Abstract
Despite 2 decades of research, no clear function for annexin A1 (AnxA1) has been established. Using AnxA1-KO mice, we show that tumor growth and metastasis are significantly decreased, whereas rodent survival and tumor necrosis are greatly increased when tumors grow in AnxA1-KO mice. Systems analysis of gene expression in these tumors specifically implicates 2 related vascular functions, angiogenesis and wound healing, in this impairment. Both tumor vascular development and wound healing are greatly retarded in KO tissues. Aortic ring assays reveal induced AnxA1 expression on sprouting endothelial cells of normal mice whereas KO aortas exhibit impaired endothelial cell sprouting that is rescued by adenoviral expression of AnxA1. Key differences in specific gene regulation may define new molecular pathways mediating angiogenesis, including a reset profile of pro- versus anti-angiogenic factors, apparently distinct for physiological versus pathological angiogenesis. These studies establish novel pro-angiogenic functions for AnxA1 in vascular endothelial cell sprouting, wound healing, and tumor growth and metastasis, thereby uncovering a new functional target for repairing damaged tissue and treating diseases such as cancer. They also provide critical new evidence that the tumor stroma and its microenvironment can greatly affect tumor progression and metastasis.
Keywords: systems biology, tumor vasculature, tumor microenvironment, cancer targets
Annexin A1 (AnxA1) was originally described as an anti-phospholipase A2 and glucocorticoid-inducible 37-kDa protein (1) and later cloned and identified as a member of the annexin superfamily of calcium-dependent phospholipid binding proteins (2). Although the exact function of AnxA1 remains unknown, it likely plays an important role in inflammation, leukocyte migration and accumulation, and phagocytosis (3). Other functions have been suggested, including cell signaling, apoptosis, and membrane trafficking (3). Yet AnxA1-KO mice are born without apparent developmental abnormalities that would support any singular or predominant function (4).
Recently, AnxA1 was discovered by subtractive proteomic mapping to be selectively expressed in vivo on the outer luminal surface of tumor but not normal vascular endothelial cells (ECs), where it can interact with specific antibodies injected intravenously to allow tumor-specific immuno-targeting and imaging (5). Targeted radioimmunotherapy greatly enhanced rat survival, even with advanced solid tumors (5). Immunohistochemistry confirms selective vascular expression in human tumors. Here, we hypothesize that tumor-induced vascular expression of AnxA1 is functionally important for tumor development. Using KO mice, we show that AnxA1 expression by host tissues can significantly influence tumor growth and metastasis in vivo. Inability to express AnxA1 disrupts EC function in angiogenesis and the expression of specific genes that define distinct molecular pathways mediating angiogenesis and wound healing.
Results
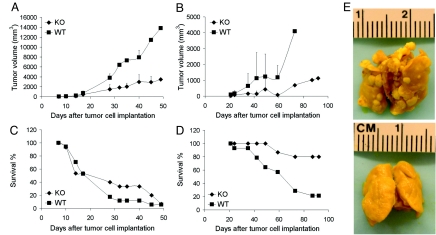
To study the effects of AnxA1 on tumor development, we injected syngeneic tumor cells into KO and WT congenic mice. Because AnxA1 can be expressed by some tumor cells (3), we chose Lewis lung carcinoma (LLC) cells, which readily express AnxA1 in cell culture and in tumors in vivo, and B16 melanoma cells, which do not [supporting information (SI) Fig. S1 A and B]. With s.c. injection of tumor cells, both tumors grow rapidly in the skin and the LLC tumors also produce spontaneous lung metastases. Western blots verified the absence of AnxA1 in lung and skin from KO mice (Fig. S1 C and D). We tracked tumor volume over time and found that growth of both B16 and LLC tumors was greatly retarded in KO mice (Fig. 1 A and B; P < 0.05). After 7 weeks, the B16 tumors in the WT mice were nearly 4 times larger than in the KO mice. The LLC tumors after 10 weeks were >5 times larger in the WT mice than in KO mice.
Fig. 1.
Impaired tumor growth and spontaneous metastasis with increased animal survival in AnxA1 KO mice. (A and B) Average tumor volume is shown for (A) the B16 tumors [KO (n = 15 mice) and WT (n = 17 mice)] and (B) LLC tumors [KO (n = 15 mice) and WT (n = 14 mice)]. (C and D) Mice surviving at indicated times post-implantation are expressed as percent of total number of mice for (C) B16 [KO (n = 15 mice) and WT (n = 17 mice)] and (D) LLC tumors [KO (n = 15 mice) and WT (n = 14 mice)]. (E) Representative photographs of lungs showing typical lack of metastases in KO but not WT mice. Lungs of mice with primary s.c. LLC tumors were excised at day 38 (WT) and day 59 (KO) after tumor cell implantation.
Tumors in the KO mice appeared to progress much less aggressively than tumors in the WT mice. Survival curves (Fig. 1 C and D) show that the percentage of surviving mice decreased more rapidly for WT than KO mice (P < 0.05). Twenty-eight days after B16 cell inoculation, < 20% of WT animals survived, whereas the KO mice did not reach 20% survival until 45 d. This effect was even more pronounced for LLC tumors, in which <20% of WT mice but >80% of KO mice survived after 85 d. Survival was clearly much greater in the KO mice.
The LLC tumor model can produce spontaneous metastases to the lung. Overall, 45% of the WT mice had multiple metastatic lesions in the lungs at the time of death, whereas no metastasis was detected in the KO mice (Fig. 1E), even when examined ≥3 weeks after the WT mice died. The weight and volume of the lungs of KO mice were normal, whereas the WT mouse lungs weighed twice as much as KO mouse lungs.
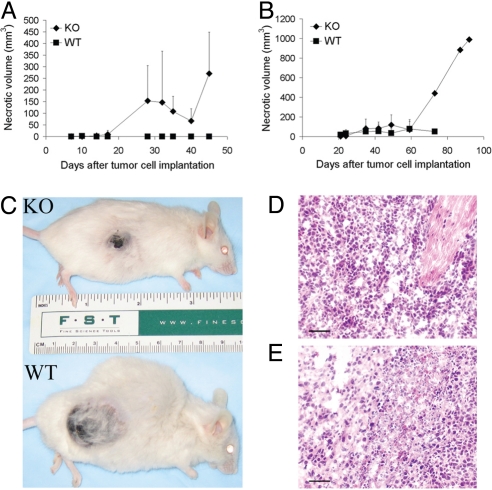
In both LLC and B16 tumor models, KO mice showed increased necrotic regions within the developing tumors (Fig. 2 A and B; P < 0.05). Although little to no necrosis was evident in the WT mice, necrosis was evident in KO mice (B16 tumors developed visible necrosis as early as 2 weeks after tumor cell implantation) and the necrotic volume increased over time (Fig. 2 A-E). Tumor section staining confirmed that cells in WT mice looked uniformly robust (Fig. 2 D) whereas significant tumor areas from KO mice exhibited shrinking and dying tumor cells (Fig. 2 E).
Fig. 2.
Increased necrosis in syngeneic tumors in AnxA1 KO mice. (A and B) Average necrotic volume in (A) B16 [KO (n = 15 mice) and WT (n = 17)] or (B) LLC [KO (n = 15 mice) and WT (n = 14 mice)] tumors of KO and WT mice (error bars indicate SD). (C) Representative KO and WT mice with B16 tumors. Note smaller tumor size with extensive necrosis in KO mouse, but larger tumor size and no necrosis in WT mouse. (D and E) Tissue staining by hematoxylin and eosin of formalin-fixed, paraffin-embedded tumor sections from WT (D) and KO (E) mice with LLC-derived tumors. (Scale bar, 100 μm.)
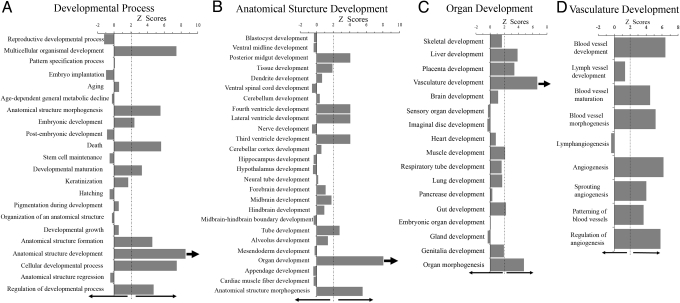
To begin in a non-biased manner to elucidate possible mechanisms underlying retarded tumor growth, we performed a systems analysis of gene expression in the B16 tumors collected from the KO and WT mice (Fig. 3). Affymetrix mouse genome expression microarrays revealed distinct expression profiles, which were further analyzed using Genomatix software and the Gene Ontology database. Among the 20 most general Gene Ontology categories for biological process, the developmental process was significantly over-represented (Z-score, 9.1). Breakdown of this process into its sub-levels showed several statistically significant over-representations (Z-score >2.0), including multicellular organismal development, anatomical structure morphogenesis, embryonic development, developmental maturation, anatomical structure formation and development, cellular developmental process, and regulation of developmental process (Fig. 3A). Death also was over-represented, consistent with our findings of increased necrosis. Most categories were underrepresented or not significantly represented, including stem cell maintenance and anatomical structure regression. Further mining into the most enriched category, anatomical structure development (Z-score, 8.52), showed outstanding over-representation in only 1 of 26 categories, organ development (Fig. 3B). Next-level breakdown of organ development revealed subcategories for the development of most organs, of which vasculature development was the most over-represented (Fig. 3C). Further downward hierarchal mining of vasculature development showed statistically significant over-representation in blood vessel development, maturation, morphogenesis, and patterning, as well as angiogenesis, sprouting angiogenesis, and regulation of angiogenesis (Fig. 3D). By contrast, lymph vessel formation and lymphangiogenesis were not represented. This analysis implicated a special, rather specific, and very interesting role for AnxA1 in distinct vascular processes.
Fig. 3.
Systems analysis of the effect of AnxA1 gene KO on biological processes in tumors. Affymetrix mouse genome expression microarray analysis was performed on the B16 tumors growing in KO and WT mice (see Materials and Methods). Categories with Z-scores >2.0 are considered significantly enriched. The biological processes are shown in each category being mined down consecutively level by level from A to B to C to D. Arrows indicate which category is further mined.
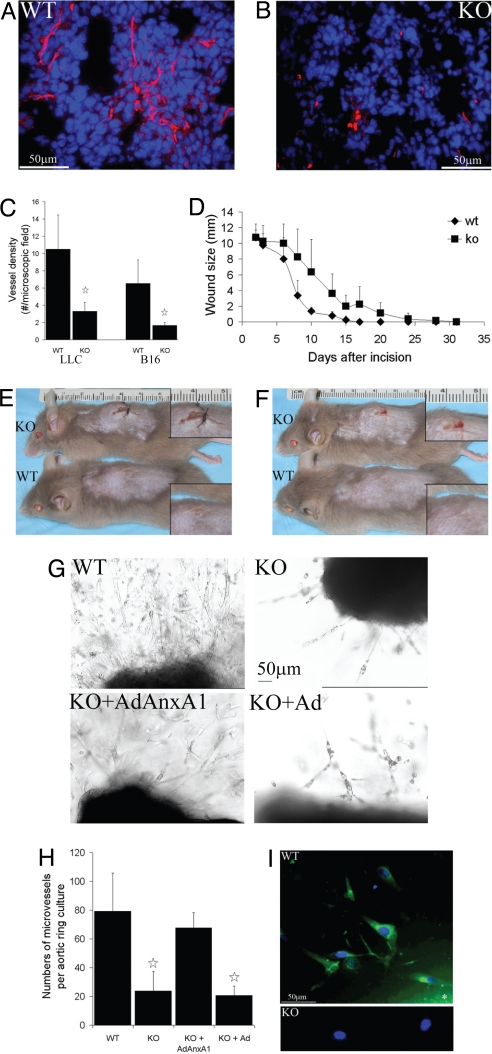
These findings support a novel role for AnxA1 in angiogenesis. AnxA1 appears to be induced in tumor endothelium (5), and the lack of AnxA1 in KO mice may impair tumor-induced angiogenesis with reduced blood supply explaining retarded tumor growth and metastasis as well as enhanced tumor necrosis and mouse survival. To test this possibility experimentally, we first compared tumor vascularity in KO and WT mice by examining tissue sections from tumors. As detected by CD31 staining, the blood vessel density in both tumor models in the KO mice was one third of that observed for tumors growing in the WT mice (Fig. 4 A-C). As expected, the WT mice, but not KO mice, exhibited AnxA1 expression in the CD31+ vasculature (data not shown).
Fig. 4.
Poor tumor vascularity, wound healing, and EC sprouting in AnxA1 KO mice. (A and B) Representative immunofluorescent staining of tumor vasculature by CD31 (red) and counterstain by DAPI (blue) on frozen tumor sections of LLC tumors implanted s.c. on WT and KO mice. (C) Tumor vessel density, measured in number of vessels per microscopic field, shown LLC-derived tumors grown in KO and WT mice. (D-F) Incisional wounds were made at the same time on WT and KO mice of the same age. Average wound size (n = 4 wounds) at the indicated time points (D). Representative wound healing at day 8 (E) and day 15 (F) post-wounding. (G) Light microscopic imaging of vascular sprouting in representative aortic ring cultures from each group indicated. Ad for adenoviral control. AdAnxA1 for adenoviral expression of AnxA1. (H) Average number of microvessels per aortic ring culture (n = 4 aortic ring cultures) in each group indicated. Error bars express SD. (I) Aortic ring cultures from WT (Top) and KO (Bottom) mice were stained for AnxA1 (green) without permeabilization to detect surface expression, and then counterstained for DAPI (blue). *Indicates location of aortic ring.
Our systems analysis also uncovered a role of AnxA1 in wound healing (after similar sequential downward hierarchal mining of the over-represented categories of another biological process, “response to stimulus,” summarized in Fig. S2). Wound healing is a multi-step process in which angiogenesis is an essential, physiological response. To determine experimentally whether AnxA1 is functionally important for physiological angiogenesis and wound healing, we made equivalent incisional skin wounds in KO and WT mice of the same age. The KO mice showed a significant delay in wound healing (P < 0.003). The wounds of the WT mice rapidly became uniformly smaller in size and healed completely within 17 d (data not shown). Equivalent wounds in KO mice failed to heal, with only a 10% reduction in the wound sizes. In other experiments, the wound was loosely sutured to hold the cut skin flaps together. All sutured wounds healed better (Fig. 4 D-F). By 7 to 8 d after the incision and suturing, the average wound length was reduced 50% in the WT mice. It took nearly twice the time in the KO mice for the wound size to be reduced equally. By 16 d, the wounds of all WT mice were completely healed; however, the KO mice required up to 32 d to heal (Fig. 4D).
ECs are normally quite quiescent and must be stimulated to proliferate and migrate to form new blood vessels. To better understand the possible effects of AnxA1 on vascular endothelium, we performed the well known aortic ring assay to assess physiological angiogenesis and EC sprouting (6). This ex vivo assay is rather selective for vascular cells because it lacks tumor cells as well as many other possible effectors that can influence angiogenesis and the local tissue microenvironment, including immune cells and inflammatory cytokines. The aortic ring cultures derived from KO mice exhibited significantly reduced EC sprouting compared with the WT mice (P < 0.01; Fig. 4 G and H). The proliferation and migration of ECs averaged >70% less in the aortas from KO versus WT mice. Adenoviral expression of AnxA1 in the aortic rings of the KO mice readily rescued EC function to nearly 90% of that of the WT mice; control adenovirus had no effect (Fig. 4 G and H). Consistent with this impairment, we also found that ECs isolated from several different KO tissues grew much more slowly in cell culture than those from WT mice (data not shown). Last, surface AnxA1 expression was induced in the WT but not KO aortic rings. AnxA1 was not detected at the surface of ECs lining freshly isolated aortas but was expressed at the surface in both the aortic rings and the sprouting ECs after 1 week in culture (Fig. 4I). These data cumulatively support an important role for AnxA1 in vascular EC function and in angiogenesis under conditions clearly independent from other reported annexin functions, including inflammation.
To further elucidate specific molecular mechanisms and possible differences between pathological and physiological angiogenic balance, we analyzed differential gene expression in the B16 tumors and the aortas collected from the KO and WT mice (Fig. 5 A-D and Fig. S3 A and B). Using the angiogenesis pathway-focused mouse oligo DNA microarray, we readily detected >80% of these genes in the tumor and/or aorta samples. Different expression profiles between WT and KO mice in both tumors and aortas were observed (Fig. S3 A). Although expression remained quite constant for most genes, differential expression of genes in angiogenesis pathways was readily evident and quite reproducible (Fig. S3B).
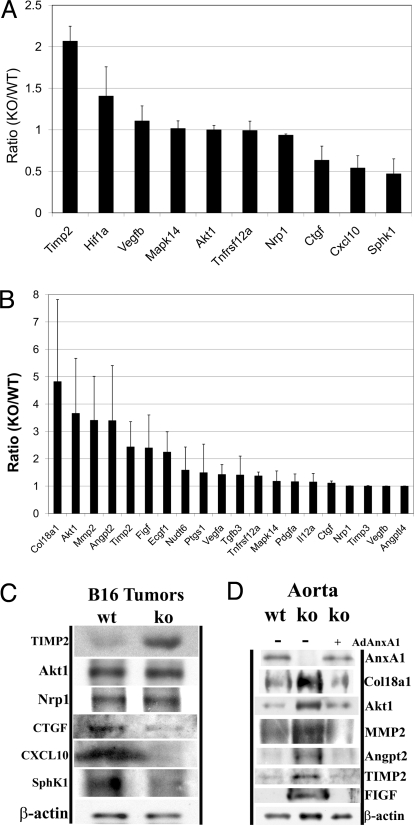
Fig. 5.
Angiogenesis related genes and proteins express differently in AnxA1 KO mice. The gene expression analysis was performed on B16 tumors (A) and aortas (B) from KO and WT mice. The expression levels are shown in averages of 3 repeat experiments (n = 3) and SD as error bars. Consistent levels of protein expression were verified by Western analysis for B16 (C) and freshly excised aortas and KO aortic ring cultures re-expressing AnxA1 using an adenoviral vector (D) from WT and KO mice. β-actin was used as a loading control.
In tumors, the anti-angiogenic gene TIMP2 was up-regulated in KO mice relative to WT mice (Fig. 5A; P < 0.01), whereas the pro-angiogenic gene SphK1 was down-regulated (Fig. 5A; P < 0.04). Other genes implicated in angiogenesis, TNFRSF12a, MAPK14, and Nrp1, showed no change in expression levels. Genes associated with tumor growth, CTGF (connective tissue growth factor) and CXCL10 [chemokine (C-X-C motif) ligand 10], were also moderately down-regulated (Fig. 5A). TIMP2 can repress angiogenesis by inhibiting metalloproteinases that degrade extracellular matrix (7), by directly suppressing the proliferation of ECs (8), and by promoting vessel stability (9). SphK1 catalyzes the phosphorylation of sphingosine to sphingosine-1-phosphate, which is a key lipid messenger promoting cellular proliferation, migration, survival, and vascular development in both physiological and pathological conditions (10–13).
We also detected increased expression for hypoxia-inducible factor 1α (HIF1α; Fig. 5A), which is likely a direct consequence of increased necrosis and presumed hypoxia (Fig. 2). Although HIF1α is clearly instrumental in promoting angiogenesis (14), its up-regulation was apparently insufficient to compensate in full for the lack of AnxA1. The expression of key HIF1α-responsive genes such as VEGF and Akt1 appeared quite constant (Fig. 5A). Consistent with the gene array analysis, Western blots of B16 tumors from KO and WT mice revealed elevated TIMP2 expression, constant levels of Akt1 and Nrp1, and decreased levels of CTGF, CXCL10, and SphK1 in tumors from KO mice (Fig. 5C). These changes likely contributed to an overall downward switch in the angiogenic profile, quite consistent with the known literature and the decreased angiogenesis and EC sprouting observed in KO mice.
Given that vascular abnormalities are not evident in the KO mice, we were curious if the KO mice compensated for the AnxA1 gene deletion by altering the expression of other genes involved in blood vessel development to reach a new homeostatic set point. The gene expression changes exhibited in freshly excised aortas were quite consistent with those observed earlier for B16 tumors. TIMP2 was significant up-regulated in aortas from KO mice (Fig. 5B; P < 0.03), whereas VEGF, TGFb3, TNFRSF12a, PTGS1, PDGFα, NUDT6, Nrp1, MAPK14, IL12a, CTGF, Angptl4, and TIMP3 showed no change. Other pro-angiogenic factors were also up-regulated in aortas from KO mice, including matrix metalloproteinase 2 (MMP2; P < 0.02), c-fos-induced growth factor (FIGF; P < 0.07), platelet-derived EC growth factor 1 (ECGF1; P < 0.06), Akt1 (P < 0.03), Angpt2 (P < 0.03), and α chain of type XVIII collagen (Col18a1; P < 0.07; Fig. 5B). These factors were not further up-regulated in the tumors and thus may be chronically compensating in the aortas as well as other blood vessels to maintain a normal homeostatic and physiological state by balancing the angiogenic switch that would otherwise be turned even more downward by the increased expression of anti-angiogenic regulator TIMP2 in the absence of AnxA1. ECGF1 acts specifically on ECs to promote angiogenesis in vivo, stimulates the growth of ECs in vitro (15), and catalyzes the reversible phosphorylation of thymidine to thymine and 2-deoxyribose-1-phosphate, which is critical for angiogenic activity (16). Col18a1 can be proteolytically cleaved to produce endostatin, an endogenous anti-angiogenic factor. Unlike tumors, HIF1α expression was not detected and SphK1 expression was low in aortas from both KO and WT mice. This is consistent with the past reports that HIF1α (17) and SphK1 (18) are more involved in pathological conditions such as hypoxia and angiogenic tumors than in physiological conditions such as aortas. FIGF is structurally and functionally similar to VEGF-C and is active in angiogenesis, lymphangiogenesis, and EC growth (19, 20). Western blotting detected increased expression of Col18a1, Akt1, MMP2, Angpt2, TIMP2, and FIGF in the aortas of KO mice; the rescue of AnxA1 using an adenoviral vector expressing AnxA1 in aortic ring cultures derived from KO aorta reversed the changes of expression of these genes (Fig. 5D), again consistent with the gene expression analysis.
Discussion
Taken together, our data suggest that AnxA1 is a key regulator of pathological angiogenesis and physiological angiogenic balance. When AnxA1 is not present, tumors develop fewer blood vessels, grow more slowly, fail to metastasize, and develop large necrotic cores, all of which likely contribute to increased animal survival. Incisional wounds on KO mice healed far slower than similar wounds on WT mice. The lack of AnxA1 also impaired EC sprouting, which could be rescued by re-expression of AnxA1 in ECs. In KO mice, the balance of pro- and anti-angiogenic factors was altered, apparently to maintain a new physiological balance that allows near-normal overall vascular functionality. However, this altered gene and protein expression appears unable to further up-regulate angiogenesis, which appears necessary for normal tumor development and wound healing.
AnxA1 expression on ECs may serve a unique function in EC sprouting, an important aspect of wound healing and angiogenesis commonly studied in the aortic ring assay. This ex vivo assay is very useful to separate effects on angiogenesis from more indirect effects of inflammation because immune cells and inflammatory factors that can influence angiogenesis and the local tissue microenvironment are not present in the culture system. Aortic rings from KO animals lacked AnxA1 expression and showed significantly fewer vascular sprouts than aortic rings from WT mice. This deficit was not subtle and could be rescued by re-expression of AnxA1. AnxA1 is induced specifically on the luminal surface of vascular ECs in tumors (5), and tumor vasculature does not develop robustly if AnxA1 is lacking. Thus, induction of AnxA1 may play a functional role in recruiting or stabilizing new vasculature, which ultimately supports tumor growth and metastasis. This effect of AnxA1 on angiogenesis is likely not exclusive. Because the AnxA1-null mouse is a global KO, it may have other more indirect effects on angiogenesis. Several functions attributed to AnxA1 may influence tumor angiogenesis and growth as well as wound healing, including inflammation and the infiltration of leucocytes and other bone marrow-derived cells (3). Future experiments will be needed to determine what role, if any, other possible AnxA1 functions may have on angiogenesis.
Currently known pathways involved in angiogenesis include VEGF, FGF, PDGF, Eph, Wnt, Ang-1, and Delta/Serrate (21, 22). We have examined the key components in these pathways in our microarray studies (Fig. 5 and Fig. S3; summarized in Table S1). None were affected by the AnxA1 KO. Based on this new evidence, we propose a novel AnxA1 pathway in regulating angiogenesis (Fig. S4). In aortas from KO mice, the increased expression of anti-angiogenic factors TIMP2 and Col18a1 appeared to be offset by increased expression of the pro-angiogenic factors MMP2, FIGF, ECGF1, Akt1, and Angpt2, apparently to maintain a new physiological balance that allows near-normal overall vascular functionality. Various angiogenic ligands interact with their cell surface receptors to initiate signaling to Akt through PI3K. AnxA1 can interact with anionic or other phospholipids and/or with a possible AnxA1 receptor and may positively regulate the PI3K-Akt pathway through a direct signaling mechanism (23). AnxA1 and MMP2 share positive regulation by transcription factor YB-1 (24). Therefore, in KO mice, MMP2 can be up-regulated by the same pro-angiogenic transcriptional control, which may be sensitized or stimulated by the absence of AnxA1, which then results in the up-regulation of TIMP2 and Col18a1 as the 2 anti-angiogenic factors both interacting with MMP2. Without the positive regulation of AnxA1 on the PI3K-Akt process, specific pro-angiogenic ligands such as FIGF may have to be up-regulated to maintain angiogenic homeostasis. Yet, the increased levels of TIMP2 and Col18a1 likely decrease angiogenesis in wound healing and aortic vessel sprouting in KO mice (Fig. 4). In tumors from KO mice, TIMP2 was up-regulated and the pro-angiogenic SphK1 was down-regulated relative to WT mice. Other pro-angiogenic factors (MMP2, FIGF, ECGF1, Akt1, and Angpt2) were not further up-regulated, consistent with the lower levels of angiogenesis. Thus, this reset angiogenic profile may be sufficient for normal functioning even during early embryonic development but cannot fully compensate for all stressors.
AnxA1 KO mice lack obvious defects; their growth is not retarded, they show no defects with blood cell composition, and both the systems analysis and the experimental data support a specific effect on vascular ECs. Thus, it is unlikely that AnxA1 plays a ubiquitous role in cell growth or homeostasis. Furthermore, the lack of obvious vascular defects in KO mice during development (4) suggests that any possible role for AnxA1 in vasculogenesis may be minor or overcome by compensating factors. Our systems analysis of gene expression supports the latter with an apparent resetting of the pro- versus anti-angiogenic gene profile switch. But, like eNOS(-/-) and caveolin(-/-) mice that are also viable despite the significant effects seen on tumor angiogenesis, growth, and progression (25, 26), this apparent overall lack of effect on vasculogenesis does not rule out a more substantial role for AnxA1 in tumor or adult angiogenesis. Although most known angiogenic factors have readily apparent effects on vasculogenesis, usually leading to embryonic lethality [VEGF (27), VEGFRs (28, 29), Tie-2 (30)], AnxA1 appears functionally more specific for angiogenesis than vasculogenesis. By functioning in EC sprouting, which at a minimum requires cell division and movement, induced AnxA1 expression helps mediate the formation and growth of blood vessels in adult tissues and tumors.
Our systems analysis suggests altered angiogenesis function in KO mice that we have confirmed experimentally. Wound healing, tumor angiogenesis, and EC sprouting are impaired without AnxA1 expression. Our microarray analyses of tumors and aortas from KO and WT mice provide further initial insights into the novel mechanisms of AnxA1 in modulating the biological processes of pathological and physiological angiogenesis. A new set point for the angiogenic switch appears to be required to maintain physiological angiogenesis and a normal vascular balance. Host tissue unable to express AnxA1 may attempt to reset the angiogenic switch, but ultimately, when stressed or stimulated, is unable to mediate WT levels of EC sprouting, wound healing, and tumor growth and metastasis. Most significantly, the TIMP2 gene was up-regulated in AnxA1 KO experiments, whereas other important angiogenesis genes such as VEGF remained unaffected. Thus, our data suggested a novel molecular mechanism that links AnxA1 and TIMP2 for first time and is independent of VEGF, HIF1, MAPK, and other growth factors and kinases except SphK1. This AnxA1–TIMP2–SphK1 pathway may shed light on the overall mechanisms that govern pathological versus physiological angiogenesis. These data indicate that AnxA1 is not just a tumor-induced vascular biomarker, but also an important factor in activated endothelium as well as a key stromal factor in tumor development, angiogenesis, and wound healing. Although VEGF and related kinase receptors have been targeted for anti-angiogenesis therapy, this novel AnxA1 pathway may serve as an alternative functional target for treating any disease in which angiogenesis is key, including cancer, retinopathies, damaged tissue repair, wound healing following surgery, transplantation, and post-ischemia tissue recovery, possibly with less toxicity and side effects.
Materials and Methods
Mice.
AnxA1 KO homozygous and congenic WT counterpart homozygous mice with 129:C57b1/6 mixed background were provided by R.J. Flower (London, United Kingdom) (4). Breeding colonies of KO and WT mice were maintained in exactly the same conditions at the Sidney Kimmel Cancer Center vivarium and genotypes were confirmed as described (4). Mice with the same coat color, age, and sex were used in any single experiment for comparison.
Tumor Models.
To obtain s.c. tumors, 5 × 106 B16 or 10 × 106 LLC mouse tumor cells in 200 μL of Dulbecco modified Eagle medium were injected s.c.
Systems Analysis and Gene Microarray.
The GeneChip mouse genome 430 2.0 array (Affymetrix) was used and the hybridization was performed in the Sidney Kimmel Cancer Center genomics core facility, followed by data analyses using WebArray (31). Further systems analysis was performed using Genomatix software (Genomatix Software) and Gene Ontology database (32). The mRNA expression of genes involved in angiogenesis was analyzed with a mouse oligo-array (SuperArray) according to the manufacturer's manual. The gene expression levels were determined using online image data acquisition and analysis software from SuperArray. Student t test was used to determine statistical significance.
More Materials and Methods are in the SI Text in Supporting Information.
Supplementary Material
Acknowledgments.
We thank Prof. R.J. Flower for providing breeding pairs of KO and WT mice, A. Wempren for cryo-sectioning of tissue blocks, and Dr. X. Xia at the Sidney Kimmel Cancer Center genomics core facility for help with the Affymetrix microarray experiments. This research was supported by National Institutes of Health Grants R01 CA115215 and P01 CA104898 (to J.E.S.). M.Y. is a recipient of a Department of Defense prostate cancer research program postdoctoral fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901324106/DCSupplemental.
References
- 1.Di Rosa M, Flower RJ, Hirata F, Parente L, Russo-Marie F. Anti-phospholipase proteins. Prostaglandins. 1984;28:441–442. doi: 10.1016/0090-6980(84)90232-6. [DOI] [PubMed] [Google Scholar]
- 2.Wallner BP, et al. Cloning and expression of human lipocortin, a phospholipase A2 inhibitor with potential anti-inflammatory activity. Nature. 1986;320:77–81. doi: 10.1038/320077a0. [DOI] [PubMed] [Google Scholar]
- 3.Lim LH, Pervaiz S. Annexin 1: the new face of an old molecule. FASEB J. 2007;21:968–975. doi: 10.1096/fj.06-7464rev. [DOI] [PubMed] [Google Scholar]
- 4.Hannon R, et al. Aberrant inflammation and resistance to glucocorticoids in annexin 1-/- mouse. FASEB J. 2003;17:253–255. doi: 10.1096/fj.02-0239fje. [DOI] [PubMed] [Google Scholar]
- 5.Oh P, et al. Subtractive proteomic mapping of the endothelial surface in lung and solid tumours for tissue-specific therapy. Nature. 2004;429:629–635. doi: 10.1038/nature02580. [DOI] [PubMed] [Google Scholar]
- 6.Nicosia RF, Zhu WH, Fogel E, Howson KM, Aplin AC. A new ex vivo model to study venous angiogenesis and arterio-venous anastomosis formation. J Vasc Res. 2005;42:111–119. doi: 10.1159/000083457. [DOI] [PubMed] [Google Scholar]
- 7.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci. 2002;115:3719–3727. doi: 10.1242/jcs.00063. [DOI] [PubMed] [Google Scholar]
- 8.Seo DW, et al. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003;114:171–180. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- 9.Saunders WB, et al. Coregulation of vascular tube stabilization by endothelial cell TIMP-2 and pericyte TIMP-3. J Cell Biol. 2006;175:179–191. doi: 10.1083/jcb.200603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee MJ, et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 11.Oyama O, et al. The lysophospholipid mediator sphingosine-1-phosphate promotes angiogenesis in vivo in ischaemic hindlimbs of mice. Cardiovasc Res. 2008;78:301–307. doi: 10.1093/cvr/cvn002. [DOI] [PubMed] [Google Scholar]
- 12.Schwalm S, et al. Sphingosine kinase-1 is a hypoxia-regulated gene that stimulates migration of human endothelial cells. Biochem Biophys Res Commun. 2008;368:1020–1025. doi: 10.1016/j.bbrc.2008.01.132. [DOI] [PubMed] [Google Scholar]
- 13.Vadas M, Xia P, McCaughan G, Gamble J. The role of sphingosine kinase 1 in cancer: oncogene or non-oncogene addiction? Biochim Biophys Acta. 2008;1781:442–447. doi: 10.1016/j.bbalip.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 15.Heldin CH, Usuki K, Miyazono K. Platelet-derived endothelial cell growth factor. J Cell Biochem. 1991;47:208–210. doi: 10.1002/jcb.240470304. [DOI] [PubMed] [Google Scholar]
- 16.Akiyama S, et al. The role of thymidine phosphorylase, an angiogenic enzyme, in tumor progression. Cancer Sci. 2004;95:851–857. doi: 10.1111/j.1349-7006.2004.tb02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu ZH, Wright JD, Belt B, Cardiff RD, Arbeit JM. Hypoxia-inducible factor-1 facilitates cervical cancer progression in human papillomavirus type 16 transgenic mice. Am J Pathol. 2007;171:667–681. doi: 10.2353/ajpath.2007.061138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granata R, et al. Insulin-like growth factor binding protein-3 induces angiogenesis through IGF-I- and SphK1-dependent mechanisms. J Thromb Haemost. 2007;5:835–845. doi: 10.1111/j.1538-7836.2007.02431.x. [DOI] [PubMed] [Google Scholar]
- 19.Achen MG, Williams RA, Baldwin ME, Lai P, Roufail S, et al. The angiogenic and lymphangiogenic factor vascular endothelial growth factor-D exhibits a paracrine mode of action in cancer. Growth Factors. 2002;20:99–107. doi: 10.1080/08977190290031969. [DOI] [PubMed] [Google Scholar]
- 20.Orlandini M, Marconcini L, Ferruzzi R, Oliviero S. Identification of a c-fos-induced gene that is related to the platelet-derived growth factor/vascular endothelial growth factor family. Proc Natl Acad Sci USA. 1996;93:11675–11680. doi: 10.1073/pnas.93.21.11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mi H, Guo N, Kejariwal A, Thomas PD. PANTHER version 6: protein sequence and function evolution data with expanded representation of biological pathways. Nucleic Acids Res. 2007;35:D247–D252. doi: 10.1093/nar/gkl869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas PD, et al. PANTHER: a library of protein families and subfamilies indexed by function. Genome Res. 2003;13:2129–2141. doi: 10.1101/gr.772403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gavins FN, Dalli J, Flower RJ, Granger DN, Perretti M. Activation of the annexin 1 counter-regulatory circuit affords protection in the mouse brain microcirculation. FASEB J. 2007;21:1751–1758. doi: 10.1096/fj.06-7842com. [DOI] [PubMed] [Google Scholar]
- 24.Kuwano M, et al. The basic and clinical implications of ABC transporters, Y-box-binding protein-1 (YB-1) and angiogenesis-related factors in human malignancies. Cancer Sci. 2003;94:9–14. doi: 10.1111/j.1349-7006.2003.tb01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukumura D, et al. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams TM, et al. Caveolin-1 promotes tumor progression in an autochthonous mouse model of prostate cancer: genetic ablation of Cav-1 delays advanced prostate tumor development in tramp mice. J Biol Chem. 2005;280:25134–25145. doi: 10.1074/jbc.M501186200. [DOI] [PubMed] [Google Scholar]
- 27.Ferrara N, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 28.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 29.Shalaby F, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 30.Jones N, et al. Rescue of the early vascular defects in Tek/Tie2 null mice reveals an essential survival function. EMBO Rep. 2001;2:438–445. doi: 10.1093/embo-reports/kve093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia X, McClelland M, Wang Y. WebArray: an online platform for microarray data analysis. BMC Bioinformatics. 2005;6:306. doi: 10.1186/1471-2105-6-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashburner M, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.