Abstract
Oligomerization of members of the p53 family of transcription factors (p53, p63, and p73) is essential for their distinct functions in cell-cycle control and development. To elucidate the molecular basis for tetramer formation of the various family members, we solved the crystal structure of the human p73 tetramerization domain (residues 351–399). Similarly to the canonical p53 tetramer, p73 forms a tetramer with D2 symmetry that can be described as a dimer of dimers. The most striking difference between the p53 and p73 tetramerization domain is the presence of an additional C-terminal helix in p73. This helix, which is conserved in p63, is essential for stabilizing the overall architecture of the tetramer, as evidenced by the different oligomeric structures observed for a shortened variant lacking this helix. The helices act as clamps, wrapping around the neighboring dimer and holding it in place. In addition, we show by mass spectrometry that the tetramerization domains of p63 and p73, but not p53, fully exchange, with different mixed tetramers present at equilibrium, albeit at a relatively slow rate. Taken together, these data provide intriguing insights into the divergent evolution of the oligomerization domain within the p53 family, from the ancestral p63/p73-like protein toward smaller, less promiscuous monomeric building blocks in human p53, allowing functional separation of the p53 pathway from that of its family members.
Keywords: crystallography, mass spectrometry, tetramer, transcription factor
The p53 family of transcription factors, which comprises the products of the TP53, TP63. and TP73 genes, is at the hub of numerous signaling pathways that determine the fate of the cell (1, 2). The first discovered family member p53 is a tumor suppressor and plays a key role in maintaining the integrity of the human genome. Its activation can lead to cell-cycle arrest, induction of senescence, differentiation, or apoptosis (3, 4). p53 is inactivated directly by mutation in ≈50% of human cancers (5, 6). The phylogenetically more ancient family members p63 and p73 were initially thought to play a predominantly developmental role (7–10). In recent years, however, there has been accumulating evidence that p63 and p73, in addition to their role in development and differentiation, can also act as tumor suppressors. Depending on their cellular context, they can exert this function either in concert with p53 or in some cases completely independent of p53 (1, 11).
Oligomerization is essential for p53 function (12), which is consistent with the observation that p53 binds DNA as a tetramer (13, 14). The same is presumed for the p63 and p73 paralogs (15). The p53 family members are found in different N- and C-terminally truncated isoforms because of two alternative promoters and multiple splicing (16). Structurally, p53 is the best-characterized family member. It has a complex domain structure (reviewed in ref. 17), consisting of a natively unfolded N-terminal transactivation domain, which is connected to the central, folded DNA-binding domain via a proline-rich region. The C-terminal region of the protein contains the tetramerization domain that controls the oligomerization state of p53 and the intrinsically disordered C-terminal regulatory domain. The overall topology of the p53 tetramerization domain is best described as a dimer of primary dimers, with the monomeric building blocks consisting of a short β-strand followed by an α-helix. The primary dimers are stabilized via an intermolecular β-sheet and helix packing. Two such dimers form a tetramer in which the helices of the primary dimers pack against each other, resulting in an orthogonal arrangement with D2 symmetry (18–21). The oligomerization domains of the invertebrate p53 homologs from Drosophila melanogaster and Caenorhabditis elegans (CEP-1) have a significantly different architecture. CEP-1 forms dimers instead of tetramers and contains an additional sterile α-motif (SAM) that is important for the stability of the domain, suggesting a possible evolutionary pathway from functional dimers to tetramers (22).
The domain structure of p63 and p73 is similar to that of p53, and particularly the DNA-binding domain shows a high degree of conservation. There are, however, notable differences in the C-terminal region of the proteins, with additional structural elements unique to p63 and p73. They both contain an extended C-terminal region, which also features a SAM domain and an inhibitory domain at the extreme C terminus (15). The oligomerization domains of p63 and p73 share ≈40% sequence identity with the corresponding region of p53 (residues 325–356). The domains independently fold into homotetramers. They are able to weakly interact with each other but do not interact with p53 (23), indicating potential differences in the topology of the oligomers. Whereas the region following the tetramerization domain in p53 is natively unfolded and the site of numerous posttranslational modifications, the canonical oligomerization domain motif in p63 and p73 is followed by a glutamine-rich region.
We report the crystal structures of different tetramerization domain variants of human p73, showing that this additional C-terminal region is helical and required for stabilizing the overall architecture of the p73 tetramer. The structural data are complemented by stability measurements and mass spectrometry studies on the formation of mixed tetramers between different members of the p53 family.
Results
Structure Determination.
For structure solution of the human p73 tetramerization domain, we used two different domain constructs: p73TET-short (residues 351–383), corresponding to the structured tetramerization domain region of the p53 paralog, and p73TET-long (residues 351–399), containing an additional C-terminal region that is conserved in p63 and p73, but not in p53 (Fig. 1). We have solved the structure of p73TET-long in two different crystal forms at 1.7- and 2.3-Å resolution and that of p73TET-short at 2.0-Å resolution (Tables S1 and S2). The highest-resolution structure of p73TET-long in the hexagonal space group H3 was solved by MAD phasing using selenomethionine-substituted crystals and contained one tetramer with D2 symmetry in the asymmetric unit (Fig. 2A). In the second crystal form (P212121), the asymmetric unit contained four independent tetramers of the same type. This structure was solved by molecular replacement using the hexagonal structure as a search model. The structure of p73TET-short was solved by MAD phasing after initial attempts to solve the structure by molecular replacement using various search models based on the p53 structure had failed. p73TET-short crystallized in a monoclinic space group (P21), containing a total of 28 independent monomers. In this crystal form, the monomers are assembled to form four hexamers and one tetramer (Fig. 2 C and D). For all observed p73 oligomers, the central building block is a primary dimer.
Fig. 1.
Multiple sequence alignment of the oligomerization domain region of p73 orthologs and paralogs. Amino acid residues are colored according to the Clustal color scheme, based on sequence conservation and similarity. Residue numbers of human p73 (636 aar), p63 (641 aar), and p53 (393 aar) are given above their sequence. The secondary structure elements of human p73 (this study) and p53 (PDB code 1C26) (19) are shown above the corresponding sequence. Sequences were collected by using the ENSEMBL genome browser (www.ensembl.org) and aligned with JALVIEW (34). Conserved p73 residues involved in H2-mediated intermolecular polar contacts are marked with asterisks.
Fig. 2.
Oligomerization domain structures of p73 and p53. Overall structure of the p73TET-long tetramer (A), the canonical p53 tetramer (B) (19), and the tetramers (C) and hexamers (D) observed in the structure of p73TET-short. All four oligomers are formed through association of dimeric building blocks (shown in different colors). The structures are shown in two orientations after superposition based on the yellow primary dimers.
Structure of the Long p73 Tetramerization Domain Variant.
The structure of p73TET-long is shown in Fig. 2A. Numbers in the following refer to the high-resolution structure in space group H3. The overall topology of the tetramer can be described as a dimer of dimers. The individual chains within this tetramer consist of a short β-strand followed by a sharp turn, an α-helix with a short 310-helical segment at the end (H1), and a second turn followed by another α-helix (H2). The first turn is facilitated by a conserved glycine residue (Gly-361), which adopts a main-chain conformation that would be energetically unfavorable in the presence of a side chain, highlighting the structural requirement for a glycine at this position (Fig. 3B). The observed dihedral angles range from ϕ = 106–112° and ψ = 124–130°. The second turn contains a conserved proline (Pro-382) which leads into helix H2.
Fig. 3.
Structure of p73TET-long (residues 351–399). (A) Overall structure of the p73 tetramer. Individual chains are shown in different colors. The same color code is used in all subsequent images. (B) Structure of the primary dimer with key residues of the hydrophobic core shown as stick models. (C) View of the central, largely hydrophobic dimer–dimer interface across the H1 helices of all four subunits. (D) Stereoview of the intermolecular contacts made by residues from the H2 helix.
Two p73 monomers form a symmetrical primary dimer via an antiparallel intermolecular β-sheet and antiparallel helix packing, with a buried surface area of 2,060 Å2 (e.g., the green and gray chains in Fig. 3A). Key residues in the hydrophobic core of this primary dimer are Leu-357, Val-359, and Leu-368 (corresponding to Leu-330, Ile-332, and Phe-341 in p53). At the center of the contact area between the two helices, Leu-368 makes hydrophobic contacts with the same residue from the adjacent subunit (Fig. 3B). At the end of the helix–helix interface, Asn-364 forms a hydrogen bond with the carboxylate group of Glu-379. In one chain, however, the glutamate side chain is disordered.
Two such primary dimers form a tetramer via helix–helix packing interactions. The two dimers are related by 2-fold symmetry and pack at an angle of approximately 65°. The tetramer is stabilized by two types of contact area: a central, largely hydrophobic contact area and a contact area with a strong polar contribution. The core of the tetramer interface is formed by the H1 helices, which are arranged in a four-helix bundle. This contact area is predominantly hydrophobic in nature. At its center, the Leu-371 side chains from all four subunits contact each other, flanked by the side chains of Ile-367 and Leu-375 (Fig. 3C). At both ends of this hydrophobic cluster, the interface is stabilized by a hydrogen bond between two Ser-374 hydroxyls. The second type of dimer–dimer contact area, which appears four times, involves the C-terminal H2 helices. These helices clasp the second primary dimer, packing against adjacent H1 helices (Fig. 3A). The H1-H2 turn region and the N-terminal part of H2 are stabilized by hydrophobic interactions (e.g., Leu-377, Val-381, and Val-386). Many of the H2-mediated interactions, however, are of a polar nature, thus providing specificity (Fig. 3D): a salt bridge between Arg-390 and Glu-373′, and charged hydrogen-bond pairs (Tyr-389-Glu-376′ and Gln-393-Lys-372′). A further hydrogen bond connects Gln-397 to the main-chain nitrogen of Gln-358″ from the β-strand region of an adjacent primary dimer, although this residue (Gln-397) is not resolved in all chains. Residues that are part of this polar network are highly conserved among p73 from different species (Fig. 1).
Structure of the Short p73 Tetramerization Domain Variant Lacking Helix H2.
The crucial role of the H2 helix in stabilizing the overall architecture of the p73 tetramer becomes clear when comparing the structures with and without this helix. There are significant differences in the architecture of the primary dimer and the assembly of primary dimers into higher-order oligomers. The structure of p73TET-short contains a total of 28 independent monomers in the asymmetric unit, which are assembled in different oligomeric species, built from primary dimer building blocks: four essentially identical hexamers with approximate D3 symmetry and one tetramer (Fig. 2 C and D). The overall assembly of these primary dimers is similar to that observed in p73TET-long; i.e., formation of an intermolecular β-strand and antiparallel packing of the α-helices with an average buried surface area of 2,150 Å2. But the two helices pack at a different angle, resulting in differences in contact geometry: θ = 160° in the long variant and θ = 134–138° in the short variant (Fig. S1).
In the hexamers, three primary dimers are aligned along an approximate, central 3-fold axis (the symmetry is not crystallographic and therefore deviations from perfect symmetry are observed as a result of crystal packing forces), with each helix packed against a helix from a neighboring dimer that runs in the opposite direction (θ = −165°). The contact area is stabilized by hydrophobic interactions involving Ile-367, Leu-371, Leu-377, and Met-378 and salt bridges between Lys-370 and Glu-373. This arrangement places the three β-sheets at the outside of the hexamer in alignment with the local 3-fold axis (Fig. 2D). It leaves a cavity at the center of the hexamer with an average volume of 580 Å3, which is lined by the hydrophobic side chains of Ile-367, Leu-371, Leu-375, and Met-378. In the tetramer, two primary dimers pack via their helices, resulting in an approximately antiparallel packing of the two primary dimers (Fig. 2C). By contrast, the packing angle of the dimers in the presence of the additional H2 helix in p73TET-long is 65°. It has to be noted that one of the four chains adopts a slightly distorted conformation, which breaks the symmetry of the tetramer in the short variant.
Stability of the Tetramerization Domains.
To investigate the contribution of the H2 helix to the thermodynamic stability of the protein, we measured the thermostability of the p53, p63, and p73 oligomerization domains by differential scanning calorimetry at a fast heating rate of 250 °C/h (Table S3). Both p63 and p73 exhibited high thermostability, with the observed melting temperature, Tm, being concentration-dependent. At a protein concentration of 400 μM (monomer), the short tetramerization domain variants, p63TET-short (p63 residues 359–388) and p73TET-short, had an apparent Tm of 89.7 °C and 89.1 °C, respectively, which was significantly higher than that of the p53 tetramerization domain under the same conditions (82.3 °C). Presence of the C-terminal helix in both p63TET-long (p63 residues 356–411) and p73TET-long resulted in a further increase in apparent Tm by 7 °C and 3 °C, respectively. According to analytical ultracentrifugation, p63TET-long and p73TET-long were tetrameric at monomer concentrations of 10 μM. By contrast, p63TET-short and p73TET-short were in equilibrium between tetramers and lower molecular species at this concentration but predominantly tetrameric when measured at higher concentration (see Table S4). This further reinforces the importance of the C-terminal helix in stabilizing the domain assembly of p63 and p73.
p63 and p73 but Not p53 Form Heterotetramers.
A previous study has shown that oligomerization domain constructs of p63 and p73 interact with each other but do not exchange with p53 tetramers (23). This study was performed by using a p73 construct that lacked helix H2, and it did not detect the stoichiometry of the heterooligomers formed. We used nanoflow electrospray ionization mass spectrometry (nESI MS) to study the heterooligomerization semiquantitatively, using p63 and p73 variants with the H2 helix intact. At 37 °C, no exchange of p53 with either p63 or p73 was observed, even after a 24-h incubation time, at a protein concentration of 10 μM each. By contrast, p63 and p73 formed mixed tetramers at 37 °C, but the exchange rate was very slow. Approximately 30 min after mixing equal amounts of p63 and p73 homotetramers, additional signals were observed in the mass spectrum corresponding to mixed tetramers with 3:1, 2:2 and 1:3 ratios (Fig. 4). With increasing incubation time, the intensity of these signals increased relative to those of the homotetramers. There was also an increase of the signal of the 2:2 complex relative to those of the 3:1 and 1:3 complexes over time. Equilibrium was reached after ≈10 h. In the case of full exchange of the monomers, an equilibrium ratio of 1:4:6:4:1 would be expected, with the homotetramers being the minor species, and the 2:2 hetero complex being the most-populated species. Exact quantification of the relative ratios was not possible because the peak intensities observed for the different species did not directly correlate with their molar ratios, as can be seen from the different signal intensities observed for the p63 and p73 homotetramers in the spectrum taken of the 1:1 mixture immediately after mixing. Nevertheless, a qualitative analysis identifies the 2:2 complex as the predominant species at equilibrium, which could adopt three different assembly geometries.
Fig. 4.
Formation of mixed tetramers of p63 and p73. Electrospray mass spectra of p63 and p73 tetramerization domains in isolation (spectra of the two homotetramers at the bottom) and at different time points after mixing equal amounts of both homotetramers at 37 °C, showing formation of mixed tetramers with 3:1, 2:2, and 1:3 ratios. For each species, different peaks were observed representing alternate charge states (see also Table S5).
Discussion
Comparison of the p53 and p73 Tetramerization Domain Structures.
The most striking difference between the p53 and p73 tetramerization domains is the presence of the additional stabilizing helix in p73, but there are also structural changes within the region of the canonical p53 tetramer (Fig. 5). In the p53 crystal structure (PDB code 1C26), the two primary dimers pack in a roughly orthogonal fashion (θ2 = 81°). By comparison, the p73 tetramer is twisted by ≈16° along the plane of the central dimer–dimer interface (θ2 = 65°). Although the twist in the helix packing of p73TET-long deviates significantly from the orthogonal packing in p53, it is still much closer to the p53 packing mode than to the antiparallel packing modes observed for p73TET-short. Because of the additional packing interactions mediated via the H2 helix, the total buried surface area within the tetramer is significantly larger in p73 than in p53 (8,420 Å2 versus 6,720 Å2). Intradimer and interdimer contacts contribute to a similar amount in p73, whereas this ratio is 2.5:1 in p53. Future studies will show how these differences in contact surfaces affect the dissociation constants and kinetics of tetramer formation.
Fig. 5.
Comparison of the tetramerization domains of p73 and p53. (A) Stereoview of the p73 tetramer (yellow and red chains) superimposed onto p53 (PDB code 1C26, green and blue chains), showing the different architecture of the tetramers. Chains belonging to the same primary dimer are shown in the same color. The yellow and green dimers were used for superposition. (B) Superposition of the primary dimers of p73 (yellow) and p53 (green). Selected residues at the center and at the periphery of the helix–helix interface are shown as stick models. The helices in p53 are further apart as a result of small-to-large substitutions of key contact residues at the center of the interface.
Comparing the primary dimers, the H1 helices pack at the same angle as observed in p53, but they are further apart in p53 (Fig. 5B). Equivalent interchain Cα-distances for the H1 helices within the primary dimers are 1.1 to 2.5 Å shorter in p73 than in p53. This difference in helix packing in p73 can be attributed, at least in part, to large-to-small substitutions (Ile → Val, Phe → Leu) of two key residues in the hydrophobic core of the dimer interface. Introduction of equivalent mutations in p53 (I332V and F341L) destabilizes the domain by 5.0 and 3.3 kcal/mol, respectively, but the effects are not additive, as the corresponding double mutant of p53 is destabilized by only 2.2 kcal/mol (24). The p53 tetramer is stabilized by an intermolecular salt bridge between Arg-337 and Asp-352 at both ends of the primary-dimer helix interface (19). Mutation in the p53 gene that disrupts this salt bridge (R337H), thus destabilizing the tetramer, has been linked with high incidence of adrenocortical carcinoma in children in southern Brazil (25). Approximately 20% of reported cases of p53 germ-line mutations have a mutation at codon 337 (release R13 of the TP53 Mutation Database of the International Agency for Research on Cancer at www-p53.iarc.fr) (6), highlighting the importance of this interaction in stabilizing the primary dimer of p53. In p73, for which no disease-related mutations in the tetramerization domain have been reported so far, this salt bridge is replaced by the Asn-364–Glu-379 pair.
Mixed Tetramer Formation and the Dominant-Negative Effect.
We have shown by mass spectrometry that the tetramerization domains of p63 and p73 can form multiple mixed tetramer species, albeit at a relatively slow rate. By contrast, the p53 tetramerization domain did not exchange with its family members, consistent with earlier data (23), thus allowing functional separation of the p53 pathway from that of its family members. This behavior can be rationalized by comparing the structures and sequences of the three p53 family members. The p73 and p53 structures show variation of key residues in both the primary-dimer and dimer–dimer interface that significantly affect the surface complementarity of the two family members (see above), which disallows subunit exchange. By contrast, the oligomerization domains of p63 and p73 share a much higher sequence similarity (Fig. 1). They both have a conserved C-terminal helix in addition to the canonical p53 motif. Importantly, residues of H2 that interact with a neighboring dimer in p73 are highly conserved in p63. Moreover, variations in the H1–H1 interfaces are substitutions of similar amino acids, e.g., Leu → Ile substitution at the center of the dimer–dimer interface. The few nonconservative substitutions affect surface-exposed residues.
There is increasing evidence of functional cross-talk of p53 family members and its role in carcinogenesis (16, 26). Many p53 cancer mutants not only reduce the activity of wild-type p53 in heterozygous cells by forming mixed tetramers but also block p63 and p73 function (27, 28). This observation has led to strategies to reactivate tumor suppressor function in mutant p53-bearing cancer cells via a p73-dependant salvage pathway (29). It is clear from our and previous studies that the underlying mechanisms of inactivation must be different in both cases. Whereas the dominant-negative effect of mutant p53 on wild-type p53 is the result of formation of mixed tetramers with altered DNA-binding properties (30), the inactivation of p63 and p73 proceeds via an alternative, yet not fully understood, mechanism that does not directly involve the tetramerization domain but possibly core domain interactions (31). The observation of mixed p63/p73 tetramers in vitro, on the other hand, raises the question whether formation of heterotetramers of these two family members is a realistic scenario in the cellular context. The rate of heterotopic exchange of the isolated tetramerization domains we observed in vitro is relatively slow once the homotetramers have been formed, and interaction between the additional structural elements in the full-length variants may slow down the exchange rate even further. So even if p63 and p73 tetramers are colocalized in the same cellular compartment, they may not form substantial amounts of heterotetramers within the lifetime of the proteins in vivo. Transiently expressed TA isoforms of p63 in Hep3B cell lines, for example, are rapidly degraded with a half-life of 1 h or less (32). The situation may be different if the nascent proteins exchange before homotetramers are formed.
Implications for the Evolution of the p53 Family.
It is generally believed that p63 and p73 more closely resemble the common ancestral protein than p53, which appears to be a more recent product of evolution (2). Many invertebrates, such as C. elegans and Drosophila, have only one family member, which is more p63-like. The presence of three family members in vertebrates is the result of two gene duplication events and subsequent divergent evolution that occurred after the invertebrate–vertebrate transition (2). This evolutionary tree derived from comparison of the full-length proteins and the presence/absence of the SAM domain is also mirrored in the sequence and structural organization of the tetramerization domain. Structurally important residues are conserved between p63 and p73, consistent with the observed subunit exchange. During evolution of mammalian p53, the C-terminal helix that seems to be essential for stabilizing the architecture of the p63 and p73 tetramer has been lost. A likely scenario is that p53 may have acquired additional stabilizing interactions that reduce the fluidity of the interface region as a result of random drift and that the C-terminal helix was subsequently lost because there was no longer evolutionary pressure to retain it. Instead, this region is intrinsically disordered in p53, providing binding promiscuity, and is the site of numerous posttranslational modifications that regulate p53 function and cellular protein levels (17). Interestingly, the tetramerization domain of zebrafish p53 shows conservation of some key residues of the C-terminal helix (e.g., Tyr-389 at the center of the H2-mediated interface in p73; see Fig. 3D) and retains a certain, albeit weak, helix propensity in this region. The canonical p53 motif in zebrafish has some of the hallmarks of p53 (e.g., the highly conserved Arg–Asp salt bridge), but also has p63/p73-like features (e.g., smaller hydrophobic side chains in the core of the primary dimer, equivalent to Val-359 and Leu-368 in p73).
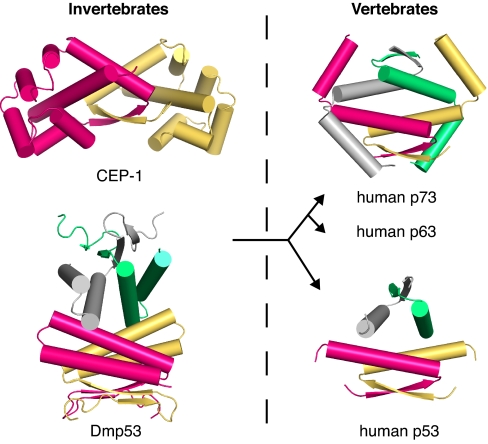
The end of the H2-helix region in the p63 and p73 genes coincides with the end of exon 10. Alternative splicing at this position gives rise to various p63 and p73 isoforms (16, 26). A common feature of all reported functionally important isoforms of p63 and p73 so far is the presence of the full tetramerization domain, including the H2 helix, supporting the structural observation that this helix is an integral part of the oligomerization domain. Interestingly, the Drosophila p53 tetramerization domain also has an additional C-terminal helix (plus an additional β-strand at the N terminus). But, this helix shares no sequence homology with H2 in p73 and displays completely different packing interactions (22). While the C-terminal helices embrace the adjacent dimer in p73, they pack against the H1 helix from the same subunit in the Drosophila homolog and make different interdimer contacts (Fig. 6). Moreover, the nature of the central dimer–dimer interface via the H1 helices is fundamentally different. It is mainly hydrophobic in p73 and human p53, but largely electrostatic in the invertebrate p53. Comparison of the different oligomerization domain structures of p53 family members, as shown in Fig. 6, suggests an intriguing evolutionary pathway from functional dimers to tetramers, whereas the actual building blocks display a loss of complexity; i.e., loss of secondary structure elements, from C. elegans and Drosophila, to vertebrate p63/p73, and eventually mammalian p53. In the process, the divergent evolution of the three family members toward less promiscuous p53 subunits has allowed uncoupling of the p53 pathway from that of its family members p63 and p73. Taken together, the oligomerization domain structures and the subunit exchange data provide insights into molecular evolution in action, which has established the three different signaling pathways found in vertebrates. Similar evolutionary scenarios may apply to other families of transcription factors with shared oligomerization domains.
Fig. 6.
Structural evolution of the oligomerization domain in the p53 family. Shown are the oligomerization domain structures of the p53 homologs from C. elegans, CEP-1, (PDB ID code 2RP5) (22) and D. melanogaster, Dmp53, (PDB ID code 2RP4) (22), as well as of human p73 and p53 (PDB ID code 1C26) (19). Individual chains are shown in different colors, highlighting a loss of secondary structure elements when going from the invertebrate structures via human p73 to human p53, which has the smallest monomeric building block.
Methods
Different tetramerization domain regions of human p63 and p73 were amplified from cDNA (Geneservice) and cloned into vector pRSET-HLT. The resulting plasmid encodes a fusion protein with an N-terminal 6xHis tag, followed by a lipoyl domain, a thrombin cleavage site, and the p63/p73 sequence of interest. The proteins were expressed in Escherichia coli strain BL21 and purified by a Ni-affinity chromatography, followed by cleavage with thrombin, purification using a second Ni-affinity column, anion exchange chromatography, and gel filtration. Subunit exchange of p53, p63, and p73 tetramerization domains was monitored by nanoflow electrospray ionization mass spectrometry (nESI-MS) on a Synapt HDMS system (Waters) optimized for the transmission of noncovalent complexes (33). p73 tetramerization domain crystals were grown by standard vapor diffusion techniques. X-ray data were collected at the Diamond Light Source (beamline I02) and the European Synchrotron Radiation Facility, Grenoble, France, (beamline ID23-1). Details of cloning, protein purification, sample preparation, differential scanning calorimetry, mass spectrometry, analytical ultracentrifugation, crystallization, structure solution, refinement, and analysis are given in SI Text. Structural figures were prepared by using PYMOL (www.pymol.org).
Supplementary Material
Acknowledgments.
We thank Antonina Andreeva, Roger Williams, and Jan van Dieck for valuable discussions; Fiona Sait for setting up crystallization trials; Christoph Müller-Dieckmann at the European Synchrotron Radiation Facility, Grenoble, France, and Thomas Sorensen at the Diamond Light Source for help with data collection. This work was supported by the Medical Research Council and by European Community FP6 funding.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 2WQI, 2WQJ, and 2WTT).
See Commentary on page 17609.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905867106/DCSupplemental.
References
- 1.Pietsch EC, Sykes SM, McMahon SB, Murphy ME. The p53 family and programmed cell death. Oncogene. 2008;27:6507–6521. doi: 10.1038/onc.2008.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang A, Kaghad M, Caput D, McKeon F. On the shoulders of giants: p63, p73 and the rise of p53. Trends Genet. 2002;18:90–95. doi: 10.1016/s0168-9525(02)02595-7. [DOI] [PubMed] [Google Scholar]
- 3.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 4.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 5.Joerger AC, Fersht AR. Structure-function-rescue: The diverse nature of common p53 cancer mutants. Oncogene. 2007;26:2226–2242. doi: 10.1038/sj.onc.1210291. [DOI] [PubMed] [Google Scholar]
- 6.Petitjean A, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: Lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28:622–629. doi: 10.1002/humu.20495. [DOI] [PubMed] [Google Scholar]
- 7.Yang A, et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- 8.Kaghad M, et al. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–819. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 9.Mills AA, et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- 10.Yang A, et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature. 2000;404:99–103. doi: 10.1038/35003607. [DOI] [PubMed] [Google Scholar]
- 11.Suh EK, et al. p63 protects the female germ line during meiotic arrest. Nature. 2006;444:624–628. doi: 10.1038/nature05337. [DOI] [PubMed] [Google Scholar]
- 12.Itahana Y, Ke H, Zhang Y. p53 Oligomerization is essential for its C-terminal lysine acetylation. J Biol Chem. 2009;284:5158–5164. doi: 10.1074/jbc.M805696200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitayner M, et al. Structural basis of DNA recognition by p53 tetramers. Mol Cell. 2006;22:741–753. doi: 10.1016/j.molcel.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Tidow H, et al. Quaternary structures of tumor suppressor p53 and a specific p53 DNA complex. Proc Natl Acad Sci USA. 2007;104:12324–12329. doi: 10.1073/pnas.0705069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scoumanne A, Harms KL, Chen X. Structural basis for gene activation by p53 family members. Cancer Biol Ther. 2005;4:1178–1185. doi: 10.4161/cbt.4.11.2254. [DOI] [PubMed] [Google Scholar]
- 16.Murray-Zmijewski F, Lane DP, Bourdon JC. p53/p63/p73 isoforms: An orchestra of isoforms to harmonise cell differentiation and response to stress. Cell Death Differ. 2006;13:962–972. doi: 10.1038/sj.cdd.4401914. [DOI] [PubMed] [Google Scholar]
- 17.Joerger AC, Fersht AR. Structural biology of the tumor suppressor p53. Annu Rev Biochem. 2008;77:557–582. doi: 10.1146/annurev.biochem.77.060806.091238. [DOI] [PubMed] [Google Scholar]
- 18.Mittl PR, Chene P, Grutter MG. Crystallization and structure solution of p53 (residues 326–356) by molecular replacement using an NMR model as template. Acta Crystallogr D. 1998;54:86–89. doi: 10.1107/s0907444997006550. [DOI] [PubMed] [Google Scholar]
- 19.Jeffrey PD, Gorina S, Pavletich NP. Crystal structure of the tetramerization domain of the p53 tumor suppressor at 1.7 angstroms. Science. 1995;267:1498–1502. doi: 10.1126/science.7878469. [DOI] [PubMed] [Google Scholar]
- 20.Clore GM, et al. Refined solution structure of the oligomerization domain of the tumour suppressor p53. Nat Struct Biol. 1995;2:321–333. doi: 10.1038/nsb0495-321. [DOI] [PubMed] [Google Scholar]
- 21.Lee W, et al. Solution structure of the tetrameric minimum transforming domain of p53. Nat Struct Biol. 1994;1:877–890. doi: 10.1038/nsb1294-877. [DOI] [PubMed] [Google Scholar]
- 22.Ou HD, Lohr F, Vogel V, Mantele W, Dotsch V. Structural evolution of C-terminal domains in the p53 family. EMBO J. 2007:3463–3473. doi: 10.1038/sj.emboj.7601764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davison TS, et al. p73 and p63 are homotetramers capable of weak heterotypic interactions with each other but not with p53. J Biol Chem. 1999;274:18709–18714. doi: 10.1074/jbc.274.26.18709. [DOI] [PubMed] [Google Scholar]
- 24.Mateu MG, Fersht AR. Mutually compensatory mutations during evolution of the tetramerization domain of tumor suppressor p53 lead to impaired hetero-oligomerization. Proc Natl Acad Sci USA. 1999;96:3595–3599. doi: 10.1073/pnas.96.7.3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DiGiammarino EL, et al. A novel mechanism of tumorigenesis involving pH-dependent destabilization of a mutant p53 tetramer. Nat Struct Biol. 2002;9:12–16. doi: 10.1038/nsb730. [DOI] [PubMed] [Google Scholar]
- 26.Petitjean A, Hainaut P, Caron de Fromentel C. TP63 gene in stress response and carcinogenesis: A broader role than expected. Bull Cancer. 2006;93:E126–135. [PubMed] [Google Scholar]
- 27.Gaiddon C, Lokshin M, Ahn J, Zhang T, Prives C. A subset of tumor-derived mutant forms of p53 down-regulate p63 and p73 through a direct interaction with the p53 core domain. Mol Cell Biol. 2001;21:1874–1887. doi: 10.1128/MCB.21.5.1874-1887.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strano S, et al. Physical interaction with human tumor-derived p53 mutants inhibits p63 activities. J Biol Chem. 2002;277:18817–18826. doi: 10.1074/jbc.M201405200. [DOI] [PubMed] [Google Scholar]
- 29.Kravchenko JE, et al. Small-molecule RETRA suppresses mutant p53-bearing cancer cells through a p73-dependent salvage pathway. Proc Natl Acad Sci USA. 2008;105:6302–6307. doi: 10.1073/pnas.0802091105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Natan E, Hirschberg D, Morgner N, Robinson CV, Fersht AR. Ultraslow oligomerization equilibria of p53 and its implications. Proc Natl Acad Sci USA. 2009;106:14327–14332. doi: 10.1073/pnas.0907840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Prives C. Are interactions with p63 and p73 involved in mutant p53 gain of oncogenic function? Oncogene. 2007;26:2220–2225. doi: 10.1038/sj.onc.1210311. [DOI] [PubMed] [Google Scholar]
- 32.Petitjean A, et al. Properties of the six isoforms of p63: p53-like regulation in response to genotoxic stress and cross talk with DeltaNp73. Carcinogenesis. 2008;29:273–281. doi: 10.1093/carcin/bgm258. [DOI] [PubMed] [Google Scholar]
- 33.Hernandez H, Robinson CV. Determining the stoichiometry and interactions of macromolecular assemblies from mass spectrometry. Nat Protocols. 2007;2:715–726. doi: 10.1038/nprot.2007.73. [DOI] [PubMed] [Google Scholar]
- 34.Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.