Abstract
Multiple entry receptors can mediate infection of cells by herpes simplex virus (HSV), permitting alternative pathways for infection and disease. We investigated the roles of two known entry receptors, herpesvirus entry mediator (HVEM) and nectin-1, in infection of neurons in the CNS and the development of encephalitis. Wild-type, HVEM KO, nectin-1 KO, and HVEM/nectin-1 double KO mice were inoculated with HSV into the hippocampus. The mice were examined for development of encephalitis or were killed at various times after inoculation for immunohistological analyses of brain slices. Nectin-1 KO mice showed no signs of disease after intracranial inoculation, and no HSV antigens were detectable in the brain parenchyma. However, HSV antigens were detected in non-parenchymal cells lining the ventricles. In the double KO mice, there was also no disease and no detectable expression of viral antigens even in non-parenchymal cells, indicating that infection of these cells in the nectin-1 KO mice was dependent on the expression of HVEM. Wild-type and HVEM KO mice rapidly developed encephalitis, and the patterns of HSV replication in the brain were indistinguishable. Thus, expression of nectin-1 is necessary for HSV infection via the intracranial route and for encephalitis; HVEM is largely irrelevant. These results contrast with recent findings that (i) either HVEM or nectin-1 can permit HSV infection of the vaginal epithelium in mice and (ii) nectin-1 is not the sole receptor capable of enabling spread of HSV infection from the vaginal epithelium to the PNS and CNS.
Keywords: HSV, pathogenesis
We have undertaken studies to determine whether the various aspects of disease caused by herpes simplex virus (HSV) are critically dependent on the same or different entry receptors. The usual manifestations of HSV disease in humans, the natural host, are perioral or genital lesions of the skin or mucosa or lesions on the cornea. The virus can spread from epithelial cells to neurons and establish latent infections in sensory and autonomic ganglia. Rarely, the virus also spreads to the CNS to cause encephalitis or meningitis (1).
Mice can be infected by HSV with manifestations of disease similar to those found in humans. In addition, the mouse entry receptors for HSV are paralogs of the human entry receptors (2). Thus, mice and mice mutated for specific HSV entry receptors provide excellent models for the study of HSV entry requirements in disease.
Binding of HSV to either mouse or human cells can be mediated by interactions of virion envelope glycoproteins gB or gC with cell surface heparan sulfate (3). These interactions are not sufficient for viral entry. Entry requires the binding of at least two envelope glycoproteins, both gD and gB, to specific cell surface receptors (4, 5). These interactions activate the fusogenic activity of gB, a homotrimer, and/or gH-gL, a heterodimer, resulting in fusion of the virion envelope with a cell membrane (6, 7).
There are multiple alternative gD receptors, including herpesvirus entry mediator (HVEM), nectin-1, nectin-2, and specific sites in heparan sulfate generated by particular 3-O-sulfotransferases (2). HSV strains differ in their ability to use human nectin-2 for cell entry (8, 9), and the mouse forms of nectin-2 tested are not active for HSV entry (10). Use of 3-O-sulfated heparan sulfate as an entry receptor seems to be restricted to HSV strains of one serotype (HSV-1 but not HSV-2), although screening of HSV-1 and HSV-2 strains has not been extensive (11). HVEM and nectin-1 appear to be the principal gD-binding entry receptors for both serotypes of HSV and for both human and mouse cells. One gB receptor, paired Ig-like receptor alpha (PILRα), has been identified, and evidence has been presented that other alternative gB receptors may exist (5).
In a previous study, wild-type, HVEM KO, nectin-1 KO, and HVEM/nectin-1 double KO mice were inoculated with HSV via the intravaginal route to assess the roles of each of these receptors in infection and disease (12). The results showed that absence of HVEM had little or no effect on the course of acute disease. Absence of nectin-1 attenuated disease while still permitting infection of the vaginal epithelium and spread of infection to the PNS and spinal cord. Infection of the vaginal epithelium required either nectin-1 or HVEM because HVEM/nectin-1 double KO mice could not be infected by this route. Spread of infection to the nervous system could not be assessed in the double KO mice. This spread was less efficient in the nectin-1 KO mice, but still occurred, and therefore must have been mediated by some other entry receptor whose identity was not determined.
To explore further the requirements for HSV replication and disease in the CNS, we have inoculated mice intracranially and directly into the hippocampus. The hippocampus was targeted because it is commonly severely affected in human encephalitis (13, 14). The results demonstrate that nectin-1 is indispensable for HSV infection of neurons and other parenchymal cells in the brain and for the encephalitis that usually results from HSV replication of the brain. HVEM was found to have a role in the infection of non-parenchymal cells of the brain but this infection had no consequences in terms of disease.
Results
Effect of Mouse Genotype on Disease Symptoms and Survival Following Intracranial HSV Inoculations.
Two methods were used to inoculate wild-type, HVEM KO, nectin-1 KO, and double KO mice. In a pilot experiment, mice were inoculated by needle and syringe through the cranium with serial dilutions of HSV in 20 μL of fluid, targeting the region of the hippocampus. In all other experiments, mice were inoculated directly into the granular layer of the hippocampus, following surgery to provide an opening in the cranium and by use of a stereotaxic apparatus to deliver an inoculum of 1 μL. All mice were monitored daily and, in most instances, two to three times per day for signs of neurological symptoms. Severe signs of disease dictated immediate sacrifice.
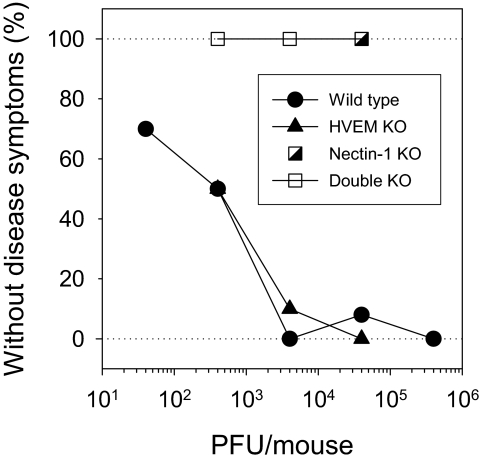
Fig. 1 shows the results of the pilot experiment. Both wild-type and HVEM KO mice were susceptible to the development of encephalitis. Approximately 400 plaque-forming units (PFU) of virus per mouse were sufficient to cause severe disease in about one-half of inoculated mice of either genotype. At the higher doses of virus, mice had to be killed within 2–4 days of inoculation because of severe neurological symptoms (seizures, uncontrolled shaking, unbalanced gait). At the lower doses, these same symptoms were observed in some mice, but not all, and at a later time (between 8–13 days following injection). The nectin-1 KO and double KO mice were totally resistant to any ill effects of virus inoculation into the brain. They survived without symptoms of disease for the duration of the experiment (14 days), at doses of HSV up to 100 times that required to cause disease in one-half of wild-type or HVEM KO mice.
Fig. 1.
Effect of mouse genotype on development of encephalitis following intracranial inoculation of HSV. Mice of each genotype (n = 10–13 mice per group) were inoculated with 20-μL samples of HSV at the doses per mouse indicated, using a needle and syringe to inject the virus through the scalp and cranium into the region of the hippocampus, right hemisphere. The mice were examined once or twice per day for symptoms of encephalitis and killed when these symptoms occurred. Most wild-type and HVEM KO mice succumbed or were killed within 4 days of inoculation at the highest two doses of virus or within 10 days for the lower doses. None of the nectin-1 KO or double KO mice showed any signs of disease for 14 days.
To target the HSV inoculum more precisely to a defined region of the brain, groups of 4 mice of each genotype were injected into the granular layer of the hippocampus by stereotaxic surgery with 2 × 106 PFU of HSV in 1 μL of fluid. This dose of virus was chosen to ensure infection of all susceptible mice (a 10-fold lower dose permitted survival of one in four wild-type mice). All wild-type and HVEM KO mice had to be killed due to their neurological symptoms within 2 days. All nectin-1 KO and double KO mice survived without any symptoms of disease for 14 days. We can conclude that development of neurological disease required the expression of nectin-1 in the mice.
Effect of Mouse Genotype on HSV Infection and Antigen Expression in the Brain.
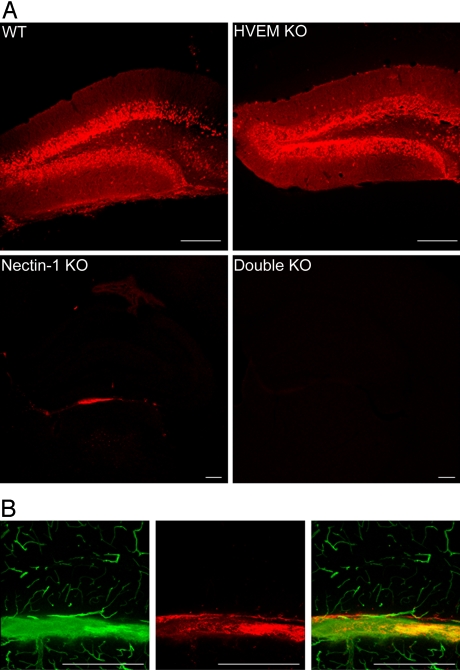
HSV infection in the brain was assessed by use of a polyclonal anti-HSV antiserum to detect viral antigens in brain sections of mice killed at 24 h after stereotaxic inoculation of virus, or at later times. Fig. 2A shows images, for mice of all four genotypes, of the hippocampal region at the injection site. Viral antigens were readily detected in brain sections from both wild-type and HVEM KO mice at 24 h, most prominently in the granular layer of the dentate gyrus and, to a lesser extent, in other regions of the brain. As described below, HSV antigen expression was readily detected in multiple areas of the brain at 36–48 h after virus inoculation. No viral antigens could be detected in brain sections from the double KO mice. Viral antigens were detected in brain sections from the nectin-1 KO mice but only in limited regions. These conclusions are based on the examination of multiple anti-HSV-stained sections obtained from the brains of many mice of each genotype (14 wild-type; 15 HVEM KO; 10 nectin-1 KO; and 8 double KO).
Fig. 2.
Effect of mouse genotype on expression of HSV antigens in the brain after stereotaxic inoculation of virus. Brain sections were prepared 24 h after virus inoculation (2 × 106 PFU per mouse) and stained with anti-HSV antibodies (red). (A) Each quadrant shows a representative section from the hippocampus of the mouse strain indicated (Scale bar, 100 μm; note that lower magnification images are shown for the nectin-1 KO and double KO mice). (B) A nectin-1 KO mouse was inoculated with virus and, after 24 h, was perfused with fluorescein-conjugated tomato lectin just before sacrifice and sectioning of the brain. Tomato lectin binds to the luminal surfaces of ventricles and blood vessels. Left: tomato lectin staining of the blood vessels and ventricular wall; middle: HSV antigens in the vicinity of the ventricular wall; right: merge of these images (Scale bar, 100 μm.)
Comparisons of bright-field and fluorescent images of stained sections from the nectin-1 KO mice indicated that the HSV antigens were localized to ventricular surfaces. To confirm that the infected cells detected in nectin-1 KO mice were non-parenchymal cells, some inoculated mice were perfused with fluorescein-conjugated tomato lectin at the time of sacrifice 24 h after HSV inoculation. Under the conditions of perfusion, tomato lectin binds to the surfaces of ventricles and blood vessels in the brain (15). Fig. 2B shows that viral antigens (red) localized to cells of a ventricular surface that was also stained with tomato lectin (green). Blood vessels in this section were stained by the tomato lectin but not by the anti-HSV antibodies. When nectin-1 KO mice were killed at 2 or 4 days after virus inoculation, no viral antigens could be detected in brain sections, suggesting that virus was cleared from the ventricular walls where HSV replication initially occurred.
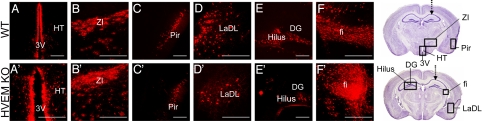
Many regions of the brain were infected in wild-type and HVEM KO mice, as observed in brain sections prepared from mice killed at 36 to 48 h after virus inoculation. No differences were noted between these two genotypes in the pattern of HSV spread in the brain as shown in Fig. 3. Infected areas included the third ventricle and hypothalamus (A and A′), zona incerta (B and B′), piriform cortex (C and C′), amygdaloid nucleus (D and D′), hilus of the dentate gyrus (E and E′), and fimbria of the hippocampus (F and F′).
Fig. 3.
HSV antigen expression in the brains of wild-type and HVEM KO mice killed at 36 or 48 h after stereotaxic virus inoculation (2 × 106 PFU per mouse). The top panels are sections from wild type mice and the bottom panels from HVEM KO mice. (A and A′) third ventricle (3V) and hypothalamus (HT); (B and B′) zona incerta (ZI); (C and C′) piriform cortex (Pir); (D and D′) lateral ventricle (LaDL) and caudate putamen; (E and E′) hilus and CA3 of dentate gyrus (DG) in the hippocampus; (F and F′) fimbria (fi) of the hippocampus. Two brain maps (29) at the right represent different coronal sections through the brain at the level of the hippocampus. The arrows point to the injection target. B–D′, F, and F′ are ipsilateral to the inoculation site and E and E′ are contralateral, as indicated by boxes on the brain maps (Scale bar, 50 μm.)
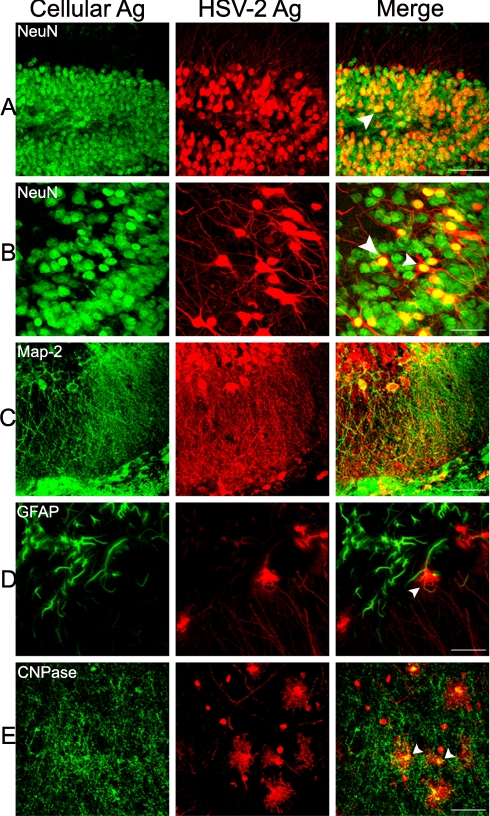
Neurons, and to a lesser extent non-neuronal cells, were found to be infected by HSV and to express viral antigens. Dual-labeling immunohistochemistry using an antibody specific for the neuronal nuclear protein, NeuN, and the anti-HSV antibodies showed that neurons in the dentate gyrus of the hippocampus as well as in the piriform cortex were infected by virus (Fig. 4 A and B). Also, antibodies specific for the microtubule-associated protein, MAP2, co-localized with the anti-HSV antibodies in dendrites and cell bodies of hippocampal neurons (Fig. 4C). There was enhanced expression of the glial fibrillary acidic protein (GFAP) in infected regions of the brain and a few GFAP-positive cells, presumably astrocytes, also expressed HSV antigen (Fig. 4D) but most did not. Oligodendrocytes also became infected by HSV, as shown by the co-localization of anti-CNPase and anti-HSV antibodies (Fig. 4E).
Fig. 4.
Use of antibodies to cell proteins to identify cell types infected by HSV. HVEM KO mice were killed at 36 or 48 h after stereotaxic inoculation of HSV (2 × 106 PFU per mouse). For each row, the left image shows staining with the antibody indicated, the middle image is labeled with the anti-HSV antibodies, and the right image shows the merge of these two images. (A) dorsal dentate gyrus of the hippocampus, ipsilateral, 36 h; (B) piriform cortex, ipsilateral, 36 h; (C) hippocampus, ipsilateral, 36 h; (D) hypothalamus and dorsal medial nucleus near the third ventricle, 48 h; (E) zona incerta and thalamic region, ipsilateral, 48 h (Scale bar, A: 200 μm; B–E: 70 μm). Arrows point to a few of the cells in which the cellular antigen and HSV antigens co-localized. Similar results were obtained with infected wild-type mice.
Discussion
We show here that nectin-1 expression is a strict requirement for HSV infection in the mouse brain parenchyma and for the development of encephalitis after intracranial inoculation. It is striking that, in the absence of nectin-1, mice were totally resistant to any deleterious effects of HSV inoculation directly into the brain, specifically into the hippocampus. This was the case even though some infection of non-parenchymal cells was observed in mice that expressed HVEM but not nectin-1. HVEM is not necessarily the only receptor capable of mediating infection of the non-parenchymal cells; nectin-1 could also be expressed in these cells in wild-type mice.
It is equally striking that nectin-1 was not required for lethal neurological disease when HSV was introduced via a peripheral route, namely by intravaginal inoculation. In this previous study (12), absence of nectin-1 attenuated disease (fewer mice suffered severe neurological disease), but nevertheless permitted infection of the vaginal epithelium (via HVEM) and permitted spread of virus infection to dorsal root ganglia and the spinal cord (via as yet unidentified receptors). There was also probably spread of virus infection to autonomic ganglia, which could have accounted for some morbidity and mortality. Thus, nectin-1 is not required for HSV to enter neurons of the PNS but is required to infect neurons of the brain.
Nectin-1 is highly conserved among mammals (16). Previous studies have shown that nectin-1 is expressed throughout the nervous system of the mouse and in many neurons of the CNS and PNS (17). Also, anti-nectin-1 antibodies have been shown to inhibit the infection of cultured rodent and human primary sensory neurons (18, 19). Thus, it is not surprising that nectin-1 has a key role in the infection of neurons. There are other entry receptors expressed in the brain and PNS, however, including 3-O-sulfotransferases (20) that generate gD-binding receptors potentially capable of substituting for nectin-1 in permitting viral entry. It remains to be determined whether peripheral neurons and CNS neurons differ in expression of potential gD receptors.
Available evidence indicates that HVEM is not highly expressed in the brain (23–26). Presumably, it is expressed in ependymal cells lining ventricles, however, to account for the infection of these cells in nectin-1 KO mice but not in double KO mice. Although nectin-1 is indispensable among the gD-binding receptors for HSV infection of cells in the brain parenchyma, it may not be the only entry receptor required. Because a gB receptor appears to be required along with a gD receptor for HSV entry, and the gB-binding receptor, PILRα, is expressed in the brain (5), the possibility exists that PILRα or another gB-binding receptor is also required.
Since the known roles of nectin-1 and HVEM in HSV infection are to mediate the entry of virus into cells, it seems reasonable to conclude from our results that, in nectin-1 KO or double KO mice, neurons or other cells at the injection site were simply resistant to viral entry. This possibility could not be directly tested by the methods described here. A previous study (21) showed that fusion of HSV with rodent synaptosomes could serve as a surrogate assay for viral entry into neurons and as a way to visualize entry by cryoelectron tomography. Therefore, we assessed the ability of HSV to fuse with synaptosomes prepared from the brains of the four strains of mice used here. The results showed that such fusion could be observed with synaptosomes from wild-type and HVEM KO mice but not with those from nectin-1 KO or double KO mice (Fig. S1 and SI Text).
We targeted the hippocampus in this study because this region of the brain is typically heavily damaged by HSV replication in human cases of encephalitis (13, 14) and also following experimental challenge of rodents (22, 23). Previous studies in mice have also targeted the hippocampus by stereotaxic inoculation (24, 25). Lower doses of virus were used resulting in less morbidity and mortality. Immunohistochemistry was carried out at later times than in the present study but gave similar results with respect to the expression of viral antigens in the hippocampus and in some of its afferent connections. In these studies and the one reported here, spread of infection was consistent with known retrograde axonal transport mechanisms of HSV. It has also been shown that HSV inoculation into the hippocampus resulted in much more extensive viral infection and pathological effects than inoculation into the cerebellum (24). The authors of this study suggested that a selective susceptibility of cells in telecephalic or limbic structures may account for the pathological features of HSV encephalitis, including those observed in acutely infected mice as well as the memory deficits observed in humans. It remains to be determined whether the distribution of nectin-1 in the brain can account for this apparent selective susceptibility.
What is the relevance of our results to human disease? Obviously, HSV is not usually introduced directly into the brains of humans. It spreads to the brain from peripheral tissues, probably via neural routes, at least in adults. Once in the brain, however, it is likely that HSV infection of neurons and other cells is largely dependent on nectin-1 expression. For some strains of HSV, human nectin-2 may substitute as an entry receptor. A survey of HSV clinical isolates revealed that some HSV-2 isolates from genital lesions and HSV-1 isolates from the brain were able to infect cells via nectin-2, whereas nearly all HSV-1 and HSV-2 strains were able to infect cells via HVEM or nectin-1 (9). It seems unlikely that HVEM plays much of a role in HSV encephalitis. The domains of gD that interact with HVEM are different from those that interact with nectin-1 and nectin-2. Thus, drugs that can block the interactions of gD with nectin-1 and nectin-2 should be effective at reducing the spread of HSV within the brain.
Materials and Methods
Mice and Animal Care.
Wild-type C57BL/6 mice were obtained from Jackson Labs. HVEM KO mice back-crossed to C57BL/6 (26) and nectin-1 KO (27) and double KO mice (12) on a mixed background have been described. PCR analysis was performed as described (12) to confirm the genotypes of all mutant mice. The mice were maintained in a specific-pathogen-free facility until just before inoculation, when they were transferred to a containment facility. Animal care and use were in accordance with institutional and National Institutes of Health guidelines, and all studies were approved by the Animal Care and Use Committee of Northwestern University.
Inoculation of the Mice.
HSV-2, strain 333, was used for inoculations of both male and female mice, aged 6–12 weeks. The virus was diluted in PBS (PBS) containing 10% glucose and 1% FCS (PBS-G-CS) to yield doses/mouse as indicated in the text. PBS-G-CS was used for inoculum in control mice. For a pilot study, groups of mice were inoculated intracranially with 20 μL of serially diluted virus, using a 26-gauge needle to penetrate the scalp and cranium over the hippocampal region of the right hemisphere with a needle guard to prevent penetration further than 5 mm. For all other experiments, mice were inoculated by stereotaxic surgery (28). Mice were anesthetized using a continuous flow of isoflurane and placed on the stereotaxic apparatus. An incision was made in the scalp and a hand-held drill used to thin the bone over the injection site. A needle was used to perforate the edges of the thinned area and to carefully remove a 1-mm diameter piece of the thinned bone. A finely pulled micropipette was used to inject the virus. Coordinates for the injection site were: Caudal (-) 2.1 mm; Lateral 1.8 mm; Ventral 2.1 mm. Mice were monitored daily (usually twice daily) for clinical symptoms of encephalitis and overall health. These symptoms included weight loss, ruffled fur, hunched posture, unbalanced gait, seizures, uncontrolled shaking, scratching of head, ocular lesions, and paralysis. Mice were killed as soon as the symptoms indicated significant discomfort.
Tissue Collection and Immunohistochemistry.
At the times indicated after virus inoculation, mice were deeply anesthetized with isoflurane and perfused transcardially first with PBS (pH 7.2), followed by 4% paraformaldehyde in PBS. The brain was removed and post-fixed in 4% paraformaldehyde solution overnight at 4 °C. Forty-micrometer-thick coronal sections were cut, using a vibratome (Leica). Every sixth section was initially used for staining with antibodies and other sections also used as needed. Sections were washed with PBS and preincubated with 5% normal goat serum (NGS, Sigma-Aldrich) diluted in 0.3% Triton-X solution in PBS for 1 h at room temperature. Sections were then incubated overnight at 4 °C with primary antibodies, including rabbit polyclonal anti-HSV-2 (Dako) 1:300; mouse monoclonal anti-NeuN, a neuronal marker (Chemicon/Millipore) 1:300; mouse monoclonal anti-GFAP, an astrocyte marker (Sigma) 1:300; mouse monoclonal anti-CNPase, an oligodendrocyte marker (Chemicon/Millipore) 1:300; mouse monoclonal anti-MAP2, a marker for neuronal cell bodies and neurites (Chemicon/Millipore)1:300 in working solution (3% NGS, 0.3%Triton-X in PBS). Sections were then washed six times for 10 min each in PBS before incubation, for 1 h at room temperature, with secondary antibodies including goat anti-rabbit IgG (H+L) Alexa 568 and several goat anti-mouse IgG Alexa 488 preparations specific for the isotype of the primary antibody used (Invitrogen/Molecular Probes). Sections were washed six times for 10 min each in PBS before mounting on VWR frost-plus charged slides with Vectashield Hard Set Mounting Media with Dapi (Vector Labs). Images were taken with an Olympus XI70 Confocal Microscope.
Supplementary Material
Acknowledgments.
This work was supported by the Midwest Sexually Transmitted Infections and Topical Microbicides Cooperative Research Center grant U19 AI031494 (P.G.S.) from the National Institute for Allergy and Infectious Diseases,
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908892106/DCSupplemental.
References
- 1.Roizman B, Knipe DM, Whitley RJ. In: Fields Virology. Knipe DM, Howley PM, editors. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 2502–2601. [Google Scholar]
- 2.Spear PG, Eisenberg RJ, Cohen GH. Three classes of cell surface receptors for alphaherpesvirus entry. Virology. 2000;275:1–8. doi: 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- 3.Shukla D, Spear PG. Herpesviruses and heparan sulfate: An intimate relationship in aid of viral entry. J Clin Invest. 2001;108:503–510. doi: 10.1172/JCI13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spear PG, Longnecker R. Herpesvirus entry: An update. J Virol. 2003;77:10179–10185. doi: 10.1128/JVI.77.19.10179-10185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satoh T, et al. PILRa is a herpes simplex virus-1 entry co-receptor that associates with glycoprotein B. Cell. 2008;132:935–944. doi: 10.1016/j.cell.2008.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krummenacher C, et al. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J. 2005;24:4144–4153. doi: 10.1038/sj.emboj.7600875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. Crystal structure of glycoprotein B from herpes simplex virus 1. Science. 2006;313:217–220. doi: 10.1126/science.1126548. [DOI] [PubMed] [Google Scholar]
- 8.Warner MS, et al. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2 and pseudorabies virus. Virology. 1998;246:179–189. doi: 10.1006/viro.1998.9218. [DOI] [PubMed] [Google Scholar]
- 9.Krummenacher C, et al. Comparative usage of herpes virus entry mediator A and nectin-1 by laboratory strains and clinical isolates of herpes simplex virus. Virology. 2004;322:286–299. doi: 10.1016/j.virol.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Shukla D, Rowe CL, Dong Y, Racaniello VR, Spear PG. The murine homolog (Mph) of human herpesvirus entry protein B (HveB) mediates entry of pseudorabies virus but not herpes simplex virus types 1 and 2. JVirol. 1999;73:4493–4497. doi: 10.1128/jvi.73.5.4493-4497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shukla D, et al. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 12.Taylor JM, et al. Alternative entry receptors for herpes simplex virus and their roles in disease. Cell Host & Microbe. 2007;2:1–10. doi: 10.1016/j.chom.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damasio AR, Van Hoesen W. The limbic system and the localization of herpes simplex encephalitis. J Neurol Neurosurg Psychiatry. 1985;48:297–301. doi: 10.1136/jnnp.48.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esiri MM. Herpes simplex encephalitis: An immunological study of the distribution of viral antigen within the brain. J Neurol Sci. 1982;55:209–219. doi: 10.1016/0022-510x(82)90183-6. [DOI] [PubMed] [Google Scholar]
- 15.Yamauchi T, Lin Y, Sharp FR, Noble-Haeusslein LJ. Hemin induces heme oxygenase-1 in spinal cord vasculature and attenuates barrier disruption and neutrophil infiltration in the injured murine spinal cord. J Neurotrauma. 2004;21:1017–1030. doi: 10.1089/0897715041651042. [DOI] [PubMed] [Google Scholar]
- 16.Milne RSB, Connolly SA, Krummenacher C, Eisenberg RJ, Cohen GH. Porcine HveC, a member of the highly conserved HveC/nectin 1 family, is a functional alphaherpesvirus receptor. Virology. 2001;281:315–328. doi: 10.1006/viro.2000.0798. [DOI] [PubMed] [Google Scholar]
- 17.Haarr L, Shukla D, Rødahl E, Dal Canto MC, Spear PG. Transcription from the gene encoding the herpesvirus entry receptor nectin-1 (HveC) in nervous tissue of adult mouse. Virology. 2001;287:301–309. doi: 10.1006/viro.2001.1041. [DOI] [PubMed] [Google Scholar]
- 18.Richart SM, et al. Entry of herpes simplex virus type 1 into primary sensory neurons in vitro is mediated by nectin-1/HveC. J Virol. 2003;77:3307–3311. doi: 10.1128/JVI.77.5.3307-3311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simpson SA, et al. Nectin-1/HveC mediates herpes simplex virus type-1 entry into primary human sensory neurons and fibroblasts. J Neurovirol. 2005;11:208–218. doi: 10.1080/13550280590924214. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence R, et al. The principal neuronal gD-type 3-O-sulfotransferases and their products in central and peripheral nervous system tissues. Matrix Biol. 2007;26:442–455. doi: 10.1016/j.matbio.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maurer E, Sodeik B, Grünewald K. Native 3D intermediates of membrane fusion in herpes simplex virus 1 entry. Proc Natl Acad Sci USA. 2008;105:10559–10564. doi: 10.1073/pnas.0801674105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ando Y, Kitayama H, Kawaguchi Y, Koyanagi Y. Primary target cells of herpes simplex virus type 1 in the hippocampus. Microbes and Infection. 2008;10:1514–1523. doi: 10.1016/j.micinf.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Webb SJ, Eglin RP, Reading M, Esiri MM. Experimental murine herpes simplex encephalitis: Immnohistochemical detection of virus antigens. Neuropathol Appl Neurobiol. 1989;15:165–174. doi: 10.1111/j.1365-2990.1989.tb01218.x. [DOI] [PubMed] [Google Scholar]
- 24.McFarland DJ, Hotchin J. Contrasting patterns of virus spread and neuropathology following microinjection of herpes simplex virus into the hippocampus or cerebellum of mice. J Neurol Sci. 1987;79:255–265. doi: 10.1016/0022-510x(87)90233-4. [DOI] [PubMed] [Google Scholar]
- 25.McFarland DJ, Sikora E, Hotchin J. The production of focal herpes encephalitis in mice by stereotaxic inoculation of virus: Anatomical and behavioral effects. J Neurol Sci. 1986;72:307–318. doi: 10.1016/0022-510x(86)90018-3. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, et al. The role of herpesvirus entry mediator as a negative regulator of T cell-mediated responses. J Clin Invest. 2005;115:711–717. doi: 10.1172/JCI200522982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inagaki M, et al. Roles of cell-adhesion molecules nectin 1 and nectin 3 in ciliary body development. Development. 2005;132:1525–1537. doi: 10.1242/dev.01697. [DOI] [PubMed] [Google Scholar]
- 28.Cetin A, Komai S, Eliava M, Seeburg PH, Osten P. Stereotaxic gene delivery in the rodent brain. Nature Protocols. 2006;1:3166–3173. doi: 10.1038/nprot.2006.450. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Elsevier Science; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.