Abstract
NK cell cytotoxicity is controlled by numerous NK inhibitory and activating receptors. Most of the inhibitory receptors bind MHC class I proteins and are expressed in a variegated fashion. It was recently shown that TIGIT, a new protein expressed by T and NK cells binds to PVR and PVR-like receptors and inhibits T cell activity indirectly through the manipulation of DC activity. Here, we show that TIGIT is expressed by all human NK cells, that it binds PVR and PVRL2 but not PVRL3 and that it inhibits NK cytotoxicity directly through its ITIM. Finally, we show that TIGIT counter inhibits the NK-mediated killing of tumor cells and protects normal cells from NK-mediated cytoxicity thus providing an “alternative self” mechanism for MHC class I inhibition.
Keywords: inhibitory receptors, natural killers
In contrast to T cells, that possess a single dominant antigen receptor (1), NK cells rely on a vast combinatorial array of receptors to initiate effector functions (2). Both activating and inhibitory receptors expressed on NK cells regulate their activity when interacting with tumors, virus infected cells and bacteria, as well as normal self-cells (2). MHC class I-expressing cells are protected from NK-mediated lysis due to the recognition of various MHC class I proteins by the inhibitory receptors KIR, LIR and CD94-NKG2A (3). Other NK inhibitory receptors which do not interact with MHC class I also exist, such as CEACAM1 and IRp60 (4–8). The significance, however, of these non-MHC class I inhibitory receptors in normal conditions is still unclear. All of the inhibitory receptors share a common immune receptor tyrosine-based inhibitory motif (ITIM) in their cytoplasmic regions, which delivers the inhibitory signal (3).
The NK cell-mediated killing is extracted by specific receptors, among which are the natural cytotoxicity receptors (NCRs), which include the NKp30 that interacts with pp65 of human cytomegalovirus (CMV), BAT3 and the recently identified B7-family member B7-H6 (9–11), and the NKp46/NKp44 receptors, which interact with various viral hemagglutinins (12, 13). The NKG2D receptor interacts with MICA, MICB and ULBP 1–5 (14) and NKp80 interacts with AICL (15). In addition, two other receptors, DNAM-1 and CD96, enhance NK cytotoxicity (16, 17). Both DNAM-1 and CD96 recognize PVR (CD155), whereas DNAM-1 also recognizes PVRL2 (CD112) (16, 17). It was recently shown that a new receptor, named TIGIT, for T cell Ig and ITIM domain, interacts with PVR and its related proteins and that TIGIT inhibits T cell activity indirectly through the manipulation of DC activity (18). Here, we show that TIGIT, through its ITIM, can directly inhibit NK cytotoxicity.
Results
TIGIT Inhibits YTS Killing Through Its ITIM Motif.
While searching for new CD28 family-like receptors, based on bioinformatics analysis, we observed that a protein named VSIG9 or VSTM3 in the databases expresses an ITIM motif. We continued to work on this protein and found that it interacts with PVR (CD155) but not with any other NK ligands tested (supporting information (SI) Figs. S1 and S2). At the same time, Yu et al. (18) identified the same protein and named it TIGIT. Because we observed that TIGIT is found on all NK cells and because it also contains an ITIM motif, we continued our analysis concentrating on the function of TIGIT in NK cells.
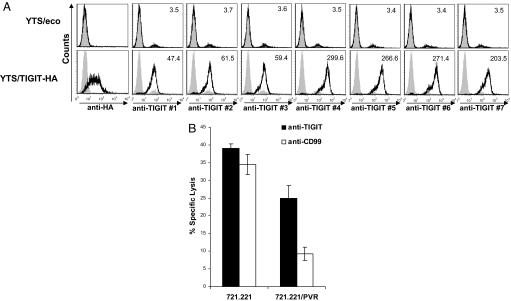
To investigate TIGIT role in controlling NK activity, we initially generated anti-TIGIT mAbs. For that purpose, we used the human YTS NK cell line and transduced it with a lentivirus containing TIGIT fused to an HA tag in its extracellular domain, to allow its detection on the cell surface (Fig. 1A). Mice were injected with the TIGIT-Ig fusion protein (described in Figs. S1 and S2) and hybridomas supernatants were tested for specific recognition of the YTS/TIGIT transfectants. Seven different mAbs were obtained that recognized YTS/TIGIT but not the parental YTS cells (Fig. 1A) in moderate (mAb 1–3) and high (mAb 4–7) modes of recognition.
Fig. 1.
TIGIT inhibits YTS killing. (A) Flow cytometry analysis of the human NK cell leukemia cell line, YTS, transfected with ecotropic receptor only (YTS/eco, Upper) or with TIGIT attached to HA tag (Lower) stained with anti-HA mAb (left histograms). The same cells TIGIT were stained with seven different antibodies directed against TIGIT. Gray filled histograms, background staining with the secondary fluorescein-conjugated antibody only. Numbers indicate median fluorescence intensity. (B) Killing of 721.221 cells or 721.221 cells expressing PVR (721.221/PVR), by YTS/TIGIT preincubated with mAb #4 directed against TIGIT (black column) or a control anti-CD99 mAb (white column). The effector-to-target (E:T) ratio was 4:1.
To test whether TIGIT could directly inhibit NK cell cytotoxicity, we evaluated the killing of YTS and YTS/TIGIT-HA cells. Because YTS cells manifest a restricted killing toward 721.221 cells which is mediated mainly through the interaction between the 2B4 receptor on YTS cells and its ligand, CD48, on the target cells (19), it was important to demonstrate, as shown in Fig. S3, that 2B4 is expressed at equal levels on the parental YTS/eco and on YTS/TIGIT cells. In addition, to allow the examination of TIGIT activity on NK cells we expressed PVR in 721.221 cells and demonstrated that it is indeed recognized by the anti-PVR mAb and by the TIGIT-Ig (Fig. S3). Finally, we verified that CD48 is present in equal levels on all 721.221 cells (Fig. S3). All of these reagents were used in killing assays and as demonstrated in Fig. 1B a strong inhibition of YTS/TIGIT killing is observed when PVR is expressed on 721.221 cells and this inhibition could be blocked with mAb #4. The increased killing observed with mAb #4 was due to blockage of TIGIT and not due to ADCC, because 721.221 cells do not express Fc receptors.
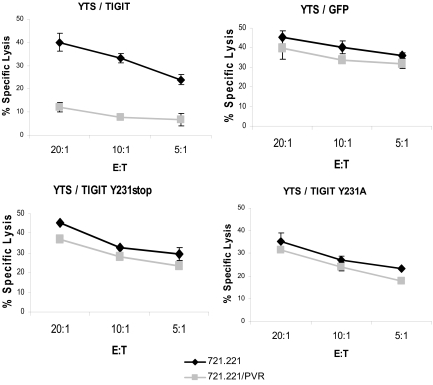
It was shown that the ITIM of the inhibitory receptors is critical for their inhibitory activity (20, 21). To test whether the ITIM of TIGIT is responsible for its inhibitory activity, we generated a truncated form of the TIGIT receptor at position 231 in the ITIM motif (named Y231stop) and also a point mutation in the 231 tyrosine reside mutating it into alanine (named Y231A). These TIGIT proteins were expressed in YTS cells, and in addition, we generated control-transfected YTS cells expressing GFP. Expression levels of all TIGIT proteins were similar. The various YTS transfectants were next assayed for killing of 721.221 and 721.221/PVR cells. Importantly, although a strong PVR-mediated inhibition was observed with YTS/TIGIT, no major difference in the killing of 721.221 and 721.221/PVR was noticed when the control YTS/GFP, YTS/TIGIT Y231stop and YTS/TIGIT Y231A were used (Fig. 2). Thus TIGIT is a direct inhibitory receptor for NK cells and its inhibitory activity is depending on its ITIM.
Fig. 2.
The ITIM of TIGIT delivers the inhibitory signal. Killing of 721.221 cells or 721.221/PVR transfectants by the various YTS cells. The E:T ratios are indicated in the x axis.
Biochemical Characterization of TIGIT.
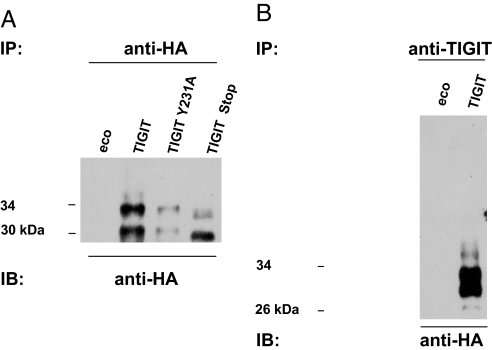
Next, we used our YTS transfectants for biochemical analysis of TIGIT and immunoprecipitated TIGIT from YTS/TIGIT-HA cells by using anti-HA-agarose beads, followed by immunobloting with anti-HA antibodies. Two protein bands in sizes of ≈30 and 34 kDa, probably representing different glycosylation forms of TIGIT, were noticed in the YTS/TIGIT and in the YTS/TIGIT Y231A cells, whereas, as expected, lower-weight protein bands were observed in the YTS/TIGIT Y231stop cells (Fig. 3A). To further strengthen our analysis, we used a specific mouse anti-TIGIT Ab for the immunoprecipitation and again detected these two main protein products only in YTS/TIGIT and not in YTS/eco cells. These products were approximately the same size as observed above (Fig. 3B).
Fig. 3.
Biochemical characterization of TIGIT. YTS/eco cells and YTS-transfectants expressing HA-tagged TIGIT were lysed with either 1% Nonidet P-40 or RIPA lysis buffer. Immunoprecipitation of TIGIT was performed by adding either anti-HA-agarose (A) or anti-TIGIT mAb (B). Membranes were immunoblotted with POD-labeled anti-HA mAbs (Roche).
TIGIT Function on Primary Immune Cells.
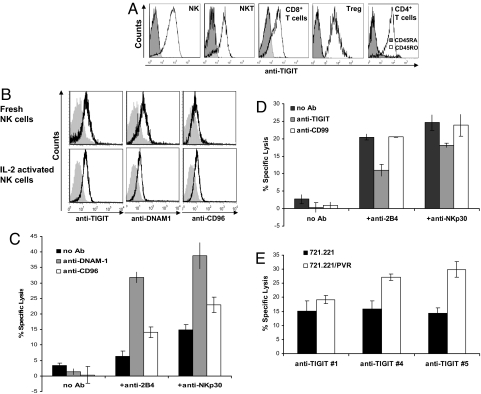
We next analyzed TIGIT expression on various immune cells by using our anti-TIGIT mAbs and observed that TIGIT is expressed on NK, NKT, CD8+, Treg, and memory CD4+ T cells (Fig. 4A). Because it was previously shown that PVR is also a ligand for the receptors DNAM-1 and CD96 (16, 17), we determined the expression and function of all three PVR-binding receptors on NK cells. We initially evaluated their expression on freshly isolated and on IL-2 activated bulk NK cells, and the expression of all of the PVR-binding receptors did not significantly change after IL-2 activation, as can be seen in Fig. 4B. Next, we performed a redirected killing assay to address the direct function of each of the three PVR-binding receptors on NK cells. As shown in Fig. 4C, both DNAM-1 and CD96 failed to independently redirect NK cytotoxicity, whereas, in contrast, direct cytotoxicity was induced by 2B4 and NKp30 (Fig. 4C). Thus, in agreement with previous publications (22), DNAM-1 and CD96 are coactivating receptors. On the other hand, TIGIT was shown to be an inhibitory receptor on NK cells, as the redirected killing of the IL-2 activated bulk NK cultures induced by anti-2B4 or anti-NKp30 mAbs was inhibited by cross-linking of TIGIT with anti-TIGIT mAb #4 (Fig. 4D).
Fig. 4.
TIGIT function on primary immune cells. (A) TIGIT is expressed by NK, NKT, Treg, CD8+ T cells and memory CD4+ T cells. FACS analysis of freshly isolated peripheral blood lymphocytes subsets (indicated in the histograms) stained for TIGIT expression. NK cells were characterized by CD3−CD56+, NKT cells by CD3+CD56+, T cells either by CD8+CD56− or by CD4+CD56−, Treg cells are CD4+CD25+FOXp3+ and memory CD4+ T cells were characterized by CD45RO staining (right histogram). (B) Flow cytometry of freshly isolated or IL-2 -activated NK cells stained for TIGIT, DNAM1, and CD96 expression. Gray shaded histograms, background staining with the secondary fluorescein-conjugated antibody only. (C) DNAM1 and CD96 are costimulatory receptors. P815 cells were incubated either with no mAb or with the combinations of anti-2B4 or anti-NKp30 mAbs (indicated in the x axis) and anti-DNAM1 or with anti-CD96 mAb (indicated by the different column colors). Redirected NK cytotoxicity against P815 cells was determined at E:T ratio of 3:1. (D) P815 cells were incubated either with no mAb or with combinations of anti-2B4 or anti-NKp30 mAbs (indicated in the x axis) with and without anti-TIGIT, or with anti-CD99 (indicated by the different column colors) to redirect NK cytotoxicity. Effector IL-2 activated bulk NK cells were then added in E:T ratio of 5:1. The two redirected killing assays presented in C and D were done independently. (E) Killing of the target cells 721.221 and 721.221/PVR by primary NK cells at E:T ratio of 5:1. NK cells were preincubated with the various mAb indicated in the x axis.
Next, we studied the TIGIT activity as part of the complex killing machinery of NK cells when encountering tumor cells expressing PVR. IL-2-activated bulk NK cell cultures were incubated with 721.221 and 721.221/PVR, and, as demonstrated in Fig. 4E, the killing of 721.221/PVR was only slightly induced, indicating that the PVR-CD96/DNAM-1 interactions are too weak in the context of 721.221/PVR cells to strongly up-regulate NK cytotoxicity. Importantly, blocking TIGIT-PVR interaction by mAb #4 and #5, but not with #1 (which did not bind the NK cells, Fig. S4), resulted in a significantly increased killing of the PVR expressing 721.221 cells, indicating that TIGIT inhibition is indeed dominant over the coactivation of CD96 and DNAM-1.
PVR and PVRL2 but Not PVRL3 Are Ligands for TIGIT.
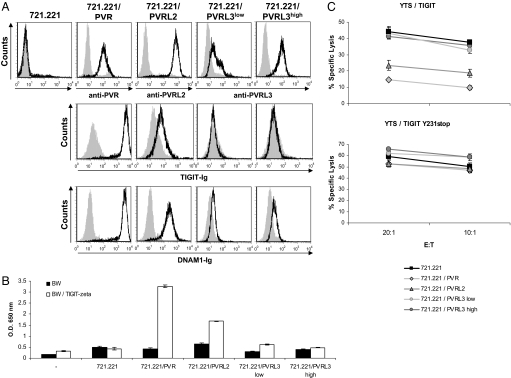
We next assayed whether other PVR-like proteins, PVRL2 (CD112) and PVRL3 (CD113), would be recognized by TIGIT and whether such recognition will lead to inhibition of NK cell killing. PVRL2 or PVRL3 proteins were expressed in 721.221 cells (Fig. 5A Top) and the various transfectants were stained with TIGIT-Ig and with DNAM-1-Ig. As can be seen in Fig. 5A and in agreement with Yu et al. observations (18), TIGIT-Ig bound PVRL2 but with much lower affinity as compared to PVR. Surprisingly, in contrast to the results of Yu et al., TIGIT-Ig did not interact with PVRL3, despite the fact that several PVRL3 transfectants were used (Fig. 5A Middle). As was previously reported (19), DNAM1-Ig bound both PVR and PVRL2, and, surprisingly, we also detected a weak interaction that to the best of our knowledge was not observed before, of DNAM-1 to PVRL3 (Fig. 5A Bottom). Next, to confirm the staining results, we used a cell-based reporter assay which utilizes the TIGIT-ζ chimeric protein expressed in BW cells (BW/ TIGIT-ζ, shown in Fig. S2). The BW parental cells and the BW/ TIGIT- ζ cells were coincubated for 48 h with the different 721.221 transfectants and in agreement with the Ig-fusion protein binding results (Fig. 5A), a significant amount of mIL-2 was detected in the supernatant of BW/ TIGIT-ζ cells coincubated with 721.221/PVR and to a lesser extent with 721.221/PVRL2 (Fig. 5B). In contrast, little or no mIL-2 secretion was observed with 721.221/PVRL3 cells.
Fig. 5.
PVR and PVRL2 but not PVRL3 are ligands for TIGIT. (A) Flow cytometry analysis of 721.221 parental cell line and the various transfectants stained with the indicated mAbs and with the fusion proteins TIGIT-Ig and DNAM1-Ig (black lines). Gray filled histograms are the background staining with the secondary fluorescein- (for mAbs) or phycoerythrin- (for fusion proteins) conjugated antibody only. The parental 721.221 cells were stained by all three anti-PVR, PVRL2 and PVRL3 antibodies (B) Mouse IL-2 secretion from parental BW cells or BW cells expressing the TIGIT ζ chimeric protein, incubated for 48 h with the 721.221 transfectants presented in A. Mouse IL-2 secretion was measured by ELISA. (C) Killing of the indicated 721.221 transfectant cells by YTS/TIGIT and YTS/TIGIT Y231stop cells. The E:T ratios are indicated in the x axis.
Finally, we tested the functional relevance of the PVR-like proteins-TIGIT interactions by using the YTS/TIGIT and YTS/TIGIT Y231stop cells. As seen in Fig. 5C, whereas PVR showed the strongest inhibition, the inhibition mediated by PVRL2 was less efficient, and no inhibition was observed when PVRL3, was used (Fig. 5C). The inhibition was ITIM dependent because no inhibition was observed when YTS/TIGIT Y231stop (Fig. 5C) cells were used. Thus, these combined results indicate that PVRL2 is a low-affinity ligand for TIGIT as compared with PVR, whereas PVRL3 is not a ligand for TIGIT.
TIGIT Provides an “Alternative Self” Mechanism for MHC Class I Inhibition.
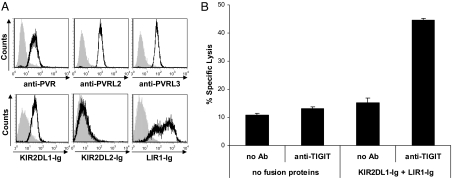
The expression of PVR and PVRL2 is up-regulated on tumor cells (16, 23), and thus it was logical to assume that the interactions of PVR and PVRL2 with their coactivating receptors will lead to enhanced tumor killing. On the other hand, PVR and PVRL2 are widely expressed on healthy normal cell types of epithelial origin and on peripheral blood monocytes (24, 25) and it is of course undesirable for NK cells to kill these normal cells. We therefore hypothesized that the inhibitory interactions of TIGIT would be dominant over the coactivating ones of DNAM-1 and CD96 to prevent self killing. For that purpose, we used the primary human foreskin fibroblasts cells which endogenously express PVR, PVRL2 and PVRL3 (Fig. 6A Upper) and also MHC class I, detected by KIR2DL1-Ig and LIR1-Ig (Fig. 6A Lower) and incubated them with bulk NK cell cultures. No staining of the fibroblasts cells was observed with KIR2DL2-Ig, indicating that the fibroblasts we used from this particular infant do not expressed HLA-C proteins characterized by the presence of the asparagine residue at position 80. As can be seen in Fig. 6B, the killing of the fibroblasts cells was low even when MHC class I proteins were blocked by the fusion proteins or when TIGIT was blocked by the anti-TIGIT mAb. Importantly, however, when both MHC class I and TIGIT were blocked (by the KIR2DL1-Ig and LIR1-Ig fusion proteins and anti-TIGIT mAb, respectively), a significant killing was induced (Fig. 6B). Thus, TIGIT is an inhibitory receptor which prevents killing of self-normal cells by NK cells.
Fig. 6.
TIGIT protects normal cells expressing PVR and PVR-like proteins from NK cell-mediated killing. (A) Primary fibroblasts characterization. Flow cytometry analysis of primary human foreskin fibroblast (HFF) cells stained with the indicated mAbs and fusion proteins (black lines). Gray filled histograms are the background staining with the secondary fluorescein- (for mAbs) or phycoerythrin- (for fusion proteins) conjugated antibody only. (B) Killing of the primary human foreskin fibroblasts by primary NK cells at an E:T ratio of 20:1. NK cells were either preincubated or not with anti-TIGIT mAb #5 or with no mAb. Fibroblasts were preincubated either with no fusion proteins or with the indicated fusion proteins.
Discussion
The current manuscript, together with the findings of Yu et al. (18), place TIGIT as a vital immunomodulator protein, able to control the activities of both NK and T cells.
In NK cells, the inhibitory signal of TIGIT is mediated via its ITIM. In addition, as TIGIT binds PVR with the highest affinity compared with DNAM-1 and CD96 (18), TIGIT should also physically interfere with DNAM-1 and CD96 binding. We still do not know why, in T cells, TIGIT is not a direct inhibitory receptor (18). However, it seems as if the inhibitory activity of TIGIT might be different from other NK inhibitory receptors as we could not precipitate TIGIT under nonreducing conditions (suggesting that it might be found in complexes) and we could not precipitate SHP1 with TIGIT.
In agreement with Yu et al. data, we also observed that TIGIT interacts with PVRL2 and that this interaction leads to the inhibition of NK cell cytotoxicity. Surprisingly, and in contrast to Yu et al. data, we demonstrate in our systems that TIGIT bind PVRL3, even when using transfectants expressing high levels of PVRL3. We currently have no explanation for this discrepancy but since we used several systems, including functional assays, we think that the PVRL3-TIGIT interactions, if exist, are not functional.
Remarkably, two additional coactivating receptors expressed on NK and T cells, DNAM-1 and CD96, also bind PVR, and DNAM-1 shares another ligand with TIGIT, PVRL2 (16, 17). This situation is a reminiscent of T cells, in which the coinhibitory receptor CTLA-4 binds the same ligands (B7-1 and B7-2) as the coactivating receptor CD28 (26–29). This apparent conflict is resolved in T cells due to the fact that expression of CTLA-4 is increased after T cell activation (30). In addition, CTLA-4 binds its ligands in a much higher affinity than CD28, resulting in the inhibition of T cell functions (27). Another example for pairwise receptors is the killer Ig-like receptor (KIR) family (31) that also includes activating counterparts named KARs (32). Currently, few KARs share the same MHC class I ligands as the KIR (33), but they interact with the appropriate MHC class I proteins with lower affinity (34, 35). Thus, in both CTLA-4/CD28 and KIR/KAR pairs, inhibition is dominant and as we and Yu et al. (18) show by functional and by binding assays, the TIGIT-PVR interaction is dominant over that of CD96/DNAM1-PVR.
Interestingly, staining 721.221 transfectants with TIGIT-Ig and with DNAM1-Ig, unexpectedly demonstrated weak binding of DNAM1-Ig to 721.221 cells expressing PVRL3, suggesting that PVRL3 might be an additional ligand for DNAM-1. Thus, the PVR-binding receptors might be balanced not only by the strength of their binding but also by the variegated expression of their ligands.
In the original paper describing DNAM-1-PVR interaction (19), PVR blockade resulted in a strong reduction of cytolysis by DNAM-1-expressing YT cells. Here, we used the YT derivative YTS and, surprisingly, observed no increase in the killing of 721.221/PVR cells by the parental YTS cells. To resolve this discrepancy, we stained YTS cells with an anti-DNAM-1 antibody and, surprisingly, observed that indeed YTS cells express DNAM-1 (Fig. S5). However, when we stained YTS cells with PVR-Ig, no binding was observed, whereas YTS/TIGIT cells were recognized by PVR-Ig (Fig. S5). Thus, it seems as if, although DNAM-1 is expressed on YTS cells, for an as yet unknown reason, it cannot interact with PVR.
As we show here, TIGIT is an inhibitory receptor expressed by all NK cells and PVR, PVRL2 and even PVRL3 are expressed on normal cells of an epithelial origin, such as endothelial cells. Endothelial cells are continuously encountered by NK cells when, for example, NK cells extravasate to the tissues. Thus, we suggest that under normal conditions, PVR and PVRL2 provide an alternative self mechanism preventing self destruction of normal cells by NK cells.
Interestingly, enhanced expression of PVR and PVRL2 is also observed in various tumors (16, 23, 36) and several recent reports demonstrated that PVR and PVRL2 expression on tumors might enhance NK cytotoxicity (37–40). Furthermore, it was recently demonstrated that knockout of DNAM-1 resulted in enhanced tumorgenicity (22, 41). In light of our observations, we think that these previous works should be reevaluated as it seems as if the function of PVR and PVRL2 on tumor cells is not to be better recognized by NK killer and inhibitory receptors, but maybe, as suggested previously to aid tumor invasion and migration (36).
Materials and Methods
Cells and Transfectants.
The cell lines used in the present study were YTS cells, transfected with the ecotropic murine retrovirus receptor (YTS/eco), the human EBV-transformed B-cell line 721.221, 721.221 transfectants (33), the mastocytoma cell line P815 and P815 stably expressing PVR (a kind gift from M. Colonna, Washington University.) and the murine thymoma BW cell line. Human fibroblasts were obtained from primary cultures of foreskins. NK cells were isolated from peripheral blood lymphocyte samples.
Antibodies.
The mAbs used in this work were anti-CD155/PVR clone 300907 (R&D Systems), anti-PVRL2/CD112 clone TX31 (BioLegend), anti-PVRL3/CD113 clone N3.12.4 (Santa Cruz Biotechnology), 12E7 directed against CD99 (used as an isotype control, was a gift from A. Bernard, INSERM, France), 12CA5 directed against HA, anti-2B4 clone C1.7 (Beckman–Coulter), anti-NKp30 clone 210845 (R&D Systems), and anti-DNAM-1 clone 102511 (R&D Systems).
BW Assay and Cytotoxicity Assay.
For measurement of IL-2 production resulting from the interaction between TIGIT and the PVR-family proteins, we used the BW assay as described previously (42), and in SI Text. The cytotoxic activity of primary NK cells, YTS/eco and YTS transfectants against 721.221 parental cells and transfectants, P815 and primary human foreskin fibroblasts target cells was assessed in 5-h 35S release assays as previously described (42).
Immunoprecipitation and Western Blot Analysis.
YTS cells as well as YTS transfectants were lysed with 1% (vol/vol) Nonidet P-40 [140 mM NaCl, 10 mM NaPO4( pH 7.2)] containing Complete Mini protease inhibitors (Roche). In some experiments, RIPA buffer was used. Lysates were cleared by centrifugation, precleared with 40 μL of G-Sepharose, and then immunoprecipitated with either 50 μL of anti-HA agarose (Sigma) or with 5 μg of TIGIT-specific antibodies, followed by 50 μL of G-Sepharose. Immunoprecipitated proteins were separated on 11% PAGE and then immunoblotted with POD-labeled rat anti-HA mAbs (clone 3F10; Roche) followed by POD-labeled anti-mouse IgG. Membranes were developed by using a BM Chemiluminescence Western Blotting kit (Roche).
Supplementary Material
Acknowledgments.
This work was supported by grants from the U.S.–Israel Binational Science Foundation, the Israeli Cancer Research Foundation, The Israeli Science Foundation (ISF), European Consortium Grants MRTN-CT-2005 and LSCH-CT-2005-518178), the Association for International Cancer Research, the ISF (Morasha) and the Israel–Croatia Research Grant (all to O.M and S.J.), O.M is a Crown professor of Molecular Immunology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903474106/DCSupplemental.
References
- 1.Schwartz RH. Costimulation of T lymphocytes: The role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 2.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 3.Lanier LL. Up on the tightrope: Natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantoni C, et al. Molecular and functional characterization of IRp60, a member of the immunoglobulin superfamily that functions as an inhibitory receptor in human NK cells. Eur J Immunol. 1999;29:3148–3159. doi: 10.1002/(SICI)1521-4141(199910)29:10<3148::AID-IMMU3148>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 5.Markel G, et al. CD66a interactions between human melanoma and NK cells: A novel class I MHC-independent inhibitory mechanism of cytotoxicity. J Immunol. 2002;168:2803–2810. doi: 10.4049/jimmunol.168.6.2803. [DOI] [PubMed] [Google Scholar]
- 6.Kumar V, McNerney ME. A new self: MHC-class-I-independent natural-killer-cell self-tolerance. Nat Rev Immunol. 2005;5:363–374. doi: 10.1038/nri1603. [DOI] [PubMed] [Google Scholar]
- 7.Lebbink RJ, Meyaard L. Non-MHC ligands for inhibitory immune receptors: Novel insights and implications for immune regulation. Mol Immunol. 2007;44:2153–2164. doi: 10.1016/j.molimm.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Stern N, et al. Carcinoembryonic antigen (CEA) inhibits NK killing via interaction with CEA-related cell adhesion molecule 1. J Immunol. 2005;174:6692–6701. doi: 10.4049/jimmunol.174.11.6692. [DOI] [PubMed] [Google Scholar]
- 9.Arnon TI, et al. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat Immunol. 2005;6:515–523. doi: 10.1038/ni1190. [DOI] [PubMed] [Google Scholar]
- 10.Pogge von Strandmann E, et al. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity. 2007;27:965–974. doi: 10.1016/j.immuni.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Brandt CS, et al. The B7 family member B7–H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206:1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandelboim O, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 13.Arnon TI, et al. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur J Immunol. 2001;31:2680–2689. doi: 10.1002/1521-4141(200109)31:9<2680::aid-immu2680>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 14.Vivier E, Tomasello E, Paul P. Lymphocyte activation via NKG2D: Towards a new paradigm in immune recognition? Curr Opin Immunol. 2002;14:306–311. doi: 10.1016/s0952-7915(02)00337-0. [DOI] [PubMed] [Google Scholar]
- 15.Welte S, Kuttruff S, Waldhauer I, Steinle A. Mutual activation of natural killer cells and monocytes mediated by NKp80-AICL interaction. Nat Immunol. 2006;7:1334–1342. doi: 10.1038/ni1402. [DOI] [PubMed] [Google Scholar]
- 16.Bottino C, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. J Exp Med. 2003;198:557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuchs A, Cella M, Giurisato E, Shaw AS, Colonna M. Cutting edge: CD96 (tactile) promotes NK cell-target cell adhesion by interacting with the poliovirus receptor (CD155) J Immunol. 2004;172:3994–3998. doi: 10.4049/jimmunol.172.7.3994. [DOI] [PubMed] [Google Scholar]
- 18.Yu X, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 19.Chuang SS, et al. 2B4 stimulation of YT cells induces natural killer cell cytolytic function and invasiveness. Immunology. 2000;100:378–383. doi: 10.1046/j.1365-2567.2000.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maeda A, et al. Paired immunoglobulin-like receptor B (PIR-B) inhibits BCR-induced activation of Syk and Btk by SHP-1. Oncogene. 1999;18:2291–2297. doi: 10.1038/sj.onc.1202552. [DOI] [PubMed] [Google Scholar]
- 21.Muta T, et al. A 13-amino-acid motif in the cytoplasmic domain of Fc gamma RIIB modulates B-cell receptor signalling. Nature. 1994;369:340. doi: 10.1038/369340a0. [DOI] [PubMed] [Google Scholar]
- 22.Gilfillan S, et al. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. J Exp Med. 2008;205:2965–2973. doi: 10.1084/jem.20081752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masson D, et al. Overexpression of the CD155 gene in human colorectal carcinoma. Gut. 2001;49:236–240. doi: 10.1136/gut.49.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuchs A, Colonna M. The role of NK cell recognition of nectin and nectin-like proteins in tumor immunosurveillance. Semin Cancer Biol. 2006;16:359–366. doi: 10.1016/j.semcancer.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Freistadt MS, Fleit HB, Wimmer E. Poliovirus receptor on human blood cells: A possible extraneural site of poliovirus replication. Virology. 1993;195:798–803. doi: 10.1006/viro.1993.1433. [DOI] [PubMed] [Google Scholar]
- 26.Linsley PS, Clark EA, Ledbetter JA. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci USA. 1990;87:5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linsley PS, et al. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman GJ, et al. Cloning of B7–2: A CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993;262:909–911. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- 29.Azuma M, et al. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993;366:76–79. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- 30.Brunet JF, et al. A new member of the immunoglobulin superfamily–CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 31.Moretta L, Moretta A. Killer immunoglobulin-like receptors. Curr Opin Immunol. 2004;16:626–633. doi: 10.1016/j.coi.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Biassoni R, et al. The human leukocyte antigen (HLA)-C-specific “activatory” or “inhibitory” natural killer cell receptors display highly homologous extracellular domains but differ in their transmembrane and intracytoplasmic portions. J Exp Med. 1996;183:645–650. doi: 10.1084/jem.183.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katz G, et al. MHC class I-independent recognition of NK-activating receptor KIR2DS4. J Immunol. 2004;173:1819–1825. doi: 10.4049/jimmunol.173.3.1819. [DOI] [PubMed] [Google Scholar]
- 34.Biassoni R, et al. Role of amino acid position 70 in the binding affinity of p50.1 and p58.1 receptors for HLA-Cw4 molecules. Eur J Immunol. 1997;27:3095–3099. doi: 10.1002/eji.1830271203. [DOI] [PubMed] [Google Scholar]
- 35.Vales-Gomez M, Reyburn HT, Erskine RA, Strominger J. Differential binding to HLA-C of p50-activating and p58-inhibitory natural killer cell receptors. Proc Natl Acad Sci USA. 1998;95:14326–14331. doi: 10.1073/pnas.95.24.14326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sloan KE, et al. CD155/PVR plays a key role in cell motility during tumor cell invasion and migration. BMC Cancer. 2004;4:73. doi: 10.1186/1471-2407-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Sherbiny YM, et al. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res. 2007;67:8444–8449. doi: 10.1158/0008-5472.CAN-06-4230. [DOI] [PubMed] [Google Scholar]
- 38.Pende D, et al. PVR (CD155) and Nectin-2 (CD112) as ligands of the human DNAM-1 (CD226) activating receptor: involvement in tumor cell lysis. Mol Immunol. 2005;42:463–469. doi: 10.1016/j.molimm.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 39.Castriconi R, et al. Natural killer cell-mediated killing of freshly isolated neuroblastoma cells: Critical role of DNAX accessory molecule-1-poliovirus receptor interaction. Cancer Res. 2004;64:9180–9184. doi: 10.1158/0008-5472.CAN-04-2682. [DOI] [PubMed] [Google Scholar]
- 40.Pende D, et al. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: Evidence for the involvement of the poliovirus receptor (CD155) and nectin-2 (CD112) Blood. 2005;105:2066–2073. doi: 10.1182/blood-2004-09-3548. [DOI] [PubMed] [Google Scholar]
- 41.Iguchi-Manaka A, et al. Accelerated tumor growth in mice deficient in DNAM-1 receptor. J Exp Med. 2008;205:2959–2964. doi: 10.1084/jem.20081611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Achdout H, et al. Enhanced recognition of human NK receptors after influenza virus infection. J Immunol. 2003;171:915–923. doi: 10.4049/jimmunol.171.2.915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.