Abstract
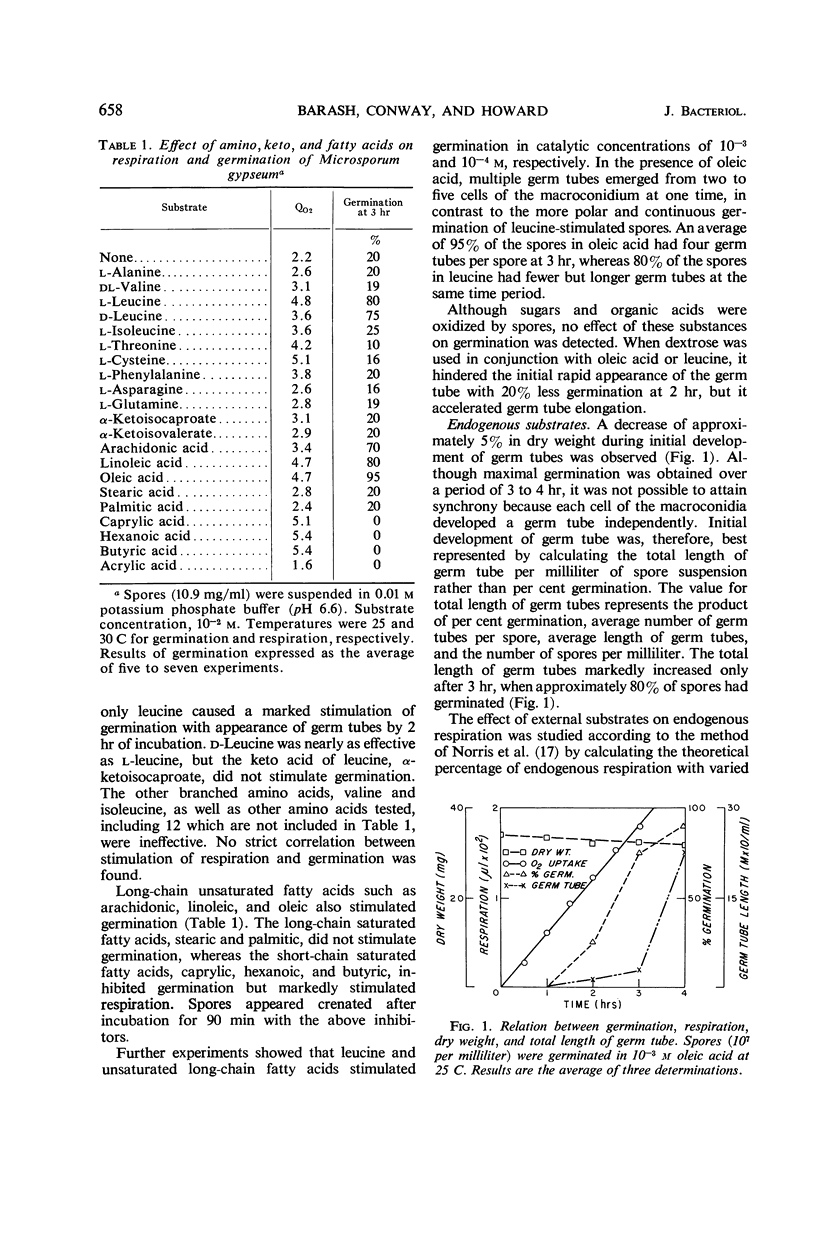
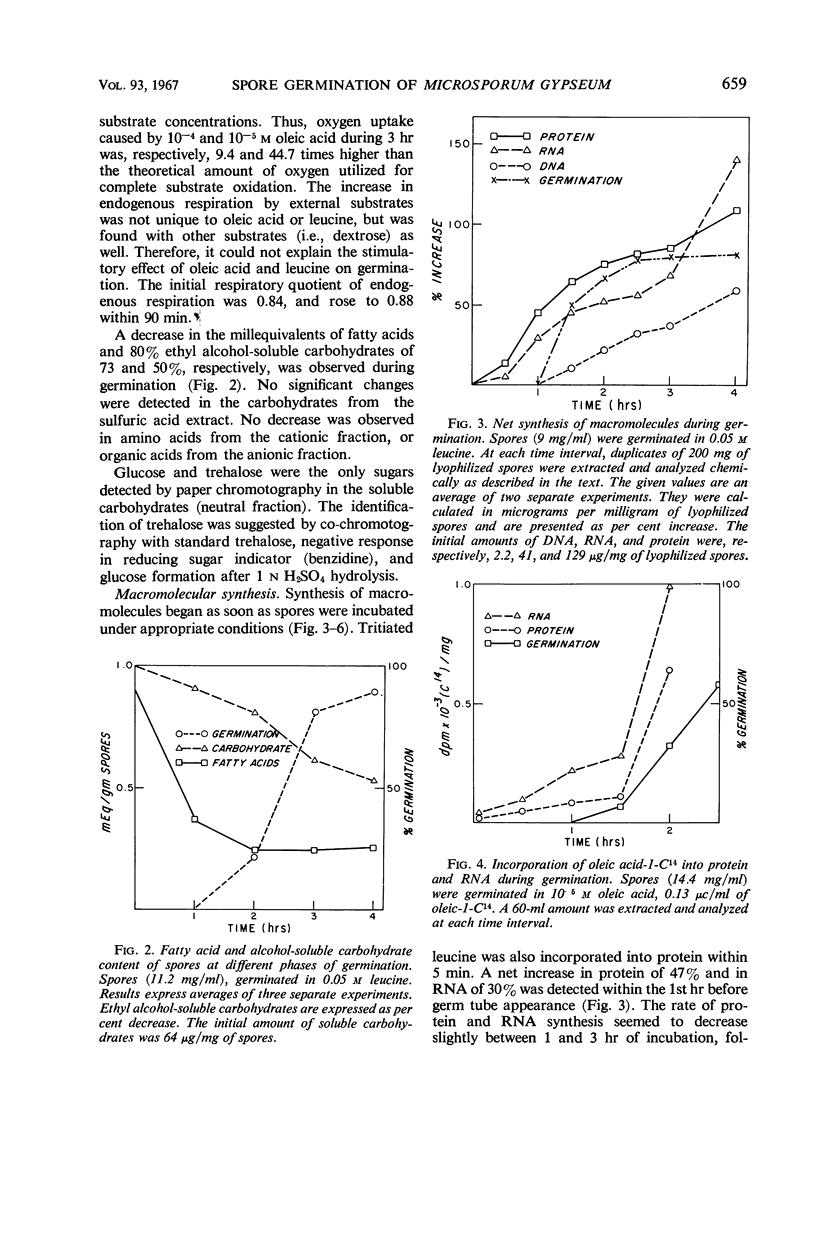
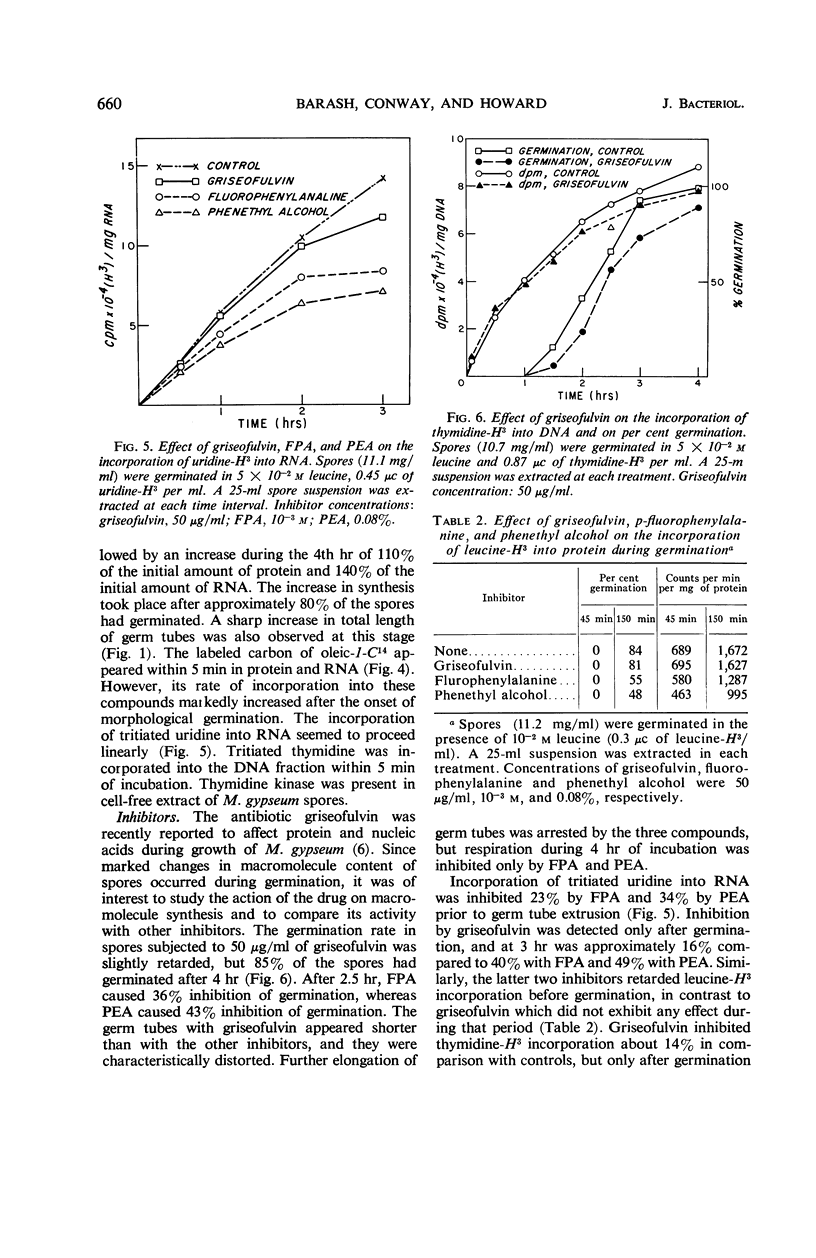
Either d- or l-leucine (10−3m) and unsaturated long-chain fatty acids such as oleic, linoleic, and arachidonic (10−4m) significantly stimulated macroconidia germination of Microsporum gypseum. Saturated long-chain fatty acids did not affect germination, whereas saturated short-chain fatty acids such as caprylic, hexanoic, and butyric were completely inhibitory. Germination was followed by an increase in endogenous respiration and a decrease in dry weight of approximately 5% at 4 hr. Endogenous fatty acids and soluble carbohydrates were utilized significantly during germination. Tritiated leucine, uridine, and thymidine were incorporated respectively into protein, ribonucleic acid (RNA), and deoxyribonucleic acid (DNA) fractions within the first 5 min of germination. Incorporation of oleic-1-C14 into RNA and protein was significantly increased after germ tube development. Net synthesis of RNA and protein started prior to germ tube protrusion. Increase in DNA could be detected only later. A significant increase in RNA and protein during the 4th hr of germination was correlated with vegetative development. Inhibition of respiration and incorporation of leucine-H3 and uridine-H3 into corresponding macromolecules by dl-fluorophenylalanine and phenethyl alcohol started before germ tube appearance. Griseofulvin significantly inhibited incorporation of uridine-H3 and thymidine-H3, but not of leucine-H3. This inhibition occurred only after initial vegetative development. In contrast to the two other inhibitors, which substantially inhibited germination, griseofulvin only slightly retarded the period of germination and did not affect respiration.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENTLEY M. L. Enzymes of pathogenic fungi. J Gen Microbiol. 1953 Jun;8(3):365–377. doi: 10.1099/00221287-8-3-365. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL NAKEEB M. A., MCLELLAN W. L., Jr, LAMPEN J. O. ANTIBIOTIC ACTION OF GRISEOFULVIN ON DERMATOPHYTES. J Bacteriol. 1965 Mar;89:557–563. doi: 10.1128/jb.89.3.557-563.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENCH R. C., WEINTRAUB R. L. Pelargonaldehyde as an endogenous germination stimulator of wheat rust spores. Arch Biochem Biophys. 1957 Nov;72(1):235–237. doi: 10.1016/0003-9861(57)90191-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lester G. Inhibition of Growth, Synthesis, and Permeability in Neurospora crassa by Phenethyl Alcohol. J Bacteriol. 1965 Jul;90(1):29–37. doi: 10.1128/jb.90.1.29-37.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa B. T., Sussman A. S. Endogenous Substrates of Dormant, Activated and Germinating Ascospores of Neurospora Tetrasperma. Plant Physiol. 1959 Jul;34(4):466–472. doi: 10.1104/pp.34.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. L. Quantitative Determination of Carbohydrates With Dreywood's Anthrone Reagent. Science. 1948 Mar 5;107(2775):254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C. The regulation RNA synthesis in bacteria. Prog Nucleic Acid Res Mol Biol. 1964;3:145–181. doi: 10.1016/s0079-6603(08)60741-2. [DOI] [PubMed] [Google Scholar]
- SUSSMAN A. S. The role of trehalose in the activation of dormant ascospores of neurospora. Q Rev Biol. 1961 Jun;36:109–116. doi: 10.1086/403332. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- YANAGITA T. Biochemical aspects on the germination of conidiospores of Aspergillus niger. Arch Mikrobiol. 1957;26(4):329–344. doi: 10.1007/BF00407583. [DOI] [PubMed] [Google Scholar]



