Abstract
Although Epstein-Barr virus (EBV) is linked to Burkitt's lymphoma (BL), the role of the virus in lymphomagenesis is unclear. LMP2A, encoded by EBV, can be detected in BL biopsies and has prosurvival functions. We generated mice expressing MYC and LMP2A in B cells. LMP2A/λ-MYC mice show greatly accelerated tumor onset. Similar to previous work, we found p53 mutations in λ-MYC tumors; however, we detected no mutations in the rapidly arising LMP2A/λ-MYC tumors. We further demonstrate that the p53 pathway is functionally intact in LMP2A/λ-MYC tumors, which have increased levels of PUMA and sensitivity to p53 activation by Nutlin. This work shows that LMP2A can permit tumorigenesis in the presence of an intact p53 pathway, identifying an important contribution of EBV to BL.
Keywords: viral oncogenesis, Burkitt's lymphoma, apoptosis
Since Epstein-Barr virus (EBV) was first identified, it has been understood to play an important role in Burkitt's lymphoma (BL) (1). However, a clear picture of the role of the virus in disease causation has not yet emerged. The first described cases of BL were the endemic form, found in equatorial Africa and Papua New Guinea where malaria is holoendemic. Endemic BL cases are nearly 100% associated with EBV. Sporadic BL is found globally with low incidence and has a 15–20% association with EBV. BL is also commonly seen in AIDS patients, with a 30–40% EBV association (2). The increased EBV association seen in patients with HIV or malaria suggests that alterations in immune function may contribute to the link between EBV and BL.
All forms of BL are characterized by a translocation between the c-MYC proto-oncogene and an immunoglobulin (Ig) locus, either IgH genes or IgL genes (3, 4). Deregulated expression of MYC results in the dual effects of induction of cell proliferation and apoptosis (5). Tumor development occurs upon disruption of one of the tumor suppressor pathways that normally induce apoptosis in response to oncogenic stimuli. Indeed, mutation of the master tumor suppressor, p53, is frequently found in BL biopsies and cell lines (6–8). One study found that 41% of BL biopsies contained a p53 mutation (9).
Mouse models of BL also demonstrate this phenomenon. The Eμ-MYC model is a transgenic mouse that expresses c-MYC under the control of the Ig heavy chain promoter and enhancer. These mice develop spontaneous pre-B and B cell lymphomas. Examination of the tumor cells revealed inactivation of the ARF-MDM2-p53 tumor suppressor pathway in ≈80% of the spontaneous tumors (10). Inactivation of any of these proteins disrupts the entire pathway, since they form a tightly regulated circuit that is responsive to oncogenic stimuli. ARF is induced by hyperproliferative signals that occur in cases such as MYC deregulation (11), and neutralizes MDM2, a negative regulator of p53 (12).
Crossing the Eμ-MYC mice with mice deficient in proapoptotic proteins such as BIM, BAX, or PUMA results in accelerated spontaneous tumor onset and a reduction in the frequency of p53 pathway mutations (13–15). Eμ-MYC tumors in BIM−/− mice show no alteration in p19ARF, in contrast to BIM +/+ Eμ-MYC tumors, where 50% demonstrated perturbation in ARF levels (13). Similarly, Eμ-MYC tumors in BAX−/− mice did not contain p53 mutations or deletion, unlike Eμ-MYC BAX+/+ tumors (14). These data demonstrate that inactivation of downstream targets of p53 relieves the strong selection pressure for functional inactivation of the p53 pathway during lymphomagenesis.
The detection of clonal EBV genomes in BL suggests that EBV may have a mechanistic role in BL pathogenesis, but viral gene expression is limited in these tumors. Therefore, immune selection may play a part in BL development. Transcripts of low levels of the EBV latency protein latent membrane protein 2A (LMP2A) have recently been detected in fresh BL biopsies, whereas transcripts of the EBV oncoprotein, LMP1, are not detected (16). These data suggest a putative role for LMP2A in BL. Like LMP1, LMP2A has been shown to have a prosurvival function in B cells (17–20). LMP2A can activate the Ras/PI3K/Akt pathway (17, 18, 21), the MAP kinase pathway (22), NF-κB (23), and Bcl-2 and Bcl-xL (18). We investigated whether LMP2A can protect B cells from apoptosis induced by MYC deregulation.
Evidence to support this hypothesis has been generated by crossing LMP2A transgenic mice (24) to λ-MYC transgenic mice (25). Accelerated tumor onset was observed in LMP2A/λ-MYC double transgenics, as well as significantly enlarged spleen size in pretumor mice (26). Here, we report similar observations using a second, phenotypically different LMP2A transgenic line (27). In addition, we demonstrate that the p53 pathway is intact in tumors arising in LMP2A/λ-MYC double transgenic mice. The ability of LMP2A to bypass p53 pathway inactivation in the presence of deregulated MYC is likely an important mechanism by which EBV contributes to BL development in humans.
Results
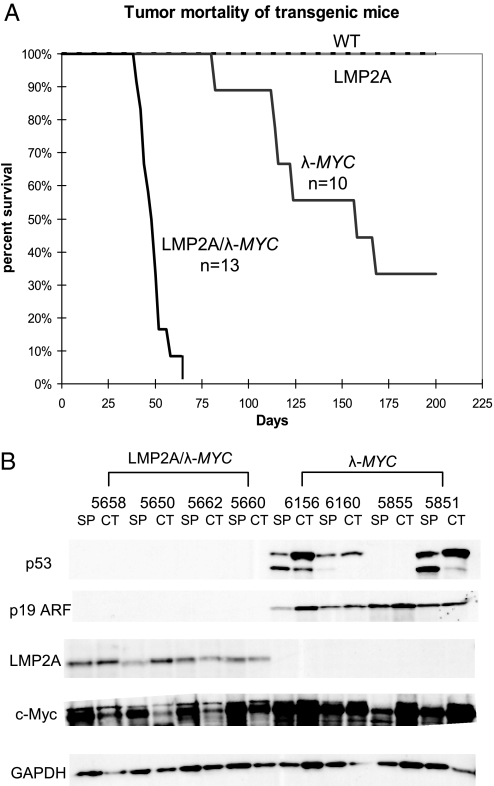
To examine the contribution of LMP2A to tumorigenesis induced by MYC deregulation and understand the involvement of this viral protein in human BL, we used the Tg6 LMP2A transgenic mouse line in which LMP2A is expressed under the control of the Ig heavy chain promoter and enhancer (27). The LMP2A transgenic mice were crossed with a MYC transgenic line in which the human MYC gene is expressed from the Ig λ locus (25). Consistent with published results (25, 26), wild-type, Tg6 LMP2A, and λ-MYC spleens were similar in size and weight. In contrast, LMP2A/λ-MYC mice had greatly enlarged spleens, 0.55 ± 0.17 g for 6-week-old LMP2A/λ-MYC compared with 0.17 ± 0.07 g for 6-week-old λ-MYC. In addition, LMP2A/λ-MYC mice developed tumors much more rapidly (40–60 days) than λ-MYC mice in which tumors arose in 100–200 days (Fig. 1A). Demonstrating that all tumors were of B cell origin, cervical lymph node tumor cells and splenocytes from tumor-bearing λ-MYC and LMP2A/λ-MYC mice were nearly 100% positive for the pan B cell marker B220 by flow cytometric analysis.
Fig. 1.
LMP2A/λ-MYC transgenic mice show accelerated tumor onset, and tumors lack p53 and p19 ARF stabilization. (A) Kaplan–Meier curve shows percentage survival of each transgenic line. Mice were killed when cervical lymph node tumors were observed, at which point the animals could generally survive for a maximum of 7 days. (B) p53 and p19 ARF are stabilized in tumors from λ-MYC mice, but not in LMP2A/λ-MYC tumors. Lysates were made from cervical lymph node tumor cells (CT) and splenocytes (SP) from tumor-bearing λ-MYC and LMP2A/λ-MYC mice. Lysates were probed for p53 and p19 ARF in addition to c-Myc and LMP2A to confirm transgene expression.
Next we examined the p53 pathway in tumors arising in our LMP2A/λ-MYC transgenic model. Analysis of MYC tumors in the Eμ-MYC model has demonstrated that the p53 pathway is frequently inactivated in these lymphomas through disruption of either p53, p19ARF, or MDM2 (10). Levels of p53 pathway proteins are tightly regulated in the cell, since the outcome of pathway activation is cell cycle arrest or apoptosis. Thus, mutations in genes such as p53 and p19ARF frequently result in stabilization of the protein due to lack of negative regulation. Such stabilization has been used as read-out for disruption of the pathway (10, 13–15). Similar to previous reports, we observed stabilization of both p53 and p19ARF in both cervical tumor and tumor-bearing spleens isolated from λ-MYC mice (Fig. 1B). We analyzed samples from 10 tumor-bearing λ-MYC mice and observed p53 stabilization in six. In a few animals, stabilization was observed only in either cervical tumor cells or splenocytes, suggesting that separate events may have given rise to each tumor (Table S1).
To confirm that stabilization observed by immunoblot correlated with p53 mutation, we also isolated DNA from cervical tumor cells and splenocytes. Exons within the DNA-binding domain of the p53 gene known to be mutational “hot spots” were amplified and sequenced (6–8). The results of this analysis are summarized in Table S1. Mutations in p53 exons 5, 6, 7, or 8 were generally detected in those tumors in which stabilization was observed by immunoblot.
Interestingly, overexpression of p53 or p19ARF was never observed in tumors from LMP2A/λ-MYC transgenic mice (Fig. 1B). We analyzed samples from 16 different LMP2A/λ-MYC tumor-bearing mice, but found no evidence of p53 stabilization. Accordingly, DNA sequencing revealed wild-type p53 in each LMP2A/λ-MYC spleen and cervical tumor sample analyzed (Table S1). This result suggests that expression of LMP2A bypasses the requirement for inactivation of the p53 pathway during lymphomagenesis induced by MYC deregulation, explaining why tumor onset is accelerated in LMP2A/λ-MYC double transgenic mice (Fig. 1A).
As we were unable to identify p53 mutations in tumors from our LMP2A/λ-MYC mice, we hypothesized that the p53 pathway is functionally intact in tumor cells from these mice. In contrast to their enhanced in vivo growth, attempts to culture continuously proliferating cell lines in vitro were unsuccessful using tumor cells isolated from LMP2A/λ-MYC mice. Tumor cells from 16 LMP2A/λ-MYC mice were unable to generate cell lines in culture whereas tumor cells from 7 of 8 λ-MYC mice readily established cell lines in culture.
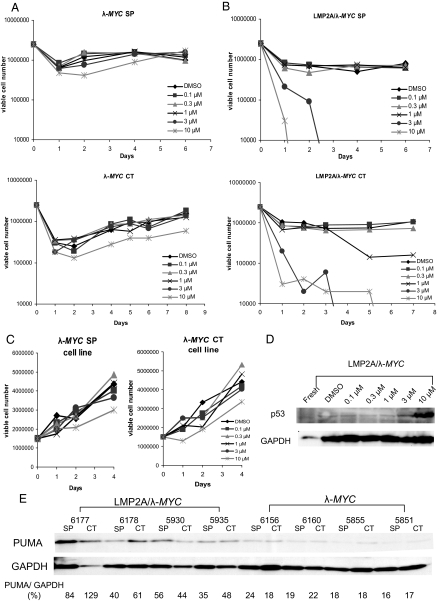
To test the functionality of the p53 pathway in tumor cells from LMP2A/λ-MYC mice, we treated the tumor cells with Nutlin 3. Nutlin 3 binds to MDM2, a negative regulator of p53, preventing MDM2-mediated degradation of the p53 protein. Nutlin 3 has been shown to stabilize p53 and lead to activation of downstream p53 targets and apoptosis (28). We treated freshly isolated tumor cells from cervical lymph nodes and spleens with increasing concentrations of Nutlin 3, and assessed cell growth and survival over time using trypan blue exclusion. The process of isolating the cells from the mice and placing them in tissue culture caused an initial rapid reduction in viable cell number in these cultures (Fig. 2 A and B). However, we found that cervical tumor or spleen cells isolated from tumor-bearing λ-MYC mice in which a p53 mutation was detected were able to grow even in the highest doses of 3 μM and 10 μM Nutlin 3 (Fig. 2A). Cells from tumor-bearing LMP2A/λ-MYC mice, however, were uniformly sensitive to both 3 μM and 10 μM Nutlin 3 treatments (Fig. 2B). This result was reproducible as the same pattern occurred in both spleen and cervical tumor cells from seven of seven different double transgenic mice. Not surprisingly, cell lines generated from λ-MYC tumors lacked the initial reduction in viability seen with freshly isolated tumor cells, and were completely insensitive to all concentrations of Nutlin 3 (Fig. 2C). To confirm that the reduction in viable cell number in the Nutlin-treated LMP2A/λ-MYC tumor cells was due to induction of p53 by the inhibitor, we harvested lysates from cells treated for 8 h with Nutlin 3 and probed for p53 by immunoblot. The drastic reduction in viability observed in the highest Nutlin 3 concentrations made time points later than 8 h impossible. We observed induction of p53 expression in LMP2A/λ-MYC cells treated with Nutlin 3 (Fig. 2D). Tumor cells from λ-MYC mice containing a p53 mutation already show p53 stabilization (Fig. 1B). Treatment with Nutlin 3 did not affect p53 levels in these cells.
Fig. 2.
The p53 pathway is intact in LMP2A/λ-MYC tumor cells. (A and B) Freshly isolated cervical lymph node (CT) or spleen (SP) tumor cells from λ-MYC and LMP2A/λ-MYC mice were plated in increasing concentrations of Nutlin 3 at 2.5 million cells per mL. Viable cell number was determined using trypan blue exclusion. (C) Tumor cells from the cervical lymph node (CT) and spleen (SP) of λ-MYC mice were grown in culture for several weeks, and then plated in increasing concentrations of Nutlin 3 at 1.5 million cells per mL. Viable cell number was determined using trypan blue exclusion. (D) Nutlin 3 treatment induces p53 in LMP2A/λ-MYC tumor cells. Splenocytes from LMP2A/λ-MYC tumor-bearing mice were isolated and immediately plated in increasing concentrations of Nutlin 3. Cells were harvested 8 h after treatment, lysed, and probed for p53. (E) PUMA levels are increased in LMP2A/λ-MYC tumor cells. Freshly isolated λ-MYC and LMP2A/λ-MYC cervical tumor cells (CT) and splenic tumor cells (SP) were lysed and probed for PUMA.
As further confirmation that the p53 apoptotic pathway is intact in tumors arising in LMP2A/λ-MYC mice, we observed increased levels of the p53 target, PUMA, in freshly isolated cervical tumor and spleen cells (Fig. 2E). Densitometry allowed for normalization of levels of PUMA to the housekeeping protein, GAPDH. The average percentage of PUMA/GAPDH in LMP2A/λ-MYC spleen samples is 53.7 ± 22.1% and in cervical tumor samples 70.5 ± 39.8%. PUMA expression was reduced in samples from λ-MYC mice, at an average of 19.2 ± 3.6% PUMA/GAPDH in spleen samples, and 18.6 ± 2.2% in cervical tumor samples.
Discussion
MYC deregulation is a common feature of many human cancers, particularly via a translocation event in BL (3). Acute MYC expression triggers the p53 response, resulting in induction of apoptosis. Specific mechanisms for bypassing this response have been documented in human and mouse models of BL. Spontaneous mutations in proteins important for triggering the p53 response, including p53 and ARF, are observed in tumors from MYC transgenic mice, and allow for accelerated development of lymphoma (10, 29, 30). Alternatively, mutations in MYC itself have also been shown to bypass p53-induced apoptosis and accelerate tumorigenesis. Two mutant MYC alleles, P57S and T58A have been observed in human tumors (31). Expression of these mutants accelerates tumor onset compared with wild-type MYC in an adoptive transfer mouse model (32). In this model, both p53 and p19ARF were wild-type in the mutant MYC tumors, but the MYC mutants were unable to activate the proapoptotic BH3-only protein, BIM (32). In addition, loss of BIM accelerates lymphomagenesis in the EμMYC mouse model in the absence of p53 pathway inactivation (13). Thus, failures to induce either p53 or BIM are two separate mechanisms to prevent apoptosis and allow lymphomagenesis in the context of de-regulated MYC. In our LMP2A/λ-MYC model, the p53 pathway is intact (Fig. 2 B and E), and we do not observe alterations in BIM protein induction in LMP2A/λ-MYC mice when compared with λ-MYC mice (Fig. S1A), and measured a slight increase in BIM induction in LMP2A/λ-MYC tumors at the mRNA level (Fig. S1B).
Here, we identify a previously undescribed mechanism by which cells containing translocated MYC can be protected from apoptosis, permitting tumor development. In the λ-MYC mouse model, MYC is expressed throughout B cell development, but tumors are only observed after a long time interval during which an inactivating mutation occurs in some step of the p53 pathway (Fig. 3A). We did not detect p53 mutations in all λ-MYC tumors, but we did not test for all mechanisms of pathway inactivation. ARF deletion, for example, has been shown in EμMYC tumors (10), but would not have been detected by our methods. In the LMP2A/λ-MYC transgenic mice, both LMP2A and MYC are expressed during B cell development. Although the MYC transgene triggers a p53 response in the B cells, LMP2A protects the cells from apoptosis, allowing early expansion of cells, observed as splenomegaly in pretumor mice, and accelerated onset of tumors in the absence of p53 pathway inactivation (Fig. 3B).
Fig. 3.
Model for the role of LMP2A in development of Burkitt's lymphoma in mice and humans. (A) In the λ-MYC mouse model, MYC is expressed in B cells throughout B cell development. Deregulated MYC expression results in induction of apoptosis through p53 pathway activation. Burkitt's lymphoma-like tumors are observed in these mice upon mutation of one or more components of the p53 pathway. (B) Both LMP2A and MYC are expressed throughout B cell development in the LMP2A/λ-MYC transgenic mouse. The MYC transgene activates the p53 pathway, but LMP2A is able to counteract this activation, likely at some step that is downstream of PUMA, perhaps through up-regulation of Bcl-XL. LMP2A causes an initial expansion of cells, observed as splenomegaly in pretumor animals (spleen masses of 0.55 ± 0.17 g for LMP2A/λ-MYC and 0.17 ± 0.07 g for λ-MYC). In the absence of immune selection, LMP2A accelerates tumor onset in this model, allowing tumorigenesis in the presence of an intact p53 pathway. (C) In human BL, translocation of MYC to an Ig locus occurs in a germinal center reaction. MYC induces p53 pathway activation, resulting in apoptosis. Cells that have inactivated the p53 pathway grow into lymphoma. (D) In EBV-positive human BL, LMP2A is present during the germinal center reaction and MYC translocation. LMP2A likely enhances survival of cells with deregulated MYC early in lymphomagenesis, causing expansion of cells and increasing the probability of secondary mutation, which leads to tumor progression. After tumor progression immune regulation selects against high levels of LMP2A in tumor cells. LMP2A is indicated by the multi spanning transmembrane protein. Constitutive MYC expression either by translocation or transgene construct is indicated by black/gray line in nucleus.
The prosurvival function of LMP2A has been well-documented. The enhancement of survival seen in LMP2A-expressing cells results in altered B cell development phenotypes in some transgenic mouse models (24, 27, 33, 34). We chose to use the Tg6 EμLMP2A transgenic line used for the experiments in this article because it has normal B cell development that is indistinguishable from wild-type mice (27, 33). Nonetheless, in the experiments described here we still observe a strong prosurvival signal from LMP2A in the Tg6 line.
Due to its apparent importance in many human malignancies, the mechanism that underlies the LMP2A survival signal has been under continual investigation. LMP2A has been shown to activate the Ras/PI3K/Akt pathway (17, 18, 21), the MAP kinase pathway (22), NF-κB (23), and Bcl-2 and Bcl-XL (18), each of which could contribute to the enhancement of cell survival observed in the LMP2A/λ-MYC model. Bcl-2 and Bcl-XL induction are likely important mechanisms as they are anti-apoptotic proteins found further downstream in the apoptotic cascade induced by p53. Induction of Bcl-2 and Bcl-XL may counteract p53 induction of proapoptotic proteins like PUMA (Fig. 2E) generating the increase in spleen size seen in the LMP2A/λ-MYC mice compared with λ-MYC mice. Increased expression of Bcl-XL has been observed in LMP2A/λ-MYC tumors (26), and Bcl-XL transgenic mice also demonstrate accelerated tumor onset in an Eμ-MYC model (35). However, we cannot exclude the possibility that a mechanism completely separate from Bcl-XL induction is responsible for the ability of LMP2A to accelerate tumorigenesis and bypass p53 inactivation in the LMP2A/λ-MYC model.
Our data illuminate an important mechanism by which EBV contributes to human BL. The EBV latency program expressed by BL cell lines was not originally thought to include LMP2A, however, recent work has demonstrated the presence of LMP2A transcripts in fresh BL biopsies (16), although the level of expression is low. Now we show that LMP2A is also functionally important in BL tumors. In EBV-negative BL (Fig. 3C), the MYC translocation occurs during a germinal center reaction. MYC induces BIM and p53, resulting in apoptosis. As such, mutations in the p53 pathway are frequently seen in EBV-negative BL. In EBV-positive BL (Fig. 3D), an LMP2A-expressing cell undergoes a germinal center reaction and acquires a MYC translocation. LMP2A provides survival signals to the B cell, such as increasing Bcl-XL levels that protect cells from apoptosis induced by an activated p53 pathway. Studies have shown no reduction in the frequency of p53 mutations in EBV-positive BL tumors when compared with EBV-negative BL tumors (7, 8). However, p53 mutations have been detected in a higher percentage of BL cell lines than in fresh biopsies, indicating an association with tumor progression (7). The prosurvival function of LMP2A may be particularly important early in lymphoma development, expanding cells to increase the probability of secondary mutation acquisition and tumor progression. Once tumor progression occurs, weak immune surveillance may select against cells expressing high levels of LMP2A (Fig. 3D).
The work presented here identifies a path to tumor development in the presence of deregulated MYC and an intact p53 pathway, illuminating a tactic that viruses have evolved for counteracting the growth suppressing function of p53. In contrast to how LMP2A inactivates the p53 pathway, human papilloma virus (HPV) directly targets p53 for degradation. Finally, our results describe a previously undescribed mechanism of how EBV may contribute to BL, but more importantly provides a model to understand the role of EBV in BL pathogenesis.
Materials and Methods
Mice.
Construction and characterization of the EμLMP2A transgenic mice has been described (24, 27). The EμLMP2A Tg6 line (27) was used in all experiments. Double transgenic mice were generated by crossing the Tg6 line with previously characterized λ-MYC mice (25). Mice were killed when cervical lymph node tumors could be observed externally and the mice were moribund. Animals were maintained at Northwestern University's Center for Comparative Medicine, in accordance with University animal welfare guidelines.
Tumor and Spleen Cell Isolation.
Cervical lymph node tumors and spleens were removed from tumor-bearing mice, and dissociated between frosted glass slides. Red blood cells were lysed using 155 mM ammonium chloride, and filtered through nytex to remove debris. Cells were frozen in dry pellets at −70 °C for later harvest of DNA or protein. For the Nutlin three experiments, cells were immediately resuspended in RPMI media containing 10% FBS, 1% penicillin/streptomycin, and 50 μM β-mercaptoethanol with the indicated concentration of Nutlin 3 or DMSO control.
Immunoblots.
Cells were lysed in Triton X-100 lysis buffer (20 mM Tris·HCl pH 8.0, 137 mM NaCl, 1% Triton X-100, 10% glycerol, 2 mM EDTA, 10 mM NaF, 1 mM Na3VO4, 0.5 mM phenylmethylsulfonyl fluoride, 10 μg/mL pepstatin, and 10 μg/mL leupeptin) at 20 million cells per mL. Lystates were cleared by centrifugation for 10 min at 15,800 × g (LMP2A blot) or sonicated 2 × 5-s pulses (all other blots). Lysates were heated for 5 min at 72 °C (LMP2A blot) or 95 °C (all other blots), then electrophoretically separated by SDS/PAGE. Protein was transferred to Immobilon-P membrane (Millipore), then probed for p53 (FL-393; Santa Cruz Inc.), p19ARF (Ab80–100; AbCam, Inc.), c-Myc (N-262; Santa Cruz Inc), PUMA/bbc3 (P 4743; Sigma–Aldrich), or anti-LMP2A 14B7 rat monoclonal antibody (36). Blots were visualized by ECL (Amersham).
Additional details of immunoblot, real time RT-PCR, and DNA sequencing are given in SI Text.
Nutlin 3 Experiments.
Nutlin 3 (Calbiochem) was suspended in DMSO at 25 mg/mL. Dilutions to 10, 3, 1, 0.3, and 0.1 μM were made in RPMI medium 1640 containing 10% FBS, 1% penicillin/streptomycin, and 50 μM β-mercaptoethanol. Cells were plated in 24-well dishes at 2.5 million (freshly isolated) or 1.5 million (cell lines) cells per well in 1 mL Nutlin 3-containing media. For cell growth experiments, cell number was measured by counting on a hemacytometer, using trypan blue to distinguish between viable cells and dead cells. Fresh media containing the appropriate concentration of Nutlin 3 was added to all wells of each experiment on day 3. For immunoblots, lysates were harvested after 8 h of treatment using methods described above.
Supplementary Material
Acknowledgments.
We thank Nicholas Huffmaster and members of the Longnecker laboratory including Michelle Swanson-Mungerson for help in the completion of these studies. R.L. is John Edward Porter Professor in Biomedical Research and is supported by Public Health Service Grants CA133063 and CA73507 from the National Cancer Institute. K.T.B. is supported by the Carcinogenesis Training Program (T32CA009560).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907994106/DCSupplemental.
References
- 1.Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1:702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 2.Kelly GL, Rickinson AB. Burkitt lymphoma: Revisiting the pathogenesis of a virus-associated malignancy. Hematology Am Soc Hematol Educ Program. 2007;2007:277–284. doi: 10.1182/asheducation-2007.1.277. [DOI] [PubMed] [Google Scholar]
- 3.Dalla-Favera R, et al. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci USA. 1982;79:7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taub R, et al. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci USA. 1982;79:7837–7841. doi: 10.1073/pnas.79.24.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pelengaris S, Khan M, Evan G. c-MYC: More than just a matter of life and death. Nat Rev Cancer. 2002;2:764–776. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]
- 6.Gaidano G, et al. p53 mutations in human lymphoid malignancies: Association with Burkitt lymphoma and chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 1991;88:5413–5417. doi: 10.1073/pnas.88.12.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia KG, Gutierrez MI, Huppi K, Siwarski D, Magrath IT. The pattern of p53 mutations in Burkitt's lymphoma differs from that of solid tumors. Cancer Res. 1992;52:4273–4276. [PubMed] [Google Scholar]
- 8.Farrell PJ, Allan GJ, Shanahan F, Vousden KH, Crook T. p53 is frequently mutated in Burkitt's lymphoma cell lines. EMBO J. 1991;10:2879–2887. doi: 10.1002/j.1460-2075.1991.tb07837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newcomb EW. P53 gene mutations in lymphoid diseases and their possible relevance to drug resistance. Leuk Lymphoma. 1995;17:211–221. doi: 10.3109/10428199509056825. [DOI] [PubMed] [Google Scholar]
- 10.Eischen CM, Weber JD, Roussel MF, Sherr CJ, Cleveland JL. Disruption of the ARF-Mdm2–p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 1999;13:2658–2669. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zindy F, et al. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–2433. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honda R, Yasuda H. Association of p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J. 1999;18:22–27. doi: 10.1093/emboj/18.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci USA. 2004;101:6164–6169. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eischen CM, Roussel MF, Korsmeyer SJ, Cleveland JL. Bax loss impairs Myc-induced apoptosis and circumvents the selection of p53 mutations during Myc-mediated lymphomagenesis. Mol Cell Biol. 2001;21:7653–7662. doi: 10.1128/MCB.21.22.7653-7662.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrison SP, et al. Selection against PUMA gene expression in Myc-driven B-cell lymphomagenesis. Mol Cell Biol. 2008;28:5391–5402. doi: 10.1128/MCB.00907-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell AI, et al. Analysis of Epstein-Barr virus latent gene expression in endemic Burkitt's lymphoma and nasopharyngeal carcinoma tumor cells by using quantitative real-time PCR assays. J Gen Virol. 2006;87:2885–2890. doi: 10.1099/vir.0.81906-0. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda M, Longnecker R. Latent membrane protein 2A inhibits transforming growth factor-beta 1-induced apoptosis through the phosphatidylinositol 3-kinase/Akt pathway. J Virol. 2004;78:1697–1705. doi: 10.1128/JVI.78.4.1697-1705.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Portis T, Longnecker R. Epstein-Barr virus (EBV) LMP2A mediates B-lymphocyte survival through constitutive activation of the Ras/PI3K/Akt pathway. Oncogene. 2004;23:8619–8628. doi: 10.1038/sj.onc.1207905. [DOI] [PubMed] [Google Scholar]
- 19.Mancao C, Altmann M, Jungnickel B, Hammerschmidt W. Rescue of “crippled” germinal center B cells from apoptosis by Epstein-Barr virus. Blood. 2005;106:4339–4344. doi: 10.1182/blood-2005-06-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mancao C, Hammerschmidt W. Epstein-Barr virus latent membrane protein 2A is a B-cell receptor mimic and essential for B-cell survival. Blood. 2007;110:3715–3721. doi: 10.1182/blood-2007-05-090142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukuda M, Longnecker R. Epstein-Barr virus latent membrane protein 2A mediates transformation through constitutive activation of the Ras/PI3-K/Akt Pathway. J Virol. 2007;81:9299–9306. doi: 10.1128/JVI.00537-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson LJ, Longnecker R. EBV LMP2A provides a surrogate pre-B cell receptor signal through constitutive activation of the ERK/MAPK pathway. J Gen Virol. 2008;89:1563–1568. doi: 10.1099/vir.0.2008/001461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swanson-Mungerson MA, Caldwell RG, Bultema R, Longnecker R. Epstein-Barr virus LMP2A alters in vivo and in vitro models of B-cell anergy, but not deletion, in response to autoantigen. J Virol. 2005;79:7355–7362. doi: 10.1128/JVI.79.12.7355-7362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caldwell RG, Wilson JB, Anderson SJ, Longnecker R. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity. 1998;9:405–411. doi: 10.1016/s1074-7613(00)80623-8. [DOI] [PubMed] [Google Scholar]
- 25.Kovalchuk AL, et al. Burkitt lymphoma in the mouse. J Exp Med. 2000;192:1183–1190. doi: 10.1084/jem.192.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bultema R, Longnecker R, Swanson-Mungerson M. Epstein-Barr virus LMP2A accelerates MYC-induced lymphomagenesis. Oncogene. 2009;28:1471–1476. doi: 10.1038/onc.2008.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caldwell RG, Brown RC, Longnecker R. Epstein-Barr virus LMP2A-induced B-cell survival in two unique classes of EmuLMP2A transgenic mice. J Virol. 2000;74:1101–1113. doi: 10.1128/jvi.74.3.1101-1113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vassilev LT, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 29.Schmitt CA, McCurrach ME, de Stanchina E, Wallace-Brodeur RR, Lowe SW. INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 1999;13:2670–2677. doi: 10.1101/gad.13.20.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitt CA, et al. Dissecting p53 tumor suppressor functions in vivo. Cancer Cell. 2002;1:289–298. doi: 10.1016/s1535-6108(02)00047-8. [DOI] [PubMed] [Google Scholar]
- 31.Chang DW, Claassen GF, Hann SR, Cole MD. The c-Myc transactivation domain is a direct modulator of apoptotic versus proliferative signals. Mol Cell Biol. 2000;20:4309–4319. doi: 10.1128/mcb.20.12.4309-4319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hemann MT, et al. Evasion of the p53 tumour surveillance network by tumor-derived MYC mutants. Nature. 2005;436:807–811. doi: 10.1038/nature03845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikeda A, Merchant M, Lev L, Longnecker R, Ikeda M. Latent membrane protein 2A, a viral B cell receptor homologue, induces CD5+ B-1 cell development. J Immunol. 2004;172:5329–5337. doi: 10.4049/jimmunol.172.9.5329. [DOI] [PubMed] [Google Scholar]
- 34.Casola S, et al. B cell receptor signal strength determines B cell fate. Nat Immunol. 2004;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- 35.Swanson PJ, et al. Fatal acute lymphoblastic leukemia in mice transgenic for B cell-restricted bcl-xL and c-myc. J Immunol. 2004;172:6684–6691. doi: 10.4049/jimmunol.172.11.6684. [DOI] [PubMed] [Google Scholar]
- 36.Fruehling S, et al. Identification of latent membrane protein 2A (LMP2A) domains essential for the LMP2A dominant-negative effect on B-lymphocyte surface immunoglobulin signal transduction. J Virol. 1996;70:6216–6226. doi: 10.1128/jvi.70.9.6216-6226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.