kunieda T. A loss-of-function mutation in natriuretic peptide receptor 2 (Npr2) gene is responsible for disproportionate dwarfism in cn/cn mouse. J Biol Chem. 2005;280:14288–14292. doi: 10.1074/jbc.C500024200. [DOI] [PubMed] [Google Scholar]
- 12.Jiao Y, et al. A single nucleotide mutation in Nppc is associated with a long bone abnormality in lbab mice. BMC Genet. 2007;8:16. doi: 10.1186/1471-2156-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma L, Tessier-Lavigne M. Dual branch-promoting and branch-repelling actions of Slit/Robo signaling on peripheral and central branches of developing sensory axons. J Neurosci. 2007;27:6843–6851. doi: 10.1523/JNEUROSCI.1479-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy TE, Tessier-Lavigne M. Guidance and induction of branch formation in developing axons by target-derived diffusible factors. Curr Opin Neurobiol. 1995;5:83–90. doi: 10.1016/0959-4388(95)80091-3. [DOI] [PubMed] [Google Scholar]
- 15.Dent EW, Barnes AM, Tang F, Kalil K. Netrin-1 and Semaphorin 3A promote or inhibit cortical axon branching, respectively, by reorganization of the cytoskeleton. J Neurosci. 2004;24:3002–3012. doi: 10.1523/JNEUROSCI.4963-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messersmith EK, et al. Semaphorin III can function as a selective chemorepellent to pattern sensory projections in the spinal cord. Neuron. 1995;14:949–959. doi: 10.1016/0896-6273(95)90333-x. [DOI] [PubMed] [Google Scholar]
- 17.Yaron A, Huang PH, Cheng HJ, Tessier-Lavigne M. Differential requirement for Plexin-A3 and -A4 in mediating responses of sensory and sympathetic neurons to distinct class 3 Semaphorins. Neuron. 2005;45:513–523. doi: 10.1016/j.neuron.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Zheng JQ, Felder M, Connor JA, Poo MM. Turning of nerve growth cones induced by neurotransmitters. Nature. 1994;368:140–144. doi: 10.1038/368140a0. [DOI] [PubMed] [Google Scholar]
- 19.Barr CS, Rhodes P, Struthers AD. C-type natriuretic peptide. Peptides. 1996;17:1243–1251. doi: 10.1016/s0196-9781(96)00110-6. [DOI] [PubMed] [Google Scholar]
- 20.Song HJ, Poo MM. Signal transduction underlying growth cone guidance by diffusible factors. Curr Opin Neurobiol. 1999;9:355–363. doi: 10.1016/s0959-4388(99)80052-x. [DOI] [PubMed] [Google Scholar]
- 21.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 22.Makita T, et al. Endothelins are vascular-derived axonal guidance cues for developing sympathetic neurons. Nature. 2008;452:759–763. doi: 10.1038/nature06859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Starr JM. Blood pressure and cognitive decline in the elderly. Curr Opin Nephrol Hypertens. 1999;8:347–351. doi: 10.1097/00041552-199905000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Salonen JT, Heinonen OP. Mental retardation and mother's hypertension during pregnancy. J Ment Defic Res. 1984;28(Pt 1):53–56. doi: 10.1111/j.1365-2788.1984.tb01601.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang KH, et al. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell. 1999;96:771–784. doi: 10.1016/s0092-8674(00)80588-7. [DOI] [PubMed] [Google Scholar]
- 26.Lohof AM, Quillan M, Dan Y, Poo MM. Asymmetric modulation of cytosolic cAMP activity induces growth cone turning. J Neurosci. 1992;12:1253–1261. doi: 10.1523/JNEUROSCI.12-04-01253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.