Ant fungus farming has become a prominent model for studying the evolution of mutualistic cooperation, with recent advances in reconstructing the evolutionary origin and elaborations of the symbiosis (1, 2), discovering additional partners and clarifying their interactions (3, 4), and analyzing host–symbiont conflict suppression (5). The attine ants are rivaled in their farming sophistication only by the Macrotermitinae, which evolved a similar obligate dependence on fungus gardens (6). The discovery of new antagonistic and mutualistic partners in the attine symbiosis has attracted wide attention, but the prevailing coevolutionary paradigm behind key components of this mutualism is now challenged by a study by Sen et al. in this issue of PNAS (7).
The attine ants originated some 50 million years ago (2). The 12 genera with >200 described species have realized a number of cultivar shifts (2, 8) and transitions in social complexity that culminated in the leafcutter ants with their enormous colonies, long-lived multiply mated queens, and extensive worker caste differentiation (9). From its very origin, the symbiosis has been haunted by a specific fungal disease caused by species of the genus Escovopsis (4, 10). The discovery of this garden pest (4) became a showcase of nature's corruptive tendencies and inventiveness when it was shown that the ants had domesticated actinomycete bacteria to help suppress this bane (3).
Sen et al. (7) present culture-independent 454-sequencing data and rearing experiments to document that the ant-associated actinomycetes are more diverse than previously thought (not only Pseudonocardia but also Amycolatopsis is found) and that they are rarely specific in their inhibition of other microbes. These findings are incompatible with a long history of specific coevolution between these actinomycetes and Escovopsis (3, 4, 8), but they are consistent with the recurrent acquisition of new strains (11, 12). Sen et al. also show that multiple strains can be isolated from the cuticle of single ants and that some of them may not be mutualists. In addition, their in vitro agar plate encounters often end up with the crop symbiont being inhibited or killed by the actinomycete strains, suggesting that the ants need to apply actinomycete-produced antibiotics with surgical precision and in doses that strictly match the infection problem. However, the pairings used were random, so this evidence does not preclude that natural combinations of resident symbionts in field colonies are better matched (13).
These results will change the way we think about the attine ant symbiosis. Extrapolations from rather limited data collected ≈10 years ago now appear to be oversimplifications in need of more explicit testing and at least partial rectification, a process that was already initiated less explicitly a few years ago (13). This changing insight mirrors the way in which we obtained our current view on coevolution between the higher attine ants and their garden symbionts. The latter were initially considered to be strictly vertically transmitted and to have cospeciated with their hosts (14), an inference that was revised into diffuse coevolution recently (15). Science and discovery proceed by hallmarks like this: We also no longer believe that phytophagous insects in general had tight coevolution with their host plants (16). There may be clades that have such reciprocity, but the burden of proof has become reversed.
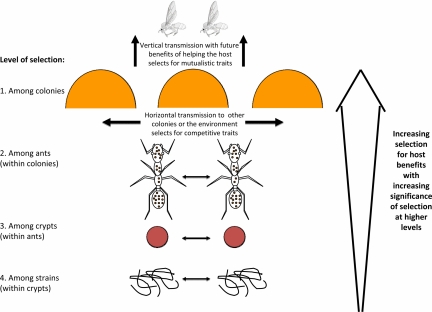
It now appears to be more straightforward (7) to interpret the actinomycete–ant association as having evolved for mutual benefits (17) than to maintain a strict antagonistic coevolutionary scenario for the actinomycete–Escovopsis interaction (3, 4, 8). Actinomycetes are slow-growing microbes that produce antibiotics for their own benefits in competition with other microorganisms, so it seems reasonable to assume that ant glandular secretions (17) produce substrates that particularly benefit slow-growing strains as they are most likely to be mutualistic. When competition among symbiont lineages harms the host, theory predicts that hosts should pursue maintaining only a single lineage (18) and it is therefore revealing that multiple strains have been isolated when richer culture media were used and that culture-independent sequencing techniques yielded even more diversity (11, 12). However, this prediction may not apply when multiple strains actually increase host fitness because they ooze out a higher collective dose of antibiotics. So, when the first attine ants started to associate with actinomycetes they would not necessarily have minded harboring more than one strain (Fig. 1). Once the ant–actinomycete relationship had become obligatory, small-scale coexistence of multiple strains would have become less likely because the cuticular crypts and tubercles that house the cultures are highly compartmentalized (i.e., easy to monopolize) unless strains were complementary in using different fractions of the ant's glandular resources. Multiple strains on the same ant are often very different (7), so it would be premature to conclude that such diversity would necessarily destabilize the mutualistic interaction until we know more about the resource use of these strains and the colonization of cuticular crypts and tubercles under field conditions. The only explicit study done so far showed that ≈40% of the actinomycete combinations derived from attine cuticles showed no mutual inhibition (19).
Fig. 1.
The relevant levels of selection and competition in interactions between attine ants and cuticular actinomycetes. The study by Sen et al. (7) indicates that we need: (i) data on how competition affects the extent of useful antibiotic production, both for the bacteria and their hosts, at each of these levels and (ii) a better understanding of the proportion of total actinomycete fitness that is obtained directly, via horizontal transmission to other workers, colonies, or the environment, and indirectly, via vertical transmission to daughter colonies (top arrows). All effects marked with horizontal arrows could harm the host ants, but do not necessarily do so, except at the lowest level where competition among actinomycete strains could select for increased antibiotics production to the benefit of the host. The general prediction is that selection for host-beneficial traits increases with increasing selection at higher levels.
Attine ants rear actinomycetes also on the cuticles of gynes (virgin prospective queens), which ensures vertical transmission when they disperse to mate and when they establish new colonies (Fig. 1). This pathway would not eliminate occasional horizontal transmission or de novo acquisition, but shapes the interaction even when domestication would not last for hundreds of generations, because founding queens with underperforming actinomycetes would be unlikely to survive the colony founding stage. Selection on gynes to pick up the most mutualistic strains possible from their worker sisters would therefore be strong, just as they are apparently able to pick up an Escovopsis-free fragment of their fungus garden symbiont before leaving for their mating flight (4).
The hypothetical scenario outlined above and in Fig. 1 is consistent with workers carrying several actinomycete strains and generates the testable prediction that gynes carry a more beneficial subset of strains than workers. It also leads to the expectation that, across colonies, the common strains would be the mutualists whereas the parasitic strains would tend to be rare even though some colonies may have lots of them. The main take-home message of the new study (7) is that Escovopsis has not been a direct and general driver of actinomycete coevolution on ant cuticles and that the ants maintain actinomycetes for less specific benefits, one of them being the control of Escovopsis. Absent specific inhibition of Escovopsis, the function of ant-reared actinomycetes becomes more comparable to general ant defenses such as metapleural gland secretions (20), making it easier to understand why resistance problems have not been rampant (11, 12). We know that some higher attine genera have almost (Sericomyrmex) or completely (Atta) lost the cuticular structures to rear actinomycetes (12, 20). That such mutualism breakdowns have taken place indicates that actinomycete parasitism may be real, but these losses could also be caused by other circumstances that render chemical defenses against Escovopsis and other diseases more profitable than maintaining ancestral cuticular actinomycetes (20).
The novel Amycolatopsis, the apparent nonspecificity of inhibition, and the risk of harmful effects of actinomycete antibiotics on fungus gardens (7) all will inspire or reinforce new research. However, the isolation of actinomycetes from some males is likely to be mostly of academic interest unless these bacteria can be shown to have more than a negligible probability of being horizontally transmitted. Likewise, the suggestion that most ant-associated actinomycete strains may be pathogens (7) seems unlikely given that most attine ants have maintained derived cuticular structures and subcuticular glands to provision actinomycete cultures.
The Sen et al. study (7) is fascinating and timely but will likely remain controversial for some time to come. For many, this work will be a welcome reminder that we need to continuously reevaluate what we know for a fact and what we merely infer. Others may feel that this study mostly addresses patterns of microbial biodiversity in laboratory colonies and in vitro experiments that may give only a partial picture of the host–symbiont interactions in natural populations. Most importantly, however, the Sen et al. study will inspire new work by a growing community of researchers that are fascinated by this tribe of ants that seems to offer everything that evolutionary biologists interested in social evolution and the evolutionary stability of mutualisms can wish for.
Footnotes
The authors declare no conflict of interest.
See companion article on page 17805.
References
- 1.Mueller UG, Rehner SA, Schultz TR. The evolution of agriculture in ants. Science. 1998;281:2034–2038. doi: 10.1126/science.281.5385.2034. [DOI] [PubMed] [Google Scholar]
- 2.Schultz TR, Brady SG. Major evolutionary transitions in ant agriculture. Proc Natl Acad Sci USA. 2008;105:5435–5440. doi: 10.1073/pnas.0711024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Currie CR, Scott JA, Summerbell RC, Malloch D. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature. 1999;398:701–704. [Google Scholar]
- 4.Currie CR, Mueller UG, Malloch D. The agricultural pathology of ant fungus gardens. Proc Natl Acad Sci USA. 1999;96:7998–8002. doi: 10.1073/pnas.96.14.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poulsen M, Boomsma JJ. Mutualistic fungi control crop diversity in fungus-growing ants. Science. 2005;307:741–744. doi: 10.1126/science.1106688. [DOI] [PubMed] [Google Scholar]
- 6.Aanen DK, et al. The evolution of fungus-growing termites and their mutualistic fungal symbionts. Proc Natl Acad Sci USA. 2002;99:14887–14992. doi: 10.1073/pnas.222313099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sen R, et al. Generalized antifungal activity and 454-screening of Pseudonocardia and Amycolatopsis bacteria in nests of fungus-growing ants. Proc Natl Acad Sci USA. 2009;106:17805–17810. doi: 10.1073/pnas.0904827106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller UG, Gerardo NM, Aanen DK, Six DL, Schultz TR. The evolution of agriculture in insects. Annu Rev Ecol Evol Syst. 2005;36:563–595. [Google Scholar]
- 9.Villesen P, Murakami T, Schultz TR, Boomsma JJ. Identifying the transition between single and multiple mating of queens in fungus growing ants. Proc R Soc London Ser B. 2002;269:1541–1548. doi: 10.1098/rspb.2002.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerardo NM, Jacobs SR, Currie CR, Mueller UG. Ancient host–pathogen associations maintained by specificity of chemotaxis and antibiosis. Plos Biol. 2006;4:1358–1363. doi: 10.1371/journal.pbio.0040235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kost C, et al. Nonspecific association between filamentous bacteria and fungus-growing ants. Naturwissenschaften. 2007;94:821–828. doi: 10.1007/s00114-007-0262-y. [DOI] [PubMed] [Google Scholar]
- 12.Mueller UG, Dash D, Rabeling C, Rodrigues A. Coevolution between attine ants and actinomycete bacteria: A reevaluation. Evolution. 2008;62:2894–2912. doi: 10.1111/j.1558-5646.2008.00501.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhang MM, Poulsen M, Currie CR. Symbiont recognition of mutualistic bacteria by Acromyrmex leaf-cutting ants. ISME J. 2007;1:313–320. doi: 10.1038/ismej.2007.41. [DOI] [PubMed] [Google Scholar]
- 14.Chapela IH, Rehner SA, Schultz TR, Mueller UG. Evolutionary history of the symbiosis between fungus-growing ants and their fungi. Science. 1994;266:1691–1694. doi: 10.1126/science.266.5191.1691. [DOI] [PubMed] [Google Scholar]
- 15.Mikheyev S, Mueller UG, Abbott P. Cryptic sex and many-to-one coevolution in the fungus-growing ant symbiosis. Proc Natl Acad Sci USA. 2006;103:10702–10706. doi: 10.1073/pnas.0601441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehrlich PR, Raven PH. Butterflies and plants: A study in coevolution. Evolution. 1964;18:586–608. [Google Scholar]
- 17.Currie CR, Poulsen M, Mendenhall J, Boomsma JJ, Billen J. Coevolved crypts and exocrine glands support mutualistic bacteria in fungus-growing ants. Science. 2006;311:81–83. doi: 10.1126/science.1119744. [DOI] [PubMed] [Google Scholar]
- 18.Frank SA. Host-symbiont conflict over the mixing of symbiotic lineages. Proc R Soc London Ser B. 1996;263:339–344. doi: 10.1098/rspb.1996.0052. [DOI] [PubMed] [Google Scholar]
- 19.Poulsen M, Erhardt DP, Molinaro DJ, Lin T-L, Currie CR. Antagonistic bacterial interactions help shape host–symbiont dynamics within the fungus-growing ant–microbe mutualism. PLOS One. 2007;9:e960. doi: 10.1371/journal.pone.0000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez-Marin H, Zimmerman JK, Nash DR, Boomsma JJ, Wcislo WT. Reduced biological control and enhanced chemical pest management in the evolution of fungus farming in ants. Proc R Soc London Ser B. 2009;276:2263–2269. doi: 10.1098/rspb.2009.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]