Abstract
Although long regarded as a conduit for the degradation or recycling of cell surface receptors, the endosomal system is also an essential site of signal transduction. Activated receptors accumulate in endosomes, and certain signaling components are exclusively localized to endosomes. Receptors can continue to transmit signals from endosomes that are different from those that arise from the plasma membrane, resulting in distinct physiological responses. Endosomal signaling is widespread in metazoans and plants, where it transmits signals for diverse receptor families that regulate essential processes including growth, differentiation and survival. Receptor signaling at endosomal membranes is tightly regulated by mechanisms that control agonist availability, receptor coupling to signaling machinery, and the subcellular localization of signaling components. Drugs that target mechanisms that initiate and terminate receptor signaling at the plasma membrane are widespread and effective treatments for disease. Selective disruption of receptor signaling in endosomes, which can be accomplished by targeting endosomal-specific signaling pathways or by selective delivery of drugs to the endosomal network, may provide novel therapies for disease.
Keywords: signal transduction, trafficking, endocytosis, receptors
Cell surface receptors allow cells to detect and respond to signals from the external environment. The binding of an extracellular ligand to a cell surface receptor initiates a cascade of signals that begins at the plasma membrane. Given the importance of this process, signaling at the plasma membrane has been intensively studied, and many drugs target signaling by cell surface receptors. However, upon activation, many receptors enter the endosomal system, a large, dynamic tubulovesicular network extending throughout the cytoplasm. Trafficking of a ligand-receptor complex within this system provides a mechanism to either terminate signaling through degradation of the receptor in lysosomes and proteasomes, or to sustain signaling through recycling of the receptor back to the cell surface where it can rebind extracellular ligands. Although the endocytic system has traditionally been viewed as a conduit that transports receptors to a degradative or recycling fate, endosomes are also a site at which receptor signaling can be initiated, sustained, and terminated. Activated receptors accumulate in endosomes, and certain essential signaling components are confined to endosomes. Different signals can arise from receptors at endosomal and plasma membranes, resulting in distinct physiological responses. Moreover, different mechanisms regulate signaling of receptors at endosomal and plasma membranes. These disparate mechanisms of signaling and regulation raise the possibility of novel therapies based on targeting endosomal rather than plasma membrane signaling. In this article, we review the mechanisms of receptor signaling from endosomes and summarize how this signaling is regulated. We discuss the physiological relevance of endosomal signaling and speculate on whether drugs that target endosomal signaling could be new therapies for disease.
Diverse Receptor Families Signal From Endosomes.
(Summarized in Table S1.)
Receptor Tyrosine Kinases (RTKs).
The view that RTK signaling occurs solely at the plasma membrane was challenged when subcellular fractionation and coimmunoprecipitation studies revealed that epidermal growth factor (EGF) induced accumulation of activated EGF receptor (EGFR) and its downstream signaling factors (SOS, Grb2, SHC) in early endosomes of liver parenchymal cells (1). A similar analysis of insulin-treated adipocytes revealed that internalized insulin receptors were more highly phosphorylated than those at the plasma membrane and that insulin receptor substrate (IRS-1) was associated with internal membranes where IRS-1 phosphorylation paralleled that of the insulin receptor (2). Insulin also preferentially activates PI3K in internal rather than plasma membranes (3) and causes accumulation of mitogen-activated protein kinase (MAPK) signaling components in endosomes isolated from fibroblasts by using insulin-coated magnetic microbeads (4). Nerve growth factor (NGF) similarly causes accumulation of NGF, activated TrkA receptor, phospholipase C-γ1 (PLC-γ1), and components of MAPK and PI3K signaling pathways in endosomes of pheochromocytoma PC12 cells (5) and nociceptive neurons (6).
Although these studies indicated that endosomes contain signaling machinery, it was less clear if signals could arise from endosomes themselves. Initial studies that addressed this issue focused on EGF-induced activation of MAPK and PI3K/Akt signaling pathways, which regulate cell proliferation and survival. Disruption of EGFR endocytosis, by expression of a mutant of the endocytic protein dynamin, suppressed EGF-induced activation of ERK 1/2 and PI3K, suggesting that EGFR internalization is required for the full spectrum of EGF signaling (7). However, a subsequent study reported that trafficking of the activated downstream kinase MEK, rather than of activated EGFR, from the plasma membrane is the critical step of endosomal signaling (8). To further establish—without the use of endocytic inhibitors—whether activated EGFR per se can signal from endosomes, a pharmacological approach was used to selectively activate EGFR in endosomes (9). Treatment of cells with the EGFR tyrosine kinase inhibitor AG-1478 blocked activation of EGFR at the plasma membrane but allowed endocytosis to proceed. Withdrawal of AG-1478 caused activation of endosomal EGFR, induced recruitment of signaling factors (SHC, Grb2, p85 subunit of PI3K) to endosomes, and led to the activation of ERK1/2 and Akt. This endosome-specific signaling of activated EGFR was sufficient to promote cell survival by the PI3K/Akt pathway. Thus, signaling pathways can originate from EGFR activated within endosomes. However, AG-1478 can attenuate EGFR internalization (10, 11), which may limit its usefulness in studying endosome-specific EGFR signaling. Signaling of the insulin receptor in endosomes was demonstrated by using a peroxovanadium compound (bpV(phen)) that activates insulin receptor kinase by inhibiting receptor-associated phosphotyrosine phosphatases, together with colchicine, which inhibits receptor recycling (12). Treatment of rats with bpV(phen) and colchicine allowed selective activation of the insulin receptor kinase in hepatic endosomes, which was accompanied by phosphorylation of IRS-1, thus demonstrating the signaling potential of the endosomal insulin receptor. Endocytosis mediates the full biological activity of other RTKs including the platelet-derived growth factor receptor (13) and vascular endothelial growth factor receptor-2 (14).
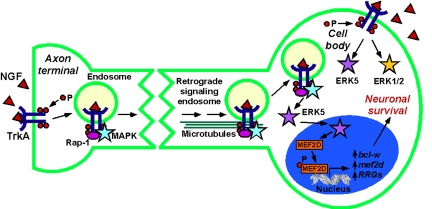
Given that receptors can signal at the cell surface and in endosomes, what is the relevance of endosomal signaling? Insight into this question is provided by consideration of NGF signaling in the nervous system. For growth factors released from target tissues to promote survival of innervating neurons, signals that arise from axon terminals must be sufficiently sustained and robust to travel long distances (>1,000-fold soma diameter) to the soma. The identification of NGF, activated TrkA, and signaling components in endosomes led to the hypothesis that NGF signals are transmitted in an axon to the cell body by retrograde transport of “signaling endosomes” (5) (Fig. 1). NGF-containing endosomes are retrogradely transported at ≈1.3 μs/s in axons of nociceptive neurons (15). In PC12 cells, NGF causes assembly of a stable endosomal signaling complex comprising TrkA, MAPK, and Rap-1 (Ras GTPase that causes sustained activation of MEK/MAPK) (16). Disruption of endosomes inhibits sustained activation of Rap-1 and MAPK, suggesting that NGF-TrkA signals in endosomes by Rap-1 to cause persistent MAPK activation. Thus, endosomes can provide a platform for sustained and robust signaling that can be transported to distant sites.
Fig. 1.
Signaling endosomes transport NGF signals from axon terminals to the cell body of neurons, resulting in activation of ERK5 in the cell body and neuronal survival. In contrast, TrkA activated directly at the cell body activates both ERK5 and ERK1/2. P, phosphate.
Do receptors in endosomes transmit signals that are distinct from those originating from receptors at the plasma membrane? Studies of the TrkA receptor provide evidence for endosome-specific signaling of NGF. Whereas NGF signaling in endosomes causes sustained MAPK activation, NGF-activated TrkA at the plasma membrane activates Ras transiently (16). In highly differentiated cells, such as neurons, the site of receptor activation can influence the nature of the endosomal signal. NGF-induced activation of TrkA in axon terminals of dorsal root ganglia neurons leads to retrograde transport of signaling endosomes to the cell body, where TrkA activates ERK5 (17). ERK5 translocates to the nucleus to activate CREB and enhance neuronal survival. In contrast, TrkA activated directly at the cell body signals through both ERK1/2 and ERK5 pathways (Fig. 1). Addition of neurotrophins to distal axons, but not cell bodies, also enhances activation of the transcription factor MEF2D by a Trk-dependent ERK5 pathway (18). Together, ERK5 and MEF2D increase expression of the antiapoptotic protein bcl-w, MEF2D, and other retrograde response genes. Thus, retrograde signaling from endosomes has a different outcome from that of direct stimulation at the soma and is required to activate an ERK5/MEF2D transcriptional response that enables neurons to survive in the presence of target-derived neurotrophins.
G Protein-Coupled Receptors (GPCRs).
GPCRs, or 7 transmembrane receptors, are the largest family of cell surface receptors. They participate in physiological control and disease and are the targets of many drugs. GPCRs signal at the plasma membrane by coupling to heterotrimeric G proteins. Although GPCRs are rapidly uncoupled from G proteins at the plasma membrane by receptor desensitization, these “desensitized” receptors can continue to signal at the plasma membrane and in endosomes by G protein-independent mechanisms. Arrestins are critically important for desensitization, endocytosis, and G protein-independent signaling of GPCRs (19). Arrestins were discovered as inhibitors of GPCR signaling; β-arrestin (βarr) 1 and 2 were identified as inhibitors of β2 adrenergic receptors (β2AR) but were subsequently found to regulate many GPCRs. βarrs interact with agonist-occupied, G protein-coupled receptor kinase (GRK)-phosphorylated GPCRs. This interaction sterically uncouples receptors from G proteins to mediate desensitization and couples receptors to clathrin and AP2 to mediate endocytosis. However, βarrs also recruit diverse signaling proteins to activated receptors at plasma and endosomal membranes and are essential mediators of signaling.
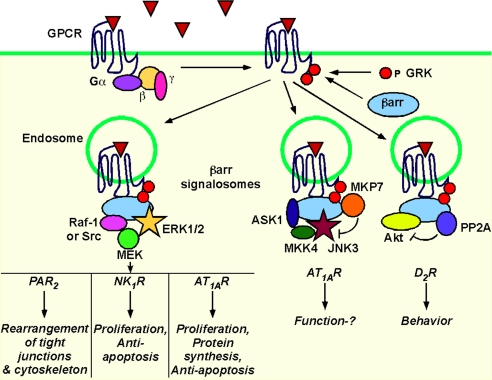
The MAPK cascades [ERK, c-Jun amino-terminal kinase (JNK), p38] are the most thoroughly characterized βarr-dependent signaling pathways (19, 20) (Fig. 2). The first evidence that βarrs are active participants in signaling was the observation that dominant negative mutants of βarr inhibited β2AR-induced activation of ERK1/2 (21). Subsequently, βarrs were found to couple β2AR to c-Src and mediate ERK1/2 activation (22). βarrs similarly participate in ERK1/2 signaling by other GPCRs, including neurokinin-1 receptor (NK1R), protease-activated receptor 2 (PAR2), angiotensin II type 1A receptor (AT1AR), and vasopressin V2 receptor (V2R) (23–26). These observations led to the view that βarrs are scaffolds that couple activated GPCRs with MAPK signaling complexes or “signalosomes”. βarrs thereby mediate a second wave of GPCR signaling that is distinct from G protein-dependent signaling at the plasma membrane. The importance of this mechanism depends on the affinity with which GPCRs interact with βarrs, which varies depending on the extent of GPCR phosphorylation by GRKs. “Class A” GPCRs (e.g., β2AR, α1bAR) have few phosphorylation sites, and transiently interact with βarr1 and βarr2, mostly at the plasma membrane, with a higher affinity for βarr2. “Class B” GPCRs (e.g., AT1AR, V2R, NK1R, PAR2) are phosphorylated at multiple sites and interact with both βarr1 and 2 with high affinity for prolonged periods at plasma and endosomal membranes. “Class C” GPCRs (e.g., bradykinin B2 receptor) internalize with βarrs into endosomes followed by rapid dissociation of βarr upon agonist removal (27). The extent of βarr-induced MAPK signaling depends on the affinity of the receptor for βarrs, which depends on the receptor structure and on which of the seven mammalian GRKs phosphorylate the receptor. Thus, activation of AT1AR and V2R causes greater phosphorylation of βarr-bound ERK1/2 than activation of α1bAR and β2AR, suggesting that the class B receptors signal more robustly through this pathway (26). Phosphorylation of the AT1AR by GRK5 and 6 is required for βarr-mediated ERK activation, whereas phosphorylation by GRK2 and 3 inhibits this signaling pathway (28). Different receptor agonists can lead to differential stimulation of GRKs with distinct outcomes. The chemokine receptor CCR7 has two endogenous agonists, CCL19 and CCL21. Whereas both agonists activate G proteins and induce recruitment of βarr2 and βarr2-dependent ERK activation, only CCL19 causes redistribution of βarr2 to endosomes and CCR7 desensitization (29). CCL19 induces GRK3- and 6-dependent phosphorylation of CCR7, whereas CCL21 activates GRK6 only, which may explain the different functional effects of these agonists.
Fig. 2.
βarrs recruit signaling complexes to endosomes. Complexes can include activators and inhibitors of signaling. Overlapping symbols specify direct interaction with βarr.
By recruiting receptors and MAPK to endosomes, βarrs can determine the subcellular location and function of activated ERKs. As is the case for RTKs, receptors in endosomes may activate signals that differ from those originating from G proteins at the plasma membrane, resulting in distinct physiological responses. These distinct mechanisms of signaling have been evaluated by disrupting βarr or G proteins, by studying mutant receptors that are unable to interact with βarrs, or by using agonists that selectively activate particular pathways. PAR2 and AT1AR coupling to Gαq activates conventional isoforms of PKC and stimulates rapid Ras-mediated activation of the Raf-1/MEK1/ERK1/2 module; activated ERK1/2 translocate to the nucleus to regulate proliferation and transcription (23, 30). PAR2 and AT1AR also activate ERK1/2 by βarr-dependent mechanisms. PAR2 activation induces assembly of a signaling complex comprising PAR2/βarr/Raf-1/MEK1/ERK1/2, which retains ERK activity in the cytosol rather than the nucleus (23). A PAR2 mutant that was unable to interact with βarrs failed to promote formation of this complex or cause cytosolic retention of activated ERK1/2, which instead translocated to the nucleus to promote proliferation. βarr-dependent mechanisms mediate delayed and sustained activation of ERK1/2 that accumulates in endosomes with AT1AR and βarrs (30). V2R activation also results in βarr-dependent activation of ERK1/2, which are mostly retained in the cytosol (26). Substance P (SP) also induces the formation of a signaling complex comprising SP/NK1R/βarr/Src/MEK1/ERK1/2 (24). When βarr1 is fused to the NK1R C terminus, the receptor is constitutively associated with a c-Raf/MEK1/2/ERK1/2 complex in endosomes, leading to robust activation of cytosolic but not nuclear ERK1/2 (31).
βarrs similarly participate in activation of the JNK MAPK cascade, a regulator of stress-induced apoptosis, cell survival, and morphogenesis. Stimulation of AT1AR promotes assembly of a signaling complex in endosomes comprising βarr2, the upstream kinases MAP kinase kinase (MKK4), and apoptosis signaling kinase (ASK1), and active JNK3 (32, 33). Whereas JNK3 and ASK1 directly interact with βarr2, MKK4, although part of the complex, interacts indirectly with βarr2. As is the case with ERK1/2, βarr2 retains activated JNK3 in the cytosol. Notably, the complex includes MAP kinase phosphatase 7 (MKP7), which interacts with βarr2 and can dephosphorylate JNK3 (34) (Fig. 2). Thus, the complex contains machinery to both initiate and terminate JNK3 activation. The p38 MAPK mediates transcriptional responses to stress and inflammation. βarrs are necessary for p38-dependent signaling of the chemokine receptor CXCR4 (35) and the κ-opioid receptor (36).
βarrs also control PI3K, a regulator of cell growth, movement, and apoptosis. Activation of PAR2 promotes interaction of βarrs and PI3K, which inhibits PI3K catalytic activity (37, 38). This mechanism opposes PAR2-induced stimulation of PI3K, which is mediated by Gαq. The result of these opposing mechanisms depends on the level of βarr expression, with PI3K inhibition predominating in cells that highly express βarrs.
In keeping with inhibition of PI3K, βarrs can also inhibit Akt, a downstream target of PI3K that controls transcription, apoptosis, and the cell-cycle (Fig. 2). Phosphatidylinositol-dependent kinase 1 and target of rapamycin complex 2 kinase phosphorylate and activate Akt, whereas protein phosphatase 2A (PP2A) dephosphorylates and inactivates Akt. Dopamine 2 receptor (D2R) stimulation induces formation of a βarr2/Akt/PP2A complex, identified in striatal extracts by pull-down assays (39, 40). Whether this complex forms at the plasma membrane or endosomes is unknown. Sustained stimulation of D2R in the mouse striatum inactivates Akt by a βarr2-dependent mechanism (39, 40). This mechanism is another example, along with regulation of JNK3, of βarr recruiting both activators and inhibitors (PP2A, MKP7) to signaling complexes. Ghrelin, a regulator of food intake and metabolism, also activates Akt, here in a biphasic fashion with an early Gi/o-dependent pathway and a late βarr-dependent pathway involving recruitment of Src and Akt (41).
Despite the focus on βarr-mediated signaling in endosomes, G proteins can also signal from endosomes. In the mating pheromone response pathway of Saccharomyces cerevisiae, the GPCR Ste2 transduces signals to secreted α-factor, which were thought to depend on the plasma membrane bound Gβγ subunits. However, Gα subunits translocate to endosomes to stimulate PI3K activity (42). Gβγ subunits can also mediate signals from endosomes in mammalian cells (43). Gβγ interact with Rab11a, lysophosphatidic acid promotes association of Gβγ, PI3K, and Akt with Rab11a-positive endosomes in HEK cells. Disruption of these associations attenuates effects of lysophosphatidic acid on cell survival and proliferation, suggesting that endosomal signaling of G proteins is functionally important.
Toll-Like Receptors (TLRs).
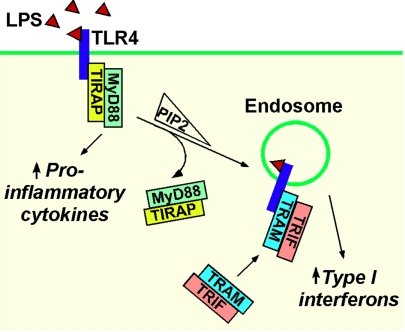
Endosomes are a platform for signaling of TLRs, major mediators of innate immunity. TLR9 binding to its ligand CpG oligodeoxynucleotide (CpG-A) induces IFN by activating the transcription factor IRF-7 via the adaptor protein myeloid differentiation primary response gene 88 (MyD88) (44). However, this response only occurs in the plasmacytoid subset of dendritic cells. The reason for this cell-type specificity has been attributed to the ability of plasmacytoid dendritic cells to retain the TLR9-bound CpG-A and MyD88-IRF-7 complex in endosomes for long periods, which is required for a robust IFN response. In conventional dendritic cells, CpG-A is rapidly degraded in lysosomes. Inducing endosomal retention of CpG-A in conventional dendritic cells by using a cationic lipid activates the TLR9-MyD88-IRF-7 pathway, causing IFN production. Endosomes also play a vital role in the antiviral responses triggered by double-stranded RNA binding to TLR3 (45). Stimulation of dendritic cells with double-stranded RNA induces redistribution of TLR3 from the endoplasmic reticulum to endosomes. TLR3 and c-Src accumulate in endosomes containing double-stranded RNA, and c-Src is essential for antiviral signaling. Endosomes are a site for the coordinated activation of signaling pathways by TLR4, a receptor for lipopolysaccharide from bacterial cell walls (46). TLR4 activates two pathways: the Toll-interleukin 1 receptor domain-containing adaptor protein (TIRAP)-MyD88 pathway that induces cytokines, and the Toll-receptor-associated molecule (TRAM)–Toll-receptor-associated activator of interferon (TRIF) pathway that induces IFN. Inhibiting TLR4 endocytosis disrupts the TRAM–TRIF pathway, and localization of TRAM to endosomes is necessary for TLR4 signaling (46). Thus, TIRAP-MyD88 signaling is initiated by TLR4 at the plasma membrane, whereas TRAM–TRIF signaling is initiated by endocytosed TLR4. The switch between the two pathways may be caused by depletion of phosphatidylinositol-4,5-bisphosphate from the membrane during endocytosis, which releases the TIRAP-MyD88 complex from TLR4, thereby enabling TLR4 to interact with TRAM–TRIF in endosomes (Fig. 3).
Fig. 3.
Endosomes are key to TLR signaling. The figure depicts the requirement of TLR4 internalization to endosomes for the exchange of the TIRAP-MyD88 signaling complex with the TRAM–TRIF signaling complex.
Other Mechanisms of Endosomal Signaling.
Although many signal-transduction cascades are propagated by phosphorylation, signaling can also require proteolysis, which may activate a substrate or allow a product to translocate to a different cellular location to exert its effect. Proteases are essential for Notch signaling, a regulator of development. Notch receptors exist in the plasma membrane as heterodimers composed of the Notch extracellular domain and the membrane-anchored intracellular domain. Ligand binding results in cleavage of the membrane-anchored intracellular domain at an extracellular site by metalloproteases (Fig. S1). Notch then undergoes intramembrane cleavage of the membrane-anchored intracellular domain by γ-secretase to liberate the Notch intracellular domain, which translocates to the nucleus to regulate gene transcription (Fig. S1). Although the role of endocytosis in Notch cleavage and signaling is poorly understood, observations of Drosophila melanogaster mutants with defects in the endocytic pathway indicate that entry of Notch into early endosomes is required for efficient γ-secretase-mediated cleavage of Notch and Notch signaling (47). Alterations in Notch trafficking in endosomes may underlie developmental abnormalities that are related to defects in Notch signaling.
Although mostly studied in metazoans, endosomes are a site for receptor signaling in plants. Increasing the endosomal localization of the steroid receptor BRI1 in Arabidopsis thaliana by overexpression enhances transcriptional signaling and genomic responses, suggesting that in plants, as in animal cells, endosomes play an essential role in receptor signaling (48).
Endosomal Signaling Is Tightly Controlled.
Receptor signaling at the plasma membrane is precisely regulated by mechanisms that control agonist availability, receptor coupling to signal-transduction machinery, and subcellular distribution of signaling components. Defects in these mechanisms can cause disease, and drugs that target these mechanisms have powerful effects. Considerably less is known about the mechanisms that regulate receptor signaling from endosomes.
Endosomal Proteolysis Attenuates Signaling.
The mechanisms that terminate endosomal signaling are not fully understood, and it is uncertain whether endosomal signaling ceases before receptor degradation or recycling. The EGFR is substantially phosphorylated in endosomes but is dephosphorylated and deactivated before trafficking to lysosomes (49). EGFR dephosphorylation coincides with a loss of EGF from endosomes, suggesting that ligand dissociation from the internalized receptor attenuates EGFR signaling. This conclusion is supported by the observations that cathepsin B in soluble endosome extracts degrades EGF and that cathepsin B inhibition enhances EGFR phosphorylation in endosomes (50). The importance of ligand dissociation in terminating EGFR signaling is further illustrated by comparing signaling of TGF-α and EGF. Whereas both agonists bind EGFR with equal affinity at the plasma membrane where the pH is neutral, TGF-α more readily dissociates from EGFR at the acidic pH of endosomes and exhibits diminished EGFR mitogenic signaling (51). Thus, EGFR signaling in endosomes is regulated by the rate of ligand dissociation in the acidic endosomal environment and subsequent degradation.
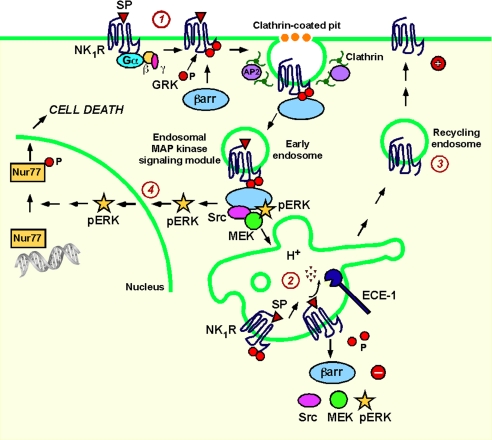
Ligand dissociation from GPCRs and subsequent degradation by endosomal peptidases also controls trafficking and signaling of neuropeptide receptors (Fig. 4). Endothelin-converting enzyme 1 (ECE-1) is a membrane-associated metalloendopeptidase that shuttles between plasma and endosomal membranes (52). ECE-1 rapidly degrades SP, calcitonin gene-related peptide (CGRP) and somatostatin in endosomes to disrupt the peptide-receptor-βarr complex, allowing βarrs to return to the cytoplasm and receptors, freed from βarrs, to recycle and resensitize (52–54). This mechanism promotes recycling and resensitization of receptors for SP (NK1R), CGRP, and somatostatin (somatostatin receptor 2A). For these class B GPCRs, dissociation from βarrs in endosomes is necessary for recycling and resensitization. ECE-1 does not regulate resensitization of the class B AT1AR because angiotensin II is not an ECE-1 substrate. Similarly, ECE-1 does not regulate recycling and resensitization of the bradykinin B2 receptor, which transiently interacts with βarrs and rapidly recycles and resensitizes. Neuropeptide degradation in endosomes also regulates βarr-mediated MAPK signaling. Inhibition of ECE-1 or treatment with the vacuolar H+-ATPAse inhibitor bafilomycin A1 causes retention of the SP/NK1R/βarr/MEK1/ERK1/2 complex in endosomes and sustained ERK1/2 activation (55).
Fig. 4.
Endosomal ECE-1 regulates SP-induced ERK activation and cell death. (1) SP binding to the NK1R leads to recruitment of βarr to the receptor, assembly of a MAPK signalosome, and ERK1/2 activation. (2) Degradation of SP by ECE-1 in acidified endosomes disrupts the SP/NK1R/βarr/MAPK signalosome. (3) NK1R recycles to the plasma membrane for resensitization. (4) Inhibiting ECE-1 activity causes sustained ERK1/2 activation and SP-induced cell death.
Deubiquitinating Proteases (DUBs) Control Endosomal Trafficking and Perhaps Signaling.
Attachment of ubiquitin to lysines of target proteins is a critical determinant of their subcellular distribution and function. Agonist-induced ubiquitination of RTKs and GPCRs at the plasma membrane and possibly in endosomes controls receptor trafficking throughout the endosomal system. For some receptors, the ubiquitin E3 ligase that mediates agonist-stimulated ubiquitination is known. c-Cbl ubiquitinates the EGFR (56) and PAR2 (57), AIP4 ubiquitinates CXCR4 (58) and δ-opioid receptor (59), and Nedd4 ubiquitinates the β2AR (60). β2AR agonists also promote interaction between βarr2 and the E3 ligase Mdm2, which ubiquitinates βarr2 (61). The importance of ubiquitination for trafficking varies between receptors. Ubiquitination is necessary for endocytosis of the yeast GPCRs Ste2p and Ste3p (62, 63). However, ubiquitination-defective mutants of β2AR, CXCR4 and PAR2 internalize normally but are instead retained in early endosomes, escaping lysosomal trafficking and degradation (57, 61, 64). Similarly, ubiquitination is not necessary for EGFR endocytosis because mutation of lysine residues in the EGFR kinase domain does not impair internalization (65) and EGFR internalization is unaltered in fibroblasts lacking c-Cbl (66). Mdm2-mediated ubiquitination of βarr is required for endocytosis of the β2AR and perhaps other GPCRs (61, 67) and is necessary for high-affinity interactions of βarr and GPCRs that determine receptor endocytosis and downstream signal transduction (68).
Before receptors are incorporated into the intralumenal vesicles of multivesicular bodies en route to lysosomes, they are deubiquitinated, which maintains levels of free ubiquitin. Two endosomal DUBs, associated molecule with the Src homology 3 (SH3) domain of signal transducing adapter molecule (STAM) (AMSH) and ubiquitin-specific protease Y (UBPY or USP8), control EGFR deubiquitination and postendocytic trafficking. AMSH and UBPY interact directly with STAM through a common binding site within its SH3 domain (69, 70). Whereas c-Cbl promotes lysosomal degradation of the EGFR (56), AMSH opposes c-Cbl action and promotes EGFR recycling (71), and UBPY is required for lysosomal sorting and degradation of EGFR (72–74). It is unclear whether AMSH and UBPY act in opposition or in a coordinated fashion (75). AMSH and UBPY also deubiquitinate δ-opioid receptor and PAR2 and are required for lysosomal trafficking and degradation of these receptors (59, 76). USP33 and USP20 deubiquitinate the β2AR, which inhibits receptor degradation and promotes recycling from late endosomes (77).
Lysosomal degradation irrevocably terminates receptor signaling, and disruption of this process would be expected to prolong signaling of receptors at the plasma membrane or in endosomes. Fusing the C-tail of the recycling NK1R to PAR1, which normally traffics to lysosomes, generates a receptor that recycles and continues to signal at the plasma membrane (78). Ubiquitination-defective PAR2 mutants also recycle and resensitize at the cell surface (57). DUBs regulate recycling and resensitization of the β2AR. Knockdown of both USP33 and USP20 inhibits β2AR recycling and resensitization of cAMP responses but increases agonist-induced β2AR ubiquitination, lysosomal trafficking and degradation of the receptor (77). Little is known about the role of endosomal DUBs in controlling signaling of endocytosed receptors. However, disruption of AMSH and UBPY does not affect the association of PAR2 with βarrs in endosomes and does not influence the duration or magnitude of PAR2-induced activation of ERK1/2 (76). In contrast, the balance of βarr ubiquitination and deubiquitination regulates the association of βarr with GPCRs and βarr-dependent ERK1/2 activation (79). Whereas the E3 ligase Mdm2 mediates agonist-induced ubiquitination of βarr2, the DUB USP33 interacts with and deubiquitinates βarr2. Overexpression of Mdm2 or knockdown of USP33 stabilizes the endosomal interaction of βarr2 with β2AR (transiently interacts with βarrs), leading to prolonged and enhanced ERK1/2 activation. Conversely, overexpression of USP33 destabilizes the interaction of βarr2 with V2R (stably interacts with βarrs in endosomes), which attenuates ERK1/2 activation. Thus, βarr ubiquitination and deubiquitination regulate stability of the βarr MAPK signalosome to control the duration of ERK1/2 signaling.
The Mechanism of Endocytosis Specifies the Outcome of Endosomal Signals.
Differences in the mechanism of endocytosis of Trk and EGFR explain the quandary that whereas Trk promotes neuronal differentiation and survival, other growth factors do not (80). NGF induces endocytosis of Trk in PC12 cells by a mechanism involving the Rho GTPase Rac and the trafficking protein Pincher, termed “macroendocytosis”. This results in accumulation of Trk in immature multivesicular bodies containing Rab5 but lacking the late endosome protein Rab7. In contrast, EGF stimulates clathrin-dependent endocytosis of EGFR into Rab5 endosomes, with rapid exchange of Rab7 for Rab5 and transition to late endosomes and lysosomes. Whereas NGF/Trk induce sustained ERK1/2 activation, EGF/EGFR transiently activate ERK1/2 because EGFR is rapidly degraded. Thus, endosomes provide a specialized NGF/TrkA platform for sustained signaling required for neuronal survival.
Receptor Transit Through the Endosomal Network Refines Signals.
The importance of endosomal transit for signaling is illustrated by the finding that disruption of trafficking of c-Met (hepatocyte growth factor receptor) from peripheral to perinuclear endosomes inhibits nuclear accumulation of the transcription factor STAT3 (81). Similarly, redirecting endosomes containing EGFR between peripheral and perinuclear locations affects EGFR degradation, MAPK activation and transcription (82).
Physiological Outcomes of Endosomal Signaling.
The signals that emanate from diverse families of receptors in endosomes control essential processes of growth, differentiation, survival, inflammation, and immunity. However, most information about endosomal signaling derives from studies of cell lines that often overexpress receptors and signaling components at supraphysiological levels. Although such studies provide important mechanistic insights, the physiological outcomes of endosomal signaling in functionally important cells or in intact animals are not fully understood.
The importance of endosomes to the physiological outcomes of RTK signaling is illustrated by neurotrophin signaling to sensory nerves (Fig. 1). Neurotrophins from innervated tissues stimulate endocytosis of TrkA in axon terminals, and endosomes convey signals to the nucleus to induce transcriptional events that promote neuronal growth. Disruption of this process enhances lysosomal degradation of TrkA, inhibits NGF-induced ERK activity in endosomes, and attenuates effects of NGF on gene expression and neurite outgrowth (83).
βarrs couple GPCRs to multiple signaling pathways and may therefore mediate many physiological responses (Fig. 2). For some receptors, βarrs retain activated ERK1/2 in endosomes or the cytosol, thereby restricting nuclear translocation and effects on transcription and proliferation. The precise downstream targets of βarr-activated ERK1/2 are not fully characterized. However, PAR2 controls tight junction assembly and paracellular permeability of colonocytes by βarr-dependent activation of ERK1/2, with implications for intestinal inflammatory diseases characterized by bacterial and macromolecule translocation from the lumen (84). A PAR2/βarr/ERK1/2 complex is enriched in pseudopodia of migrating cells and is required for cytoskeletal reorganization, pseudopodia extension, and chemotaxis (85). This mechanism may mediate the migration of breast cancer cells induced by the release of trypsin and autocrine activation of PAR2 (86). A SP/NK1R/βarr/Src/ERK1/2 complex is required for the proliferative and antiapoptotic actions of the SP (24). Inhibition of ECE-1 stabilizes this complex and results in markedly sustained ERK1/2 signaling, where activated ERK1/2 translocates to the nucleus and activates the death receptor Nur77, causing neurodegeneration (55).
The physiological consequences of βarr signaling have been characterized in βarr-deficient mice (87). βarr2-deficient mice fail to develop antinociceptive tolerance to morphine due to diminished βarr-mediated desensitization of the μ-opioid receptor (88). However, other effects of morphine (for example, induction of constipation) are diminished in these mice, suggesting a role for βarr2 in μ-opioid receptor signaling in enteric neurons by mechanisms that remain to be explored (87). βarr2 deficiency impedes formation of the βarr2/Akt/PP2A complex, disrupts the effects of dopamine on Akt activity, and abrogates the behavioral effects of dopaminergic drugs without affecting G protein signaling (39, 40). Given the importance of dopamine in locomotion, reward, and affect, and its involvement in Parkinson's disease, Huntington's disease, and schizophrenia, this mechanism of βarr2-dependent signaling could be of considerable importance. Although studies of βarr2-deficient mice illustrate the physiological importance of βarr2 for opioid and dopamine signaling, it remains to be determined whether this role depends on formation of endosomal signaling complexes.
The use of agonists that selectively activate G protein- or βarr-dependent signaling (biased agonists) has provided further insight into the physiological importance of βarr signaling. An analogue of angiotensin II, SII-angiotensin, is a specific agonist of the βarr-dependent pathway of AT1R signaling and does not activate G protein-dependent signaling. This analogue induces proliferation, protein synthesis, and antiapoptotic signals in vascular smooth muscle cells by βarr and ERK1/2-dependent processes, demonstrating the functional relevance of this pathway (89–91).
βarrs also regulate signaling in primary cilia, hair-like extensions of cells that detect environmental stimuli (92). The GPCR Smoothened is a component of the Hedgehog signaling pathway that is essential for development, stem cell function, and cancer. Translocation of Smoothened to cilia is necessary for regulation of gene transcription. βarrs couple Smoothened to the kinesin motor protein Kif3A and thereby promote the translocation of this complex to primary cilia where Smoothened regulates transcription. Because other GPCRs are found in cilia (93, 94), βarrs may control the location and activity of several signaling pathways in this location.
Endosomal mechanisms also regulate signaling of TLRs (Fig. 3) and Notch (Fig. S1), and may therefore be essential for innate immunity and development.
Targeting Endosomal Signaling: New Opportunities for Therapy?
Many drugs target receptor signaling at the cell surface. Given the importance of signals that originate from receptors in endosomes, which are sometimes quite distinct from those that derive from cell surface receptors, therapies specifically directed to endosomal signals will likely offer a novel and important pharmacological approach to disease. The concept that receptors can signal differently at the cell surface and in endosomes emphasizes the importance of screening multiple pathways during drug discovery. Specific inhibition of endosomal signaling may be achieved by targeting drugs to disrupt only endosomal signaling pathways or by designing drugs that are selectively delivered to endosomes. Both strategies have been successful.
The observation that therapeutic actions of lithium depend on disrupting βarr function illustrates the feasibility of targeting βarr. Lithium is used to treat certain psychiatric disorders, including schizophrenia, bipolar disorder, and depression. Lithium disrupts the ability of βarr2 to assemble the Akt/PP2A complex, which mediates some of its therapeutic actions (95). Importantly, lithium does not disrupt other actions of βarr2 at the plasma membrane and in endosomes, including βarr2 interaction with receptors, clathrin, and Raf-1, βarr-mediated desensitization of receptors, and βarr-dependent activation of ERK1/2. Whereas chronic administration of lithium to WT mice has effects on tail suspension and dark–light emergence behaviors, it is completely without effect in mice deficient in βarr2. Thus, lithium specifically targets βarr-dependent interactions of Akt/PP2A to exert its effects on behavior. Given that distinct domains of βarr interact with various proteins at plasma and endosomal membranes, it should be possible to target specific domains to influence particular actions of βarrs.
The differential effects of drugs on G protein- and βarr-mediated signaling can explain some of their beneficial and detrimental effects. Deletion of βarr2 enhances morphine analgesia due to diminished desensitization of the μ-opioid receptor but reduces the detrimental side effect of constipation (87, 88). Deletion of βarr1 does not affect the beneficial actions of nicotinic acid on lowering circulating triglycerides and raising high-density lipoproteins but attenuates the side effect of flushing, which is associated with burning and itching of the skin (96).
Drugs that target mechanisms that regulate endosomal signaling may also be useful. Endosomal ECE-1 is a target for disorders of inflammation and pain. By degrading SP and CGRP in acidified endosomes, ECE-1 promotes recycling and resensitization of receptors that mediate neurogenic inflammation and pain (52, 53). An ECE-1 inhibitor prevents resensitization of SP-induced plasma extravasation, providing evidence for an antiinflammatory effect of ECE-1 inhibitors (53, 97). Thus, in the case of βarrs and ECE-1, therapies that specifically target GPCR signaling in endosomes without affecting signaling at the plasma membrane are a viable and novel pharmacological strategy.
Although most drug development focuses on drug interaction with active sites of target proteins, strategies that target drugs to specific subcellular regions can be effective. Endosomal β-secretase is critical for formation of β-amyloid protein and is a therapeutic target for Alzheimer's disease. By synthesizing a β-secretase inhibitor coupled to a sterol moiety, an inhibitor was developed that concentrated the inhibitor at the endosomal membrane (98). This “endosomally targeted” inhibitor was more effective than the free inhibitor. Similar strategies could be used to target other drugs to the endosomal system.
Concluding Remarks and Future Directions.
Endocytosis was originally viewed as a mechanism that delivered receptors to degradatory or recycling pathways. It is now clear that diverse families of receptors cells can signal from the endosomal network to control essential cellular responses. These endosomal signals differ from those originating from receptors at the plasma membrane, both mechanistically and temporally, and endosomal signaling is tightly regulated by mechanisms that are not fully understood.
A major challenge is to understand the physiological relevance of endosomal signaling: Why do receptors signal from endosomes? and Do endosomes transmit unique and functionally important signals? Studies of model systems, typically cell lines overexpressing receptors, have provided a wealth of information about the potential mechanisms of receptor signaling in endosomes, but whether they faithfully replicate signaling in complex, highly differentiated cells such as neurons is not completely clear. Difficulties in studying endosomal signaling in cells in primary culture or in intact animals include detection of signaling molecules, which are often expressed at low levels, and discrimination between plasma membrane and endosomal signaling events. Promising approaches include studies of mice expressing fluorescently tagged receptors (99) or lacking key endosomal signaling proteins (87), siRNA knockdown of signaling intermediates in neurons (18), use of innovative methods to isolate endosomal signaling complexes (4, 55), and studies of agonists that selectively activate endosomal rather than plasma membrane signaling pathways (89–91).
The knowledge that receptors can signal in endosomes by mechanisms that are distinct from those at the plasma membrane raises the possibility of developing drugs that specifically target endosomal signaling. This strategy may offer improved selectivity with fewer side effects than targeting more proximal steps of receptor signaling. Indeed, drugs that either target endosomal signaling events (53, 95–97) or that are specifically delivered to endosomes (98) have powerful effects. The challenge will be to design drugs that target endosomal signaling relevant to disease. Given the success of drugs that target signals generated at the plasma membrane, this challenge is likely to be worthwhile.
Supplementary Material
Acknowledgments.
This work was supported by National Institute of Health Grants DK39957, DK43207, and DK57840 (to N.W.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0906541106/DCSupplemental.
References
- 1.Di Guglielmo GM, Baass PC, Ou WJ, Posner BI, Bergeron JJ. Compartmentalization of SHC, GRB2 and mSOS, and hyperphosphorylation of Raf-1 by EGF but not insulin in liver parenchyma. EMBO J. 1994;13:4269–4277. doi: 10.1002/j.1460-2075.1994.tb06747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kublaoui B, Lee J, Pilch PF. Dynamics of signaling during insulin-stimulated endocytosis of its receptor in adipocytes. J Biol Chem. 1995;270:59–65. doi: 10.1074/jbc.270.1.59. [DOI] [PubMed] [Google Scholar]
- 3.Kelly KL, Ruderman NB. Insulin-stimulated phosphatidylinositol 3-kinase. Association with a 185-kDa tyrosine-phosphorylated protein (IRS-1) and localization in a low density membrane vesicle. J Biol Chem. 1993;268:4391–4398. [PubMed] [Google Scholar]
- 4.Li HS, Stolz DB, Romero G. Characteriza-tion of endocytic vesicles using magnetic micro-beads coated with signalling ligands. Traffic. 2005;6:324–334. doi: 10.1111/j.1600-0854.2005.00274.x. [DOI] [PubMed] [Google Scholar]
- 5.Grimes ML, et al. Endocytosis of activated TrkA: Evidence that nerve growth factor induces formation of signaling endosomes. J Neurosci. 1996;16:7950–7964. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delcroix JD, et al. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron. 2003;39:69–84. doi: 10.1016/s0896-6273(03)00397-0. [DOI] [PubMed] [Google Scholar]
- 7.Vieira AV, Lamaze C, Schmid SL. Control of EGF receptor signaling by clathrin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 8.Kranenburg O, Verlaan I, Moolenaar WH. Dynamin is required for the activation of mitogen-activated protein (MAP) kinase by MAP kinase kinase. J Biol Chem. 1999;274:35301–35304. doi: 10.1074/jbc.274.50.35301. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Pennock S, Chen X, Wang Z. Endosomal signaling of epidermal growth factor receptor stimulates signal transduction pathways leading to cell survival. Mol Cell Biol. 2002;22:7279–7290. doi: 10.1128/MCB.22.20.7279-7290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt MH, Furnari FB, Cavenee WK, Bogler O. Epidermal growth factor receptor signaling intensity determines intracellular protein interactions, ubiquitination, and internalization. Proc Natl Acad Sci USA. 2003;100:6505–6510. doi: 10.1073/pnas.1031790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, Ahn S, Guo R, Daaka Y. Regulation of epidermal growth factor receptor internalization by G protein-coupled receptors. Biochemistry. 2003;42:2887–2894. doi: 10.1021/bi026942t. [DOI] [PubMed] [Google Scholar]
- 12.Bevan AP, et al. Selective activation of the rat hepatic endosomal insulin receptor kinase. Role for the endosome in insulin signaling. J Biol Chem. 1995;270:10784–10791. doi: 10.1074/jbc.270.18.10784. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Pennock SD, Chen X, Kazlauskas A, Wang Z. Platelet-derived growth factor receptor-mediated signal transduction from endosomes. J Biol Chem. 2004;279:8038–8046. doi: 10.1074/jbc.M311494200. [DOI] [PubMed] [Google Scholar]
- 14.Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174:593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui B, et al. One at a time, live tracking of NGF axonal transport using quantum dots. Proc Natl Acad Sci USA. 2007;104:13666–13671. doi: 10.1073/pnas.0706192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu C, Lai CF, Mobley WC. Nerve growth factor activates persistent Rap1 signaling in endosomes. J Neurosci. 2001;21:5406–5416. doi: 10.1523/JNEUROSCI.21-15-05406.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watson FL, et al. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat Neurosci. 2001;4:981–988. doi: 10.1038/nn720. [DOI] [PubMed] [Google Scholar]
- 18.Pazyra-Murphy MF, et al. A retrograde neuronal survival response: Target-derived neurotrophins regulate MEF2D and bcl-w. J Neurosci. 2009;29:6700–6709. doi: 10.1523/JNEUROSCI.0233-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 20.Defea K. Beta-arrestins and heterotrimeric G-proteins: Collaborators and competitors in signal transduction. Br J Pharmacol. 2008;153(Suppl 1):S298–S309. doi: 10.1038/sj.bjp.0707508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daaka Y, et al. Essential role for G protein-coupled receptor endocytosis in the activation of mitogen-activated protein kinase. J Biol Chem. 1998;273:685–688. doi: 10.1074/jbc.273.2.685. [DOI] [PubMed] [Google Scholar]
- 22.Luttrell LM, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 23.DeFea KA, et al. beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeFea KA, et al. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta -arrestin-dependent scaffolding complex. Proc Natl Acad Sci USA. 2000;97:11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luttrell LM, et al. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci USA. 2001;98:2449–2454. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tohgo A, et al. The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J Biol Chem. 2003;278:6258–6267. doi: 10.1074/jbc.M212231200. [DOI] [PubMed] [Google Scholar]
- 27.Simaan M, Bedard-Goulet S, Fessart D, Gratton JP, Laporte SA. Dissociation of beta-arrestin from internalized bradykinin B2 receptor is necessary for receptor recycling and resensitization. Cell Signal. 2005;17:1074–1083. doi: 10.1016/j.cellsig.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, et al. Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci USA. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zidar DA, Violin JD, Whalen EJ, Lefkowitz RJ. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc Natl Acad Sci USA. 2009;106:9649–9654. doi: 10.1073/pnas.0904361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- 31.Jafri F, et al. Constitutive ERK1/2 activation by a chimeric neurokinin 1 receptor-beta-arrestin1 fusion protein. Probing the composition and function of the G protein-coupled receptor “signalsome”. J Biol Chem. 2006;281:19346–19357. doi: 10.1074/jbc.M512643200. [DOI] [PubMed] [Google Scholar]
- 32.McDonald PH, et al. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- 33.Miller WE, et al. Identification of a motif in the carboxyl terminus of beta -arrestin2 responsible for activation of JNK3. J Biol Chem. 2001;276:27770–27777. doi: 10.1074/jbc.M102264200. [DOI] [PubMed] [Google Scholar]
- 34.Willoughby EA, Collins MK. Dynamic interaction between the dual specificity phosphatase MKP7 and the JNK3 scaffold protein beta-arrestin 2. J Biol Chem. 2005;280:25651–25658. doi: 10.1074/jbc.M501926200. [DOI] [PubMed] [Google Scholar]
- 35.Sun Y, Cheng Z, Ma L, Pei G. Beta-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem. 2002;277:49212–49219. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- 36.Bruchas MR, Macey TA, Lowe JD, Chavkin C. Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J Biol Chem. 2006;281:18081–18089. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang P, DeFea KA. Protease-activated receptor-2 simultaneously directs beta-arrestin-1-dependent inhibition and Galphaq-dependent activation of phosphatidylinositol 3-kinase. Biochemistry. 2006;45:9374–9385. doi: 10.1021/bi0602617. [DOI] [PubMed] [Google Scholar]
- 38.Wang P, Kumar P, Wang C, Defea KA. Differential regulation of class IA phosphoinositide 3-kinase catalytic subunits p110 alpha and beta by protease-activated receptor 2 and beta-arrestins. Biochem J. 2007;408:221–230. doi: 10.1042/BJ20070483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beaulieu JM, et al. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 40.Beaulieu JM, et al. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci. 2007;27:881–885. doi: 10.1523/JNEUROSCI.5074-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lodeiro M, Theodoropoulou M, Pardo M, Casanueva FF, Camina JP. c-Src regulates Akt signaling in response to ghrelin via beta-arrestin signaling-independent and -dependent mechanisms. PLoS ONE. 2009;4:e4686. doi: 10.1371/journal.pone.0004686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slessareva JE, Routt SM, Temple B, Bankaitis VA, Dohlman HG. Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein alpha subunit at the endosome. Cell. 2006;126:191–203. doi: 10.1016/j.cell.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 43.Garcia-Regalado A, et al. G protein-coupled receptor-promoted trafficking of Gbeta1gamma2 leads to AKT activation at endosomes via a mechanism mediated by Gbeta1gamma2-Rab11a interaction. Mol Biol Cell. 2008;19:4188–4200. doi: 10.1091/mbc.E07-10-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Honda K, et al. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434:1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 45.Johnsen IB, et al. Toll-like receptor 3 associates with c-Src tyrosine kinase on endosomes to initiate antiviral signaling. EMBO J. 2006;25:3335–3346. doi: 10.1038/sj.emboj.7601222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kagan JC, et al. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaccari T, Lu H, Kanwar R, Fortini ME, Bilder D. Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J Cell Biol. 2008;180:755–762. doi: 10.1083/jcb.200708127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geldner N, Hyman DL, Wang X, Schumacher K, Chory J. Endosomal signaling of plant steroid receptor kinase BRI1. Genes Dev. 2007;21:1598–1602. doi: 10.1101/gad.1561307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burke P, Schooler K, Wiley HS. Regulation of epidermal growth factor receptor signaling by endocytosis and intracellular trafficking. Mol Biol Cell. 2001;12:1897–1910. doi: 10.1091/mbc.12.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Authier F, Metioui M, Bell AW, Mort JS. Negative regulation of epidermal growth factor signaling by selective proteolytic mechanisms in the endosome mediated by cathepsin B. J Biol Chem. 1999;274:33723–33731. doi: 10.1074/jbc.274.47.33723. [DOI] [PubMed] [Google Scholar]
- 51.Reddy CC, Wells A, Lauffenburger DA. Receptor-mediated effects on ligand availability influence relative mitogenic potencies of epidermal growth factor and transforming growth factor alpha. J Cell Physiol. 1996;166:512–522. doi: 10.1002/(SICI)1097-4652(199603)166:3<512::AID-JCP6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 52.Padilla BE, et al. Endothelin-converting enzyme-1 regulates endosomal sorting of calcitonin receptor-like receptor and beta-arrestins. J Cell Biol. 2007;179:981–997. doi: 10.1083/jcb.200704053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roosterman D, et al. Endothelin-converting enzyme 1 degrades neuropeptides in endosomes to control receptor recycling. Proc Natl Acad Sci USA. 2007;104:11838–11843. doi: 10.1073/pnas.0701910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roosterman D, et al. Endothelin-converting enzyme-1 degrades internalized somatostatin-14. Endocrinology. 2008;149:2200–2207. doi: 10.1210/en.2007-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cottrell GS, et al. Endosomal endothelin-converting enzyme-1: A regulator of beta-arrestin-dependent ERK signaling. J Biol Chem. 2009;284:22411–22425. doi: 10.1074/jbc.M109.026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levkowitz G, et al. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 1998;12:3663–3674. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacob C, et al. c-Cbl mediates ubiquitination, degradation, and down-regulation of human protease-activated receptor 2. J Biol Chem. 2005;280:16076–16087. doi: 10.1074/jbc.M500109200. [DOI] [PubMed] [Google Scholar]
- 58.Marchese A, et al. The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein-coupled receptor CXCR4. Dev Cell. 2003;5:709–722. doi: 10.1016/s1534-5807(03)00321-6. [DOI] [PubMed] [Google Scholar]
- 59.Hislop JN, Henry AG, Marchese A, von Zastrow M. Ubiquitination regulates proteolytic processing of G protein-coupled receptors after their sorting to lysosomes. J Biol Chem. 2009;284:19361–19370. doi: 10.1074/jbc.M109.001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shenoy SK, et al. Nedd4 mediates agonist-dependent ubiquitination, lysosomal targeting, and degradation of the beta2-adrenergic receptor. J Biol Chem. 2008;283:22166–22176. doi: 10.1074/jbc.M709668200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- 62.Hicke L, Riezman H. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell. 1996;84:277–287. doi: 10.1016/s0092-8674(00)80982-4. [DOI] [PubMed] [Google Scholar]
- 63.Roth AF, Davis NG. Ubiquitination of the yeast a-factor receptor. J Cell Biol. 1996;134:661–674. doi: 10.1083/jcb.134.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276:45509–45512. doi: 10.1074/jbc.C100527200. [DOI] [PubMed] [Google Scholar]
- 65.Huang F, Goh LK, Sorkin A. EGF receptor ubiquitination is not necessary for its internalization. Proc Natl Acad Sci USA. 2007;104:16904–16909. doi: 10.1073/pnas.0707416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duan L, et al. Cbl-mediated ubiquitinylation is required for lysosomal sorting of epidermal growth factor receptor but is dispensable for endocytosis. J Biol Chem. 2003;278:28950–28960. doi: 10.1074/jbc.M304474200. [DOI] [PubMed] [Google Scholar]
- 67.Shenoy SK, Lefkowitz RJ. Trafficking patterns of beta-arrestin and G protein-coupled receptors determined by the kinetics of beta-arrestin deubiquitination. J Biol Chem. 2003;278:14498–14506. doi: 10.1074/jbc.M209626200. [DOI] [PubMed] [Google Scholar]
- 68.Shenoy SK, et al. Ubiquitination of beta-arrestin links seven-transmembrane receptor endocytosis and ERK activation. J Biol Chem. 2007;282:29549–29562. doi: 10.1074/jbc.M700852200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tanaka N, et al. Possible involvement of a novel STAM-associated molecule “AMSH” in intracellular signal transduction mediated by cytokines. J Biol Chem. 1999;274:19129–19135. doi: 10.1074/jbc.274.27.19129. [DOI] [PubMed] [Google Scholar]
- 70.Kato M, Miyazawa K, Kitamura N. A deubiquitinating enzyme UBPY interacts with the Src homology 3 domain of Hrs-binding protein via a novel binding motif PX(V/I)(D/N)RXXKP. J Biol Chem. 2000;275:37481–37487. doi: 10.1074/jbc.M007251200. [DOI] [PubMed] [Google Scholar]
- 71.McCullough J, Clague MJ, Urbe S. AMSH is an endosome-associated ubiquitin isopeptidase. J Cell Biol. 2004;166:487–492. doi: 10.1083/jcb.200401141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Row PE, Prior IA, McCullough J, Clague MJ, Urbe S. The ubiquitin isopeptidase UBPY regulates endosomal ubiquitin dynamics and is essential for receptor down-regulation. J Biol Chem. 2006;281:12618–12624. doi: 10.1074/jbc.M512615200. [DOI] [PubMed] [Google Scholar]
- 73.Bowers K, et al. Degradation of endocytosed epidermal growth factor and virally ubiquitinated major histocompatibility complex class I is independent of mammalian ESCRTII. J Biol Chem. 2006;281:5094–5105. doi: 10.1074/jbc.M508632200. [DOI] [PubMed] [Google Scholar]
- 74.Mizuno E, et al. Regulation of epidermal growth factor receptor down-regulation by UBPY-mediated deubiquitination at endosomes. Mol Biol Cell. 2005;16:5163–5174. doi: 10.1091/mbc.E05-06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Urbe S, et al. Control of growth factor receptor dynamics by reversible ubiquitination. Biochem Soc Trans. 2006;34:754–756. doi: 10.1042/BST0340754. [DOI] [PubMed] [Google Scholar]
- 76.Hasdemir B, Murphy JE, Cottrell GS, Bunnett NW. Endosomal deubiquitinating enzymes control ubiquitination and down-regulation of protease-activated receptor 2. J Biol Chem. 2009;284:28453–28466. doi: 10.1074/jbc.M109.025692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berthouze M, Venkataramanan V, Li Y, Shenoy SK. The deubiquitinases USP33 and USP20 coordinate beta2 adrenergic receptor recycling and resensitization. EMBO J. 2009;28:1684–1696. doi: 10.1038/emboj.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trejo J, Hammes SR, Coughlin SR. Termination of signaling by protease-activated receptor-1 is linked to lysosomal sorting. Proc Natl Acad Sci USA. 1998;95:13698–13702. doi: 10.1073/pnas.95.23.13698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shenoy SK, et al. Beta-arrestin-dependent signaling and trafficking of 7-transmembrane receptors is reciprocally regulated by the deubiquitinase USP33 and the E3 ligase Mdm2. Proc Natl Acad Sci USA. 2009;106:6650–6655. doi: 10.1073/pnas.0901083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valdez G, et al. Trk-signaling endosomes are generated by Rac-dependent macroendocytosis. Proc Natl Acad Sci USA. 2007;104:12270–12275. doi: 10.1073/pnas.0702819104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kermorgant S, Parker PJ. Receptor trafficking controls weak signal delivery: A strategy used by c-Met for STAT3 nuclear accumulation. J Cell Biol. 2008;182:855–863. doi: 10.1083/jcb.200806076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taub N, Teis D, Ebner HL, Hess MW, Huber LA. Late endosomal traffic of the epidermal growth factor receptor ensures spatial and temporal fidelity of mitogen-activated protein kinase signaling. Mol Biol Cell. 2007;18:4698–4710. doi: 10.1091/mbc.E07-02-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wan J, et al. Endophilin B1 as a novel regulator of nerve growth factor/ TrkA trafficking and neurite outgrowth. J Neurosci. 2008;28:9002–9012. doi: 10.1523/JNEUROSCI.0767-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jacob C, et al. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. J Biol Chem. 2005;280:31936–31948. doi: 10.1074/jbc.M506338200. [DOI] [PubMed] [Google Scholar]
- 85.Ge L, Ly Y, Hollenberg M, DeFea K. A beta-arrestin-dependent scaffold is associated with prolonged MAPK activation in pseudopodia during protease-activated receptor-2-induced chemotaxis. J Biol Chem. 2003;278:34418–34426. doi: 10.1074/jbc.M300573200. [DOI] [PubMed] [Google Scholar]
- 86.Ge L, Shenoy SK, Lefkowitz RJ, DeFea K. Constitutive protease-activated receptor-2-mediated migration of MDA MB-231 breast cancer cells requires both beta-arrestin-1 and -2. J Biol Chem. 2004;279:55419–55424. doi: 10.1074/jbc.M410312200. [DOI] [PubMed] [Google Scholar]
- 87.Schmid CL, Bohn LM. Physiological and pharmacological implications of beta-arrestin regulation. Pharmacol Ther. 2009;121:285–293. doi: 10.1016/j.pharmthera.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- 89.Kim J, Ahn S, Rajagopal K, Lefkowitz RJ. Independent beta-arrestin2 and Gq/protein kinase Czeta pathways for ERK stimulated by angiotensin type 1A receptors in vascular smooth muscle cells converge on transactivation of the epidermal growth factor receptor. J Biol Chem. 2009;284:11953–11962. doi: 10.1074/jbc.M808176200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.DeWire SM, et al. Beta-arrestin-mediated signaling regulates protein synthesis. J Biol Chem. 2008;283:10611–10620. doi: 10.1074/jbc.M710515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ahn S, Kim J, Hara MR, Ren XR, Lefkowitz RJ. {beta}-Arrestin-2 Mediates Anti-apoptotic Signaling through Regulation of BAD Phosphorylation. J Biol Chem. 2009;284:8855–8865. doi: 10.1074/jbc.M808463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kovacs JJ, et al. Beta-arrestin-mediated localization of smoothened to the primary cilium. Science. 2008;320:1777–1781. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Handel M, et al. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience. 1999;89:909–926. doi: 10.1016/s0306-4522(98)00354-6. [DOI] [PubMed] [Google Scholar]
- 94.Brailov I, et al. Localization of 5-HT(6) receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Res. 2000;872:271–275. doi: 10.1016/s0006-8993(00)02519-1. [DOI] [PubMed] [Google Scholar]
- 95.Beaulieu JM, et al. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- 96.Walters RW, et al. beta-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J Clin Invest. 2009;119:1312–1321. doi: 10.1172/JCI36806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cattaruzza F, Cottrell GS, Vaksman N, Bunnett NW. Endothelin-converting enzyme 1 promotes re-sensitization of neurokinin 1 receptor-dependent neurogenic inflammation. Br J Pharmacol. 2009;156:730–739. doi: 10.1111/j.1476-5381.2008.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rajendran L, et al. Efficient inhibition of the Alzheimer's disease beta-secretase by membrane targeting. Science. 2008;320:520–523. doi: 10.1126/science.1156609. [DOI] [PubMed] [Google Scholar]
- 99.Scherrer G, et al. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci USA. 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.