Abstract
Fibrocytes are collagen-type-I-producing cells that arise at low frequency from hematopoietic cells. We have analyzed in mice which leukocyte subsets are required for generation of fibrocytes and show that murine fibrocytes develop from the subpopulation of CD11b+ CD115+ Gr1+ monocytes under the control of CD4+ T cells. In the absence of CD4+ T cells, differentiation of fibrocytes was markedly reduced in vitro and in vivo. In the presence of CD4+ T cells, the characteristics of T-cell activation critically determined development of fibrocytes. Polyclonal activation of CD4+ T cells induced the release of soluble factors that completely prevented the outgrowth of fibrocytes and could be identified as IL-2, TNF, IFN-γ, and IL-4. Application of IL-2 and TNF significantly reduced the appearance of fibrocytes and the severity of fibrosis in the model of unilateral ureteral obstruction. In contrast, activation of CD4+ T cells in the presence of calcineurin inhibitors, but not mTOR inhibitors, markedly enhanced the outgrowth of fibrocytes and renal deposition of collagen I. Taken together, we show that differentiation of fibrocytes is critically dependent on CD4+ T cells and that the context of T-cell activation determines whether development of fibrocytes is supported or blocked. Our data may have implications for prevention of organ fibrosis in autoimmune diseases and transplantation.
Keywords: fibrosis, transplantation, kidney, cytokines
Fibrosis, defined as excess deposition of extracellular matrix, especially collagen type I, is often the result of incomplete healing after tissue destruction. In the kidney, three cell types have been described to develop into collagen-type-1-producing cells: resident mesenchymal cells (fibroblasts), epithelial or endothelial cells undergoing mesenchymal transformation, and collagen-I-producing hematopoietic cells called fibrocytes (1–4). Fibroctyes were described by Bucala et al. (5) and arise under appropriate conditions at a low frequency from CD45+ leukocytes. The bone marrow-derived fibrocyte precursors are present within the blood and attracted to sites of injury, where they differentiate into spindle-shaped fibrocytes and at least in part, mediate tissue repair and fibrosis. Fibrocytes have been described to develop with the help of CD4+ T cells from peripheral blood monocytes (5–10). Fibrocytes express CD11b and chemokine receptors such as CCR7, CXCR4, and CCR2 (6, 11–13) that are involved in their recruitment to sites of inflammation and fibrosis (11–13). Fibrocytes were detected in mouse models of pulmonary fibrosis, bronchial asthma, renal fibrosis, and skin wounds and in human interstitial pulmonary fibrosis, burns, and nephrogenic systemic fibrosis (6, 10, 12–18). The contribution of fibrocytes to tissue fibrosis is still unclear. Early work in the model of bleomycin-induced pulmonary fibrosis suggested that fibrocytes constitute a major part of all collagen type I producing cells in the injured lung (12). In the model of unilateral ureteral obstruction (UUO) blockade of CCR7 reduced the numbers of fibrocytes in the kidney and the degree of fibrosis (13). However, there are also data challenging the contribution of fibrocytes to collagen production. In transgenic mice carrying a reporter gene under the control of the collagen promoter, no significant numbers of bone marrow-derived reporter gene-positive cells were found in the UUO model (19). In a similar approach, bone marrow-derived reporter-gene-positive cells were found in the UUO model, but were considered to play only a minor role compared with intrinsic mesenchymal fibroblasts (20). In bone marrow chimeric mice, not all leukocyte subpopulations may reconstitute with equal efficiency, and the reporter gene may only be expressed in a fraction of collagen-producing cells, thus resulting in underestimation of the number and diversity of collagen-producing cells.
CD4+ T cells play a prominent role in the progression of fibrotic diseases. Studies conducted with cytokine-deficient mice have demonstrated that liver fibrosis is strongly linked to the development of a Th2 cell response involving IL-4, IL-5, IL-13, and IL-21 (21–26). Some of these factors also affect the development of fibrocytes. IL-4 and IL-13 promote, whereas IFN-γ and IL-12 block differentiation of fibrocytes from their progenitors (27). In addition, TGF-β was shown to promote the differentiation and accumulation of fibrocytes (6, 12) whereas aggregated IgG or serum amyloid P block differentiation (28, 29). TNF can have pro- and anti-fibrotic effects (30) and, as a single agent, did not alter the numbers of human fibrocytes or their production of collagen I (8, 27). Production of cytokines by CD4+ T cells is regulated by the influx of Ca that interacts with calcineurin. The calcineurin-inhibitor cyclosporine A blocks the transcription of cytokines such as IL-2 and is associated with a number of toxic effects, most notably tubulointerstitial fibrosis in transplanted kidneys (31–34).
The precise origin of fibrocyte progenitors is unknown, and only few factors leading to increased or decreased differentiation are known. Using murine cells and the model of UUO that leads to a pronounced renal fibrosis, we analyzed in more detail which cells constitute the precursors of fibrocytes and which factors determine their differentiation into mature fibrocytes. Because T-cell-derived cytokines and cyclosporine A are associated with fibrosis, we investigated how T-cell activation in the absence or presence of immunosuppressive drugs and cytokines produced under these conditions influence the differentiation of fibrocytes.
Results
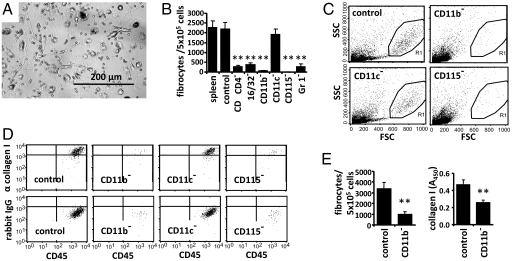
Cellular Origin of Fibrocytes in Mice.
To dissect the origin of fibrocytes in mice, we quantified the appearance of fibrocytes after depletion of various leukocyte subsets from mouse splenocytes. Culture of splenocytes for 14 days resulted in outgrowth of spindle-shaped cells that morphologically resemble fibrocytes (6) (Fig. 1A) and appear as large cells with rather homogenous light scatter properties in flow cytometry (Fig. 1C). Large cells located within gate R1 showed a homogenous expression of collagen type I by intracellular fluorescence-activated cell sorting (FACS) staining (Fig. 1D) whereas small cells outside gate R1 had similar light scatter properties as freshly prepared splenocytes or peripheral blood cells and did not express collagen I (Fig. S1 and S2). FACS-analysis also clearly showed that these collagen I+ cells strongly express the pan-leukocyte marker CD45, a typical feature of fibrocytes (5). In addition, collagen I+ CD45+ cells showed a clear expression of CD16/32 and CD11b, a weak expression of MHC-II, but no expression of CD115, CD34, or CXCR4 (Fig. S2). Using microscopy, the number of spindle-shaped cells was markedly reduced after in vitro depletion of cells expressing the monocyte markers CD16/32, CD11b, CD115, or Gr1 (Fig. 1B). Total splenocytes or sham-depleted cell populations served as control. Depletion of CD11c+ dendritic cells (Fig. 1B) had no influence on the outgrowth of fibrocytes. CD115 is a rather specific marker for murine monocytes, and Gr1 is expressed on a subpopulation of monocytes that preferentially accumulate in inflamed tissues. Light scatter analysis and intracellular staining of collagen I confirmed that depletion of CD11b+ or CD115+ cells, but not CD11c+ cells, almost completely prevents the outgrowth of fibrocytes (Fig. 1 C and D). Quantification of collagen I in total cell lysates by ELISA verified that the overall amount of collagen I in culture significantly decreases after depletion of CD11b+ cells (Fig. 1E). These results suggest that murine fibrocytes differentiate from a Gr1+ monocyte subpopulation.
Fig. 1.
Cellular origin of fibrocytes in mice. (A–E) Total splenocytes or splenocytes depleted of specific leukocyte subsets were cultured for 14 days. (A) Pictures of cultured splenocytes were taken under light microscopy. (B) Number of spindle-shaped cells was counted. Compared with culture of total splenocytes (spleen) or sham depletion (control), depletion of cells expressing CD4, CD16/32, CD11b, CD115, or Gr1 but not CD11c almost completely prevented the outgrowth of spindle-shaped cells. (C) Flow cytometric analysis of light scatter properties of splenocytes cultured after depletion of various leukocyte subpopulations. (D) Large cells located within gate R1 of C were analyzed for expression of CD45 and intracellular collagen I. Intracellular staining with an isotype control antibody (rabbit IgG) served as control. (E) Number of spindle-shaped cells per 105 splenocytes and quantification of collagen I in total cell lysates by ELISA after culture of sham-depleted splenocytes (control) or splenocytes depleted of CD11b+ cells. Results are expressed as the mean ± SEM of the number of spindle-shaped cells from three pictures of three wells/condition. Statistical significance has been determined in comparison with the control.
Conditions Supporting the Differentiation of Monocytes into Fibrocytes.
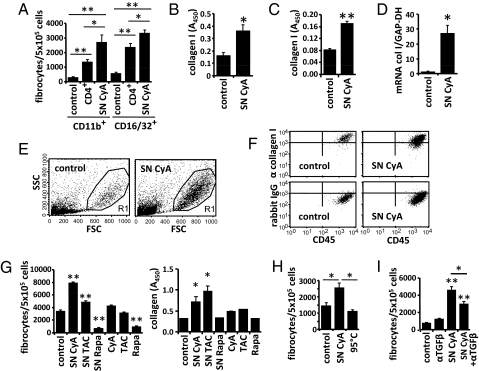
CD11b+ or CD16/32+ cells isolated from the spleen were used to study in more detail the conditions for differentiation of monocytes into fibrocytes. Addition of purified nonstimulated CD4+ T cells markedly increased the differentiation of monocytes into spindle-shaped fibrocytes (Fig. 2A). These data are consistent with the observation that depletion of CD4+ T cells from total splenocytes almost completely prevented the outgrowth of fibrocytes (Fig. 1B). To further investigate the influence of factors derived from CD4+ T cells on the differentiation of fibrocytes, we cultured purified monocytes with the supernatant of CD4+ T cells. If CD4+ T cells were polyclonally activated for 72 h in the presence of cyclosporine A, the supernatant of these cells (SN CyA) markedly increased the ability of monocytes to develop into fibrocytes during a 14-day culture. This became evident by increased numbers of spindle-shaped cells (Fig. 2A), by increased levels of collagen I in the cell lysate (Fig. 2B), by increased secretion and deposition of collagen I on the culture plate (Fig. 2C), and by an up-regulation of collagen I mRNA measured by real-time PCR (Fig. 2D). If CD11b+ monocytes were exposed to SN CyA, we also found increased numbers of collagen I+ CD45+ fibrocytes with typical light scatter properties by flow cytometry (Fig. 2 E and F). To investigate whether cyclosporine A promotes the generation of fibrocytes by acting on CD4+ T cells and changing their production of soluble mediators or by directly acting on CD11b+ monocytes, we incubated purified CD11b+ monocytes either with SN CyA or with medium containing the same concentration of cyclosporine A (Fig. 2G). SN CyA significantly increased the development of fibrocytes whereas cyclosporine A alone had no effect. We also analyzed the calcineurin inhibitor (tacrolimus) and the mTor inhibitor rapamycin. The supernatant of CD4+ T cells activated in the presence of tacrolimus (SN TAC) increased whereas the supernatant of CD4+ T cells activated in the presence of rapamycin (SN Rapa) decreased the development of fibrocytes and the deposition of collagen I. SN CyA heated to 95 °C did not enhance generation of fibrocytes, indicating that the T-cell-derived factors may consist of soluble proteins (Fig. 2H). TGF-β appeared as possible candidate, because cyclosporine A induces the expression of TGF-β from CD4+ T cells (35, 36) and TGF-β is known to support the generation of fibrocytes (6, 15). Blockade of TGF-β with an antibody partially reduced the differentiation of monocytes into fibrocytes in the presence of SN CyA whereas it had no effect in the absence of SN CyA (Fig. 2I). To demonstrate more directly that Gr1+ monocytes have the ability to differentiate into collagen-producing fibrocytes, CD11b+Gr1+ and CD115+Gr1+ monocytes were highly purified by FACS sort (purity >97%) (Fig. S3). If the purified monocytes were cultured in plain medium, no fibrocytes developed. However, when the cells were cultured in the presence of SN CyA numerous large spindle-shaped cells developed that secreted collagen I as measured by ELISA and displayed typical light scatter properties in flow cytometry (Fig. S3). In contrast, CD115+Gr1− monocytes did not develop into fibrocytes, even in the presence of SN CyA. These results demonstrate that Gr1+ monocytes are dependent on additional factors for differentiation into fibrocytes.
Fig. 2.
Conditions supporting the differentiation of monocytes isolated from splenocytes into fibrocytes. (A) CD11b+ or CD16/32+ monocytes were cultured for 14 days with medium (control), purified CD4+ T cells, or the supernatant of CD4+ T cells activated in the presence of cyclosporine A (SN CyA). Addition of CD4+ T cells or SN CyA markedly enhanced the appearance of spindle-shaped cells. (B–F) CD11b+ monocytes were incubated with medium or SN CyA. (B) Amount of intracellular collagen I in total cell lysates. (C) Amount of collagen I secreted onto the culture plate was measured by ELISA. (D) Expression of collagen I mRNA (col I) relative to GAPDH was quantified by real-time RT-PCR in cultured cells. (E and F) Light scatter properties of cultured cells (E) and expression of CD45 and intracellular collagen I compared with an isotype control (rabbit IgG) (F) were analyzed by flow cytometry. (G) CD11b+ monocytes were cultured with medium (control), with SN CyA, SN TAC, SN Rapa, or directly with cyclosporine A (CyA), tacrolimus (TAC), or rapamycin (Rapa). The number of spindle-shaped cells was counted, and the amount of secreted collagen I was measured by ELSIA. (H) Numbers of spindle-shaped cells after culture of CD11b+ monocytes with medium (control) SN CyA or boiled SN CyA (95 °C). (I) Numbers of spindle-shaped cells after culture of CD11b+ monocytes with isotype control antibody (control), anti-TGF-β, SN CyA or SN CyA preincubated with anti-TGF-β. Cell culture experiments were performed in triplicates. Results are expressed as the mean ± SEM of cell culture triplicates. Statistical significance has been determined in comparison with the medium control.
Influence of T-Cell-Derived Cytokines on the Generation of Fibrocytes.
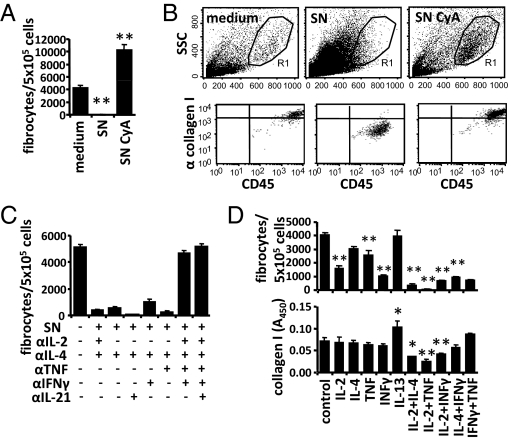
In contrast to T-cell activation in the presence of cyclosporine A, the supernatant of CD4+ T cells activated in the absence of immunosuppressive drugs (SN) completely prevented the differentiation of monocytes into spindle-shaped fibrocytes (Fig. 3A). This was confirmed by flow cytometry, where the large fibrocytes completely disappeared in light scatter plots, and collagen I+ cells were absent (Fig. 3B). We used blocking antibodies against several T-cell-derived cytokines to elucidate which cytokines suppress the development of fibrocytes. Combined blockade of four cytokines (IL-2, IL-4, TNF, and IFN-γ) was required to restore the generation of spindle-shaped fibrocytes (Fig. 3C). Blockade of only two or three of these factors (IL-2, TNF, and IFN-γ) had no or only minor effects. CD4+ T cells activated with anti-CD3 released substantial amounts of IFN-γ, TNF, IL-4, and IL-2, which was almost completely prevented by cyclosporine A or tacrolimus, whereas rapamycin had an intermediate effect (Fig. S4).
Fig. 3.
Influence of T-cell-derived cytokines on the generation of fibrocytes. (A and B) CD11b+ monocytes isolated from splenocytes were cultured for 14 days with medium (control), supernatant of CD4+ T cells activated in absence (SN) or in presence of cyclosporine A (SN CyA). (A) Addition of SN completely prevented, whereas addition of SN CyA markedly enhanced, the appearance of spindle-shaped cells. (B) Light scatter properties of cultured cells and their expression of CD45 and intracellular collagen I compared with an isotype control (rabbit IgG) were analyzed by flow cytometry. (C) CD11b+ monocytes were cultured with or without SN or SN preincubated with neutralizing antibodies against IL-2, IL-4, TNF, INFγ, and IL-21, as indicated. The number of spindle-shaped cells was counted. (D) CD11b+ monocytes were cultured for 14 days with the indicated cytokines. The numbers of spindle-shaped cells were counted, and secretion of collagen I to the culture plate was quantified by ELISA. Results are expressed as the mean ± SEM of the number of fibrocytes from three pictures of three wells/condition. Statistical significance was determined in comparison with medium control.
To analyze the suppressive effects of these cytokines more directly, we quantified the differentiation of monocytes into fibrocytes in the presence of various cytokines. Application of single cytokines showed, that IFN-γ, IL-2, and TNF suppress the generation of spindle-shaped fibrocytes. The combinations of IL-2 + TNF, IL-2 + IL-4, and IL-2 + IFN-γ were most potent and also significantly suppressed the deposition of collagen I (Fig. 3D). All tested combinations of three cytokines almost completely suppressed the appearance of fibrocytes and the deposition of collagen I (Fig. S5).
IL-2 and TNF Reduce the Numbers of Fibrocytes and the Amount of Renal Fibrosis in Vivo.
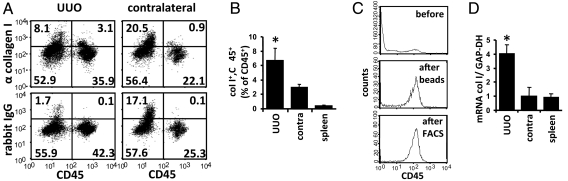
To analyze the generation of fibrocytes in vivo, we used the model of UUO in mice. After UUO, a hydronephrosis with progressive deposition of extracellular matrix in the cortex and accumulation of collagen-producing cells occurs (37–39). On day 7 after UUO CD45+, collagen I+ fibrocytes were identified in the kidneys and spleen by flow cytometry. Staining with an isotype control antibody confirmed the specificity of the intracellular staining for collagen I (Fig. 4A). UUO kidneys contained significantly more CD45+ collagen I+ cells than the contralateral kidneys or the spleens (Fig. 4B). Because of the widespread deposition of collagen I in UUO kidneys, we were unable to clearly identify CD45+ collagen I+ cells by immunohistology with double staining for CD45 and collagen I. To further demonstrate that CD45+ cells express collagen I in the UUO kidney, we performed unilateral ureteral ligation in rats, highly purified CD45+ cells from the kidneys and spleens by FACS sorting (Fig. 4C), and quantified the amount of collagen I mRNA by real-time RT-PCR. CD45+ cells isolated from the obstructed kidneys expressed significantly more collagen I mRNA than CD45+ cells isolated from the contralateral kidneys or the spleens (Fig. 4D). These data indicate that fibrocytes are present in the obstructed kidney and can reliably be identified by flow cytometry.
Fig. 4.
Detection of fibrocytes in the model of UUO. (A and B) On day 7 after induction of UUO in C57BL/6 mice, single-cell suspensions of the obstructed kidneys (UUO), the contralateral kidneys (contralateral), and the spleens were stained for surface expression of CD45 and intracellular collagen I or rabbit IgG as control and analyzed by flow cytometry. (A) Representative light scatter FACS-plots with the percentage of cells in each quadrant. (B) Number of CD45+ collagen I+ fibrocytes as percentage of total CD45+ cells in the obstructed kidneys, the contralateral kidneys, and the spleens (n = 5 per group). (C) Example of purification of CD45+ cells from UUO kidneys of rats at day 10 after UUO. Single-cell suspensions of UUO kidneys were analyzed for expression of CD45 before purification (before), after enrichment of CD45+ cells with beads (beads), and after further purification by FACS-sort (FACS). (D) Expression of collagen I mRNA (col I) relative to GAPDH in purified CD45+ cells from rat UUO kidneys (UUO), contralateral kidneys (contra), and spleens was quantified by real-time RT-PCR (n = 4 per group). Results are expressed as mean ± SEM. Statistical significance was determined compared with contralateral kidneys.
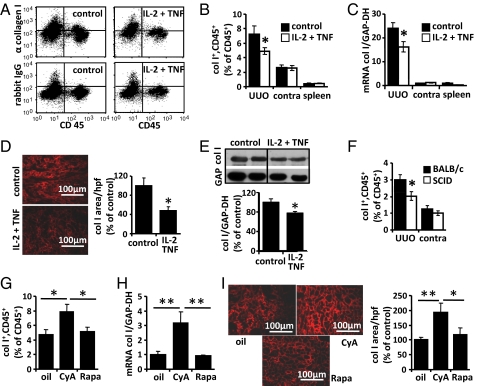
Mice were treated from days 0 to 6 after UUO with IL-2 and TNF or PBS as control. The frequency of fibrocytes was determined by flow cytometry in both kidneys and the spleen (Fig. 5 A and B), and the expression of collagen I mRNA was quantified by RT-PCR (Fig. 5C). In addition, deposition of collagen I in the obstructed kidneys was quantified by immunofluorescence (Fig. 5D) and Western blot analysis (Fig. 5E) using quantitative readouts as described in the Materials and Methods section. Treatment of mice with IL-2 and TNF resulted in a significant reduction of the numbers of fibrocytes, collagen I mRNA expression, and deposition of collagen I in the obstructed kidneys. In the contralateral kidneys or the spleens, the numbers of fibrocytes or mRNA expression of collagen I were not significantly altered by IL-2 and TNF. Treatment with IL-2 and TNF did not change the total number of infiltrating CD45+ cells in the kidneys and did not alter the number of apoptotic cells per high power field (hpf) in UUO kidney sections (Fig. S6).
Fig. 5.
Modulation of fibrocyte differentiation in vivo. (A–E) Mice were treated from day 0 to 6 after induction of UUO with IL-2 and TNF or PBS as control (n = 5 per group). (A) Flow cytometric detection of CD45+ collagen I+ cells in UUO kidneys of mice treated with PBS (control) or IL-2 plus TNF. Staining with rabbit IgG served as control. (B) Number of CD45+ collagen I+ cells as percentage of infiltrating total CD45+ cells and (C) expression of collagen I mRNA (col I) relative to GAPDH in UUO kidneys (UUO), contralateral kidneys (contra), and spleens of mice treated with PBS (black bars) or IL-2 plus TNF (white bars). (D) Immunofluorescence of collagen I (red) and quantification of the collagen I positive area in sections of UUO kidneys of mice treated with IL-2 plus TNF or PBS (control). (E) Quantification of collagen I protein relative to GAPDH protein in UUO kidneys of IL-2 plus TNF or PBS-treated mice by Western blot analysis. (F) Quantification of CD45+ collagen I+ cells as percentage of infiltrating CD45+ cells by flow cytometry in UUO and contralateral kidneys (contra) of T-cell-deficient SCID mice and BALB/c control mice. (G–I) Renal fibrocytes in mice treated from day 0 to 6 after induction of UUO with αCD3 together with cyclosporine A (CyA), rapamycin (Rapa), or olive oil as control (oil) (n = 5 per group). Number of CD45+ collagen I+ cells as percentage of infiltrating CD45+ cells (G), expression of collagen I mRNA (col I) relative to GAPDH (H), and immunofluorescence of collagen I (red) (I) and quantification of the collagen I positive area in sections of UUO kidneys of various treatment groups.
CD4+ T Cells Support Differentiation of Fibrocytes in Vivo.
In vitro, the differentiation of monocytes into fibrocytes was greatly enhanced by the presence of nonactivated CD4+ T cells (Fig. 2A). To investigate the influence of CD4+ T cells in vivo, we induced UUO in T-cell-deficient SCID mice on a BALB/c background and quantified the number of infiltrating CD45+ collagen I+ fibrocytes in both kidneys by flow cytometry (Fig. 5F). We found that the number of fibrocytes in the UUO kidneys of SCID mice was significantly lower than in wild-type BALB/c mice and that deposition of collagen was significantly reduced in SCID mice (Fig. S7A). The number of apoptotic cells per hpf was identical in UUO kidney sections of BALB/c and SCID mice (Fig. S7B).
We also used antibody-mediated depletion of CD4+ T cells as a second approach (Fig. S8). In the absence of CD4+ T cells, the numbers of CD45+ collagen I+ fibrocytes and the deposition of collagen I was significantly reduced in UUO kidneys (Fig. S8 B and E). The total number of infiltrating CD45+ leukocytes in the kidneys and the number of apoptotic cells per hpf in sections of UUO kidneys were not altered (Fig. S8 C and D). These data indicate that CD4+ T cells play an important role for differentiation of fibrocytes in vivo.
Influence of Cyclosporine A and Rapamycin on Fibrocytes and Renal Fibrosis in Vivo.
As described above, T-cell activation in the presence of cyclosporine A markedly enhanced the generation of fibrocytes in vitro. To confirm these results in vivo, UUO-treated mice were injected daily with anti-CD3 antibody for polyclonal activation of T cells and simultaneously treated with cyclosporine A, rapamycin, or olive oil as control. Flow cytometry demonstrated that the number of CD45+ CD11b+ collagen I+ fibrocytes in the UUO kidneys of mice treated with cyclosporine A was significantly increased compared with the vehicle control (Fig. 5G). Treatment with cyclosporine A also significantly increased the level of collagen I mRNA (Fig. 5H) and the severity of fibrosis (Fig. 5I) in the obstructed kidneys. Treatment of mice with rapamycin did not alter the number of fibrocytes or the expression of collagen I. Treatment with cyclosporine A or rapamycin did not significantly change the number of infiltrating CD45+ leukocytes in UUO kidneys and the number of apoptotic cells per hpf in UUO kidney sections (Fig. S9).
Discussion
The cellular origin of fibrocytes and conditions enhancing or decreasing the development of fibrocytes have mostly been analyzed in vitro with human cells. Only few data are available on differentiation of fibrocytes in vivo. We have studied in mice, which cells have the ability to differentiate into fibrocytes, and how this differentiation is regulated. We have used several complementary methods (light microscopy, flow cytometry, RT-PCR, and ELISA) to identify fibrocytes and to confirm expression of collagen I. Flow cytometry with extracellular staining of CD45 and intracellular staining of collagen I proved to be a very reliable method to identify fibrocytes in single-cell suspensions of kidneys. A clear intracellular staining for collagen I was detectable in a distinct subpopulation of CD45+ cells in the UUO kidneys but at a much lower frequency in the contralateral kidneys and the spleens. Highly purified CD45+ cells from fibrotic kidneys expressed significantly higher levels of collagen I mRNA than CD45+ cells from the spleens or contralateral kidneys.
Using cell depletion experiments and purified cells, we show that CD11b+ CD115+ Gr1+ monocytes are the precursor cells of fibrocytes in mice. Gr1+ monocytes did not spontaneously differentiate into fibrocytes in vitro, but required supporting factors (either CD4+ T cells or factors derived from them). We observed some variation in the differentiation of enriched CD11b+ monocytes into fibrocytes, which may result from the use of individual mice or the presence of cells that remained after enrichment of monocytes. A supportive role of CD4+ T cells for differentiation of fibrocytes has been described with human cells (6). Also in the mouse, depletion of CD4+ T cells almost completely prevented the development of fibrocytes from spleen cells, whereas coculture of purified monocytes with nonactivated CD4+ T cells markedly enhanced the outgrowth of fibrocytes. The presence of CD4+ T cells also increased the number of fibrocytes in the UUO model as demonstrated with T-cell-deficient SCID mice and by antibody-mediated depletion of CD4+ T cells.
The supernatant of activated CD4+ T cells had a pronounced influence on fibrocytes. Polyclonal T-cell activation in the absence of calcineurin inhibitors resulted in the release of soluble factors that completely prevented the differentiation of monocytes into fibrocytes whereas T-cell activation in the presence of calcineurin inhibitors had the opposite effect. We have identified the inhibitory factors (IL-2, IL-4, TNF, and IFN-γ) using a large panel of monoclonal antibodies against T-cell-derived cytokines. Similarly, addition of recombinant cytokines, especially the combination of IL-2 and TNF almost completely blocked the outgrowth of fibrocytes. Some differences exist between humans and mice, as IL-4 enhanced fibrocyte differentiation in humans (27), whereas it had an inhibitory effect in mice. IL-13 enhanced collagen I production in the mouse and in humans. In the model of UUO, application of IL-2 and TNF markedly reduced the number of fibrocytes and the overall amount of fibrosis in the obstructed kidneys, indicating that IL-2 and TNF also regulate the development of fibrocytes in vivo. The correlation between the number of infiltrating fibrocytes and the degree of fibrosis suggests that fibrocytes contribute to renal fibrosis in this model, although we cannot exclude that IL-2 and TNF also affect other collagen-producing cells. Release of proinflammatory cytokines during an acute immune response, may contribute to restitution without appearance of fibrosis.
Prolonged subacute activation of T cells or T-cell activation in the presence of calcineurin inhibitors is associated with the development of fibrosis. Long-term use of calcineurin inhibitors and episodes of transplant rejection enhance the risk of chronic allograft dysfunction and fibrosis in patients after kidney transplantation (31, 40, 41). In contrast, rapamycin seems to induce less or no renal fibrosis (42). Cyclosporine A, but not rapamycin, increased the development of fibrocytes and the severity of fibrosis in UUO model. We assume that T-cell activation in the presence of cyclosporine A increases the release of profibrocytic factors (e.g., TGF-β) and decreases the release of antifibrocytic cytokines.
Taken together, we have found that differentiation of fibrocytes is critically dependent on CD4+ T cells and that commonly applied immunosuppressive drugs markedly alter the ability of CD4+ T cells to provide “help” for development of fibrocytes. Our data may have implications for prevention of organ fibrosis in autoimmune diseases and transplantation.
Materials and Methods
Animals.
Female C57BL/6 mice at least 8–10 weeks of age were obtained from Elevage Janviere. Female BALB/c and CB17 SCID mice were obtained from Charles River Laboratories and only used for the experiment shown in Fig. 5F. Female Lewis rats (200 g) were obtained from Charles River Laboratories and only used for the experiment described in Fig. 4 C and D. Governmental approval was given for all animal experiments.
Antibodies and Cytokines.
A list of antibodies and cytokines is provided in the SI Text.
Cell Preparation and Cell Culture.
Isolation of leukocyte subsets from spleen and kidneys and activation of CD4+ T cells is described in the SI Text. For differentiation of fibrocytes, 5 × 105 total splenocytes, splenocytes depleted of specific leukocyte subsets, or enriched monocytes were incubated in flat-bottom 96-well plates in 250 μL RPMI medium 1640, 10% (vol/vol) heat-inactivated FCS, and 1% penicillin/streptomycin in a humidified incubator containing 5% CO2 and 37 °C for 14 days. Purified CD4+ T cells (5 × 105 per well) were added as indicated. The supernatant of activated T cells was preincubated for 1 h with blocking antibodies (20 μg/mL) against various cytokines as indicated and added to the culture at a concentration of 80% (vol/vol). Cyclosporine A, tacrolimus, or rapamycin were added at the concentrations given above. Recombinant cytokines were used at a concentration of 10 ng/mL. On day 14 of cell culture, spindle-shaped cells were counted. For that purpose, pictures of three randomly selected hpf (200× magnification) per well from three wells per condition were taken with a digital camera on a Axiovert25 microscope (Zeiss). The number of spindle-shaped cells per well is provided in relation to 5 × 105 input cells.
Flow Cytometry.
Cultured fibrocytes were removed from the culture dish on day 14 with PBS/1.5 mM EDTA for 15 min at 37 °C. Cells were stained with antibodies against CD45 and other surface molecules, permeabilized with cytofix/cytoperm (BD Biosciences), and stained with biotinylated anti-collagen I antibody or biotinylated isotype control rabbit IgG, followed by streptavidin-APC. For quantification of CD45+ cells in UUO and nonligated kidneys, counting beads were included. Cells were analyzed on a FACSCalibur with CellQuest Software (BD Biosciences).
Unilateral Ureteral Obstruction.
The left ureter was ligated at two points under general anesthesia through a low midline abdominal incision on day 0. Unobstructed contralateral kidneys served as controls. Mice were killed on day 7 after UUO, rats on day 10.
C57BL/6 mice were treated twice daily s.c. from days 0–6 with recombinant IL-2 and TNF (500 ng each, n = 5) or PBS as control (n = 5). Alternatively, C57BL76 mice were treated daily i.p. from days 0–6 with cyclosporine A (10 mg/kg in olive oil), rapamycin (1.5 mg/kg in olive oil), or olive oil as control (n = 5 per group) and 3 h later with 10-μg anti-CD3 antibody (BD Bioscience). Alternatively, C57BL/6 mice were injected i.p. on day −3, −2, and −1 with 300-μg GK1.5 or the rat IgG2b isotype control antibody (n = 5 per group). On day 7, both kidneys and the spleen were harvested, and half of each kidney and spleen was used to obtain single-cell suspensions for flow cytometry. The other halves were immediately snap-frozen in liquid nitrogen for isolation of mRNA and protein and for immunohistochemistry.
Quantification of Collagen I RNA and Protein.
Quantification of collagen I by real-time PCR, ELISA, Western blot analysis, and immunofluorescence in kidney sections is described in SI Text.
Statistical Analysis.
Error bars indicate the SEM in all figures. Cell culture experiments were performed in triplicates. If P values for significance are provided, they were calculated with a one-sided Student's t test and indicated with one asterisk (P < 0.05) or two asterisks (P < 0.01).
Supplementary Material
Acknowledgments.
We thank J. Stahl for FACS-sorting and R. Warth for helpful discussions. This work was supported by Deutsche Forschungsgemeinschaft Grant SFB699.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906070106/DCSupplemental.
References
- 1.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strutz F, Muller GA. Transdifferentiation comes of age. Nephrol Dial Transplant. 2000;15:1729–1731. doi: 10.1093/ndt/15.11.1729. [DOI] [PubMed] [Google Scholar]
- 3.Zeisberg EM, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 4.Zeisberg M, et al. Renal fibrosis: Collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. Am J Pathol. 2001;159:1313–1321. doi: 10.1016/S0002-9440(10)62518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 6.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: Differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 7.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 8.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160:419–425. [PubMed] [Google Scholar]
- 9.Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: Collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, et al. Peripheral blood fibrocytes from burn patients: Identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab Invest. 2002;82:1183–1192. doi: 10.1097/01.lab.0000027841.50269.61. [DOI] [PubMed] [Google Scholar]
- 11.Moore BB, et al. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips RJ, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakai N, et al. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci USA. 2006;103:14098–14103. doi: 10.1073/pnas.0511200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 16.Mehrad B, et al. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353:104–108. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 17.Ortonne N, et al. Presence of CD45RO+ CD34+ cells with collagen synthesis activity in nephrogenic fibrosing dermopathy: A new pathogenic hypothesis. Br J Dermatol. 2004;150:1050–1052. doi: 10.1111/j.1365-2133.2004.05900.x. [DOI] [PubMed] [Google Scholar]
- 18.Schroeder JA, et al. Ultrastructural evidence of dermal gadolinium deposits in a patient with nephrogenic systemic fibrosis and end-stage renal disease. Clin J Am Soc Nephrol. 2008;3:968–975. doi: 10.2215/CJN.00100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roufosse C, et al. Bone marrow-derived cells do not contribute significantly to collagen I synthesis in a murine model of renal fibrosis. J Am Soc Nephrol. 2006;17:775–782. doi: 10.1681/ASN.2005080795. [DOI] [PubMed] [Google Scholar]
- 20.Lin SL, Kisseleva T, Brenner DA, Duffield JS. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheever AW, et al. Anti-IL-4 treatment of Schistosoma mansoni-infected mice inhibits development of T cells and non-B, non-T cells expressing Th2 cytokines while decreasing egg-induced hepatic fibrosis. J Immunol. 1994;153:753–759. [PubMed] [Google Scholar]
- 22.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pesce J, et al. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116:2044–2055. doi: 10.1172/JCI27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reiman RM, et al. Interleukin-5 (IL-5) augments the progression of liver fibrosis by regulating IL-13 activity. Infect Immun. 2006;74:1471–1479. doi: 10.1128/IAI.74.3.1471-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wynn TA, et al. An IL-12-based vaccination method for preventing fibrosis induced by schistosome infection. Nature. 1995;376:594–596. doi: 10.1038/376594a0. [DOI] [PubMed] [Google Scholar]
- 27.Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83:1323–1333. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;171:5537–5546. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilling D, Tucker NM, Gomer RH. Aggregated IgG inhibits the differentiation of human fibrocytes. J Leukoc Biol. 2006;79:1242–1251. doi: 10.1189/jlb.0805456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Distler JH, Schett G, Gay S, Distler O. The controversial role of tumor necrosis factor alpha in fibrotic diseases. Arthritis Rheum. 2008;58:2228–2235. doi: 10.1002/art.23645. [DOI] [PubMed] [Google Scholar]
- 31.Andoh TF, Bennett WM. Chronic cyclosporine nephrotoxicity. Curr Opin Nephrol Hypertens. 1998;7:265–270. doi: 10.1097/00041552-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Olyaei AJ, de Mattos AM, Bennett WM. Nephrotoxicity of immunosuppressive drugs: New insight and preventive strategies. Curr Opin Crit Care. 2001;7:384–389. doi: 10.1097/00075198-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Opelz G, Dohler B. Cyclosporine and long-term kidney graft survival. Transplantation. 2001;72:1267–1273. doi: 10.1097/00007890-200110150-00015. [DOI] [PubMed] [Google Scholar]
- 34.Shihab FS. Cyclosporine nephropathy: Pathophysiology and clinical impact. Semin Nephrol. 1996;16:536–547. [PubMed] [Google Scholar]
- 35.Li B, et al. Differential regulation of transforming growth factor beta and interleukin 2 genes in human T cells: Demonstration by usage of novel competitor DNA constructs in the quantitative polymerase chain reaction. J Exp Med. 1991;174:1259–1262. doi: 10.1084/jem.174.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prashar Y, Khanna A, Sehajpal P, Sharma VK, Suthanthiran M. Stimulation of transforming growth factor-beta 1 transcription by cyclosporine. FEBS Lett. 1995;358:109–112. doi: 10.1016/0014-5793(94)01382-b. [DOI] [PubMed] [Google Scholar]
- 37.Bascands JL, Schanstra JP. Obstructive nephropathy: Insights from genetically engineered animals. Kidney Int. 2005;68:925–937. doi: 10.1111/j.1523-1755.2005.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vielhauer V, et al. Obstructive nephropathy in the mouse: Progressive fibrosis correlates with tubulointerstitial chemokine expression and accumulation of CC chemokine receptor 2- and 5-positive leukocytes. J Am Soc Nephrol. 2001;12:1173–1187. doi: 10.1681/ASN.V1261173. [DOI] [PubMed] [Google Scholar]
- 39.Wada T, Sakai N, Matsushima K, Kaneko S. Fibrocytes: A new insight into kidney fibrosis. Kidney Int. 2007;72:269–273. doi: 10.1038/sj.ki.5002325. [DOI] [PubMed] [Google Scholar]
- 40.Najafian B, Kasiske BL. Chronic allograft nephropathy. Curr Opin Nephrol Hypertens. 2008;17:149–155. doi: 10.1097/MNH.0b013e3282f4e514. [DOI] [PubMed] [Google Scholar]
- 41.Nankivell BJ, et al. Calcineurin inhibitor nephrotoxicity: Longitudinal assessment by protocol histology. Transplantation. 2004;78:557–565. doi: 10.1097/01.tp.0000128636.70499.6e. [DOI] [PubMed] [Google Scholar]
- 42.Morcos SK, Thomsen HS. Nephrogenic systemic fibrosis: More questions and some answers. Nephron Clin Pract. 2008;110:c24–c31. doi: 10.1159/000151228. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.