Abstract
Aims
To assess the incidence and timing of atrial fibrillation (AF), describe antithrombotic therapy use, and evaluate the association of AF with 90 day mortality and other secondary clinical outcomes.
Methods and results
We studied 5745 ST-segment elevation myocardial infarction patients treated with primary percutaneous coronary intervention (PCI) in APEX-AMI. Approximately 11% had AF during hospitalization. Atrial fibrillation prevalence at baseline and at discharge was 4.8% [confidence interval (CI) 4.3–5.4%] and 2.5% (CI 2.1–2.9%), respectively. The proportion of 5466 patients without AF at baseline who developed new onset AF was 6.3% (CI 5.6–6.9%). This corresponded to 9.3 cases of new onset AF/1000 patient days at risk. New onset AF was independently associated with 90 day mortality [adjusted hazard ratio (HR) 1.81; 95% CI 1.06–3.09; P = 0.029] after accounting for baseline covariates and in-hospital procedures and complications. New onset AF was associated with shock (adjusted HR 3.81; 95% CI 1.88–7.70; P = 0.0002), congestive heart failure (adjusted HR 2.66; 95% CI 1.74–4.06; P < 0.0001), and stroke (adjusted HR 2.98; 95% CI 1.47–6.04; P = 0.0024) in models accounting for baseline covariates. Of AF patients, 55% did not receive oral anticoagulation therapy at discharge. Among patients with coronary stents, 5.1% were discharged on triple therapy. Patients at highest risk of stroke (CHADS2 score ≥2) were least likely to receive oral anticoagulation at discharge (39%). Warfarin use in patients with AF at discharge (43.4%) was associated with lower rates of 90 day mortality and stroke.
Conclusion
Atrial fibrillation prevalence at baseline and at discharge was 4.8 and 2.5%, respectively. The proportion of patients who developed new onset AF was 6.3%. New onset AF was independently associated with 90 day mortality and was a marker of adverse outcomes in patients undergoing primary PCI.
Keywords: Atrial fibrillation, Myocardial infarction, Antithrombotic therapy, Outcomes
Introduction
Atrial fibrillation (AF) is a common complication of myocardial infarction (MI) with an incidence of between 5 and 23%.1–4 It is associated with worse in-hospital and long-term outcomes and more in-hospital complications.4–7
Although antithrombotic therapy is important in the treatment of patients with both AF and MI,8–10 the combination of different classes of drugs such as aspirin, thienopyridines, and vitamin K antagonists increases the risk of bleeding.8 Guidelines recommend the use of aspirin, clopidogrel, and warfarin [with target international normalized ratio (INR) 2.0–2.5] after stenting for patients with acute coronary syndromes and with indication for oral anticoagulation for the shortest period of time.11,12 However, little is known about how these drugs are currently used and about the risks and benefits of using these drugs when AF occurs in patients with ST-segment elevation MI (STEMI) treated with primary percutaneous coronary intervention (PCI).
The primary objectives of this study in STEMI patients treated with primary PCI were to (i) assess the incidence and timing of AF, (ii) describe the use of antithrombotic therapy according to the presence or absence of AF and the CHADS2 risk score,13 and (iii) evaluate the association of AF with 90 day mortality to determine whether this association is maintained after controlling for baseline and in-hospital covariates. In addition to this analysis, we also looked at associations between AF and other clinical outcomes.
Methods
We conducted these analyses using the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX-AMI) trial database.
The rationale and design of the APEX-AMI trial has been previously published.14,15 In brief, APEX-AMI was a randomized, double blind, placebo-controlled trial comparing the effect of pexelizumab (an inhibitor of complement) with placebo. The primary outcome was all-cause 30 day mortality in 5745 patients with STEMI treated with primary PCI. This multicentre trial involved 17 countries and 296 sites. Patients were enrolled from 2004 to 2006. Patients were deemed eligible for the study if they were at least 18 years of age and presented for primary PCI with symptom onset within 6 h with high-risk MI with electrocardiographic characteristics. Exclusion criteria included prior treatment with fibrinolytic therapy, isolated inferior MI, pregnancy or breastfeeding, complement deficiency or active serious infection, or other serious medical conditions limiting survival. Institutional review boards of participating medical centres approved the APEX-AMI protocol and all patients gave written informed consent.
Atrial fibrillation at baseline and discharge was defined as an irregular rhythm with lack of discernible P waves on the electrocardiogram (ECG) assessed by a central core lab at presentation and prior to discharge. Atrial fibrillation was also collected on the case report form under a list of clinical events experienced from the time of randomization through hospital discharge or day 14, whichever came first; this is classified as AF as a complication. In this study, new onset AF was defined as the occurrence of AF as an in-hospital complication, in the absence of AF at baseline. The day of AF onset is the day when AF is first observed as an in-hospital complication.
Statistical methods
We present descriptive statistics for baseline covariates among the different classifications of AF. Continuous variables are expressed as the median and 25th and 75th percentiles; discrete variables are expressed as percentages. P-values are provided for a non-parametric Wilcoxon rank-sum test for a difference in the distribution of baseline covariates between patients with new onset AF compared with no AF.
Our principle goals were to assess the incidence and timing of AF, describe antithrombotic therapy use, and evaluate the association of AF with 90 day mortality. We estimated the prevalence of AF at baseline by the proportion of 5745 patients who had AF at baseline. Prevalence of AF at discharge was calculated based on the proportion of patients with AF at discharge among those patients who had a discharge ECG. We describe the incidence of new onset AF in two ways. First, we report the proportion of 5745 patients who developed new onset AF in the hospital with confidence intervals (CIs). Second, to account for differing time in-hospital, we also report the incidence rate, or the number of occurrences of new onset AF per 1000 patient days at risk. We report the median along with 25th and 75th percentiles for days until the occurrence of new onset AF, length of hospital stay, and time from symptom onset to randomization. We include the empirical cumulative distribution function of the time until new onset AF to illustrate the timing when new onset AF was observed to occur. Finally, we describe the treatment of patients in our sample by the proportion of patients in various treatment categories.
Accounting for a potentially complex set of confounding variables, including multiple time-dependent covariates, we assessed the relationship between 90 day mortality and new onset AF. Patients experienced a variety of procedures and complications during their initial hospitalizations that may be associated with both AF and mortality. We used the landmark analysis to evaluate the association between AF and 90 day mortality while controlling for baseline and in-hospital variables.16 This involved fitting a Cox model using only patients who had survived to the end of a landmark period. We fit separate models for the landmark times of 0, 1, 2, 3, and 4 days. In each model, covariates were included that occurred up to the end of a landmark period. We also aggregated the results for an overall fit using a Cox proportional hazards model. Our threshold for statistical significance of new onset AF was alpha <0.05.
We used forward selection to choose important covariates at landmarks 0, 1, and 4, where the criteria to be included in the model was alpha <0.05. The inclusion of covariates with lesser statistical significance had virtually no effect on the estimated coefficient for AF. Therefore, we felt that we had sufficiently accounted for confounding variables available in our data set. The potential variables considered for inclusion were all baseline characteristics (Table 1) as well as procedures (intra-aortic balloon pump, automatic implantable cardioverter defibrillator, PCI, cardiac surgery, red blood cell transfusions, re-catheterization, re-PCI, and stents) and complications (moderate or severe bleed, congestive heart failure, shock, cardiac arrest, deep vein thrombosis, acute ventricular septal defect, recurrent MI, recurrent ischaemia, renal failure, pulmonary embolism, stroke, cardiac tamponade, ventricular fibrillation, ventricular tachycardia, ventricular rupture, pericarditis, acute mitral regurgitation, asystole, and acute atrioventricular block). Any covariates selected at either landmark 0, 1, or 4 were then included in the final landmark analysis at all landmark times, and values for in-hospital complications and procedures were updated at the beginning of each landmark period. The benefit of conducting a landmark analysis is that we could update the many time-varying covariates at each landmark time.
Table 1.
Baseline characteristics by timing of atrial fibrillation
| Parameter | No AF (n = 5108) | AF at baseline (n = 276) | New onset AF (n = 342) | AF at discharge (n = 136) | P-value |
|---|---|---|---|---|---|
| Age (year) | 60 (52, 70) | 71 (63, 79) | 70 (61, 78) | 75 (67, 81) | <0.01 |
| Female, n (%) | 1144 (22.4) | 78 (28) | 95 (28) | 39 (29) | 0.02 |
| US patients, n (%) | 1562 (30.6) | 76 (27) | 111 (32) | 38 (28) | 0.45 |
| Height (cm) | 173 (166, 178) | 171 (165, 177) | 172 (165, 178) | 172 (165, 178) | 0.19 |
| Weight (kg) | 80 (70, 91) | 78 (70, 88) | 80 (70, 90) | 80 (70, 90) | 0.07 |
| Blood pressure (mm Hg) | |||||
| Systolic | 134 (118, 150) | 130 (112, 149) | 129 (110, 146) | 137 (120, 158) | <0.01 |
| Diastolic | 80 (70, 90) | 80 (69, 90) | 78 (65, 88) | 81 (70, 93) | <0.01 |
| Heart rate (b.p.m.) | 75 (65, 86) | 87 (70, 110) | 76 (63, 88) | 80 (68, 95) | 0.34 |
| Killip class, n (%) | <0.01 | ||||
| I | 4620 (90.4) | 217 (79) | 279 (82) | 112 (83) | |
| II | 399 (7.8) | 40 (15) | 49 (14) | 20 (15) | |
| III | 47 (0.9) | 12 (4.3) | 5 (1.5) | 2 (1.5) | |
| IV | 42 (0.8) | 7 (2.5) | 9 (2.6) | 1 (0.7) | |
| MI location, n (%) | 0.01 | ||||
| Anterior | 3016 (59) | 147 (53) | 228 (67) | 80 (59) | |
| Non-anterior | 2096 (41) | 129 (47) | 114 (33) | 56 (41) | |
| History of MI, n (%) | 612 (12) | 39 (14) | 42 (12) | 16 (12) | 0.86 |
| History of CAD, n (%) | 821 (16) | 57 (21) | 61 (18) | 31 (23) | 0.38 |
| History of CHF, n (%) | 155 (3) | 30 (11) | 20 (5.8) | 23 (17) | <0.01 |
| History of diabetes, n (%) | 783 (15) | 56 (20) | 68 (20) | 35 (26) | 0.02 |
| History of hypertension, n (%) | 2485 (49) | 159 (58) | 186 (54) | 91 (67) | 0.04 |
| History of stroke, n (%) | 169 (3.5) | 23 (8.3) | 21 (6.1) | 18 (13) | 0.01 |
| History of TIA, n (%) | 64 (1.5) | 16 (5.8) | 9 (2.6) | 8 (6) | 0.05 |
| Prior PCI, n (%) | 507 (10) | 22 (8.0) | 31 (9.1) | 12 (8.8) | 0.62 |
| Prior CABG, n (%) | 117 (2) | 7 (2.5) | 4 (1.2) | 4 (2.9) | 0.17 |
| Current smoking, n (%) | 2303 (45) | 78 (28) | 96 (28) | 18 (13) | <0.01 |
| Creatinine clearance (mL/min) | 87.4 (68.8, 111.3) | 67.7 (50.7, 86.0) | 86.2 (67.7, 110.4) | 65.4 (49.4, 81.8) | <0.01 |
| CK/ULN | 0.7 (0.5, 1.5) | 0.7 (0.4, 1.5) | 0.9 (0.5, 2.0) | 0.6 (0.4, 1.4) | 0.03 |
| CK-MB/ULN | 0.8 (0.4, 2.4) | 1.5 (0.6, 6.3) | 1.1 (0.5, 4.6) | 1.8 (0.6, 9.6) | 0.02 |
| Troponin/ULN | 1.0 (0.3, 4.7) | 1.0 (0.3, 6.2) | 1.5 (0.5, 11.3) | 1.0 (0.6, 7.1) | <0.01 |
| BNP value | 187 (65, 639) | 1001 (406, 2642) | 377 (120, 1734) | 1235 (553, 2576) | <0.01 |
Continuous variables expressed as median (25th, 75th). P-values for the non-parametric Wilcoxon rank-sum test for a difference in distribution between patients with new onset AF and those with no AF.
AF, atrial fibrillation; BNP, brain natriuretic peptide; b.p.m., beats per minute; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CHF, congestive heart failure; CK, creatine kinase; MI, myocardial infarction; PCI, percutaneous coronary intervention; TIA, transient ischaemic attack; ULN, upper limit of normal.
We recognize that our model building techniques may have identified variables with less certainty of true associations than a validated model. To assure our results were not sensitive to our choice of covariates, we repeated the landmark analysis using covariates that have been previously established and validated in the GUSTO-I STEMI population.17 The predictor variables in this model were similar to the set used in our study, including baseline patient descriptors, ECG variables, and in-hospital complications.
Continuous variables were tested for linearity by comparing the fit of linear models with more flexible linear spline models. When this identified non-linear relationships, these variables were included in the landmark analysis using linear splines. All covariates selected for the final model, as well as new onset AF, were checked for proportional hazards by testing for an interaction with time at both landmarks 1 and 4. No significant deviations from the proportional hazards assumption were observed.
We considered the possibility of association between 90 day mortality and AF at baseline or discharge. Baseline AF was included in the landmark analysis. In order to consider AF at discharge, it was necessary to subset on the patients who survived to hospital discharge. There were too few deaths in this group to conduct an adjusted analysis.
A Cox proportional hazards model was used to evaluate associations between new onset AF and secondary outcomes (clinical endpoints and in-hospital complications). In these models, new onset AF was included as a time-dependent covariate. The models for 90 day clinical outcomes are adjusted for baseline covariates that were significant in the baseline 90 day mortality model. The models for in-hospital complications are unadjusted.
Results
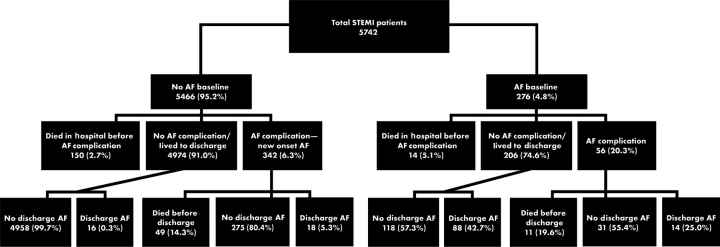
All 5745 patients were included in this analysis (Figure 1). Three had missing baseline and pre-discharge ECGs. In 11% of the patients (634/5745), AF occurred at some time during the hospitalization. Atrial fibrillation prevalence at baseline and at discharge was 4.8% (CI 4.3–5.4%) and 2.5% (CI 2.1–2.9%), respectively. The proportion of 5466 patients without AF at baseline who developed new onset AF was 6.3% (CI 5.6–6.9%). This corresponded to 9.3 cases of new onset AF per 1000 patient days at risk. Among patients who developed new onset AF and survived until discharge, the prevalence of AF at discharge was 5.3%.
Figure 1.
Timing of AF from randomization to discharge, including patients who died while hospitalized. New onset AF was defined as AF as a complication in the absence of AF at baseline. AF, atrial fibrillation; ST-segment elevation myocardial infarction, ST-segment elevation myocardial infarction.
Timing of atrial fibrillation
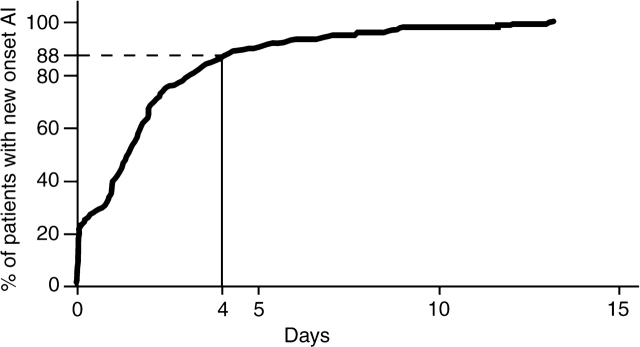
The distribution of the categories of AF is shown in Figure 1. The timing of new onset AF is illustrated in Figure 2, showing the cumulative distribution of the time until AF among patients observed to have new onset AF. This can be interpreted as the percentage of patients having experienced AF by a given number of days in hospital. The median number of days from randomization to new onset AF was 1.4 (0.24, 2.5). The median length of hospital stay in patients who developed new onset AF was 6 (4, 8) days vs. 5 (3, 7) days in those who did not. The median time from symptom onset to randomization was 3.1 (2.2, 4.2) hours in those patients who developed new onset AF compared with 2.8 (1.9, 3.9) hours in those who did not. Approximately 90% of the episodes of new onset AF occurred before 4 days following randomization (Figure 2).
Figure 2.
Cumulative distribution of the time until development of new onset atrial fibrillation. Timing is described only for patients who developed new onset atrial fibrillation at some time during hospitalization.
Baseline characteristics
The baseline characteristics according to the time of AF are shown in Table 1. When compared with patients without AF, patients with new onset AF were older, more often female, had lower systolic and diastolic blood pressures, were more often Killip class III and IV, had more anterior infarction, more prior history of congestive heart failure, diabetes, stroke, hypertension, less current smoking, and higher biomarkers such as creatine kinase (CK), CK-MB, troponin, and brain natriuretic peptide (BNP).
Antiarrhythmics, rate control drugs, procedures, and stents
Compared with patients without new onset AF, patients with AF received less beta-blockers (88.4 vs. 77.4%), more calcium channel blockers (5.3 vs. 8.5%), more digoxin (1.6 vs. 7.3%), and less other antiarrhythmic medications (97.2 vs. 90.0%) at discharge.
The type of stent used according to the presence or absence of AF at different time points is shown in Table 2. Patients with AF at baseline, new onset AF, and AF at discharge received more bare-metal stents than drug-eluting stents.
Table 2.
Stents by timing of atrial fibrillation
| Type of Stent, n (%) | No AF (n = 4790) | AF at baseline (n = 254) | New onset AF (n = 304) | No new onset AF (n = 5055) | AF at discharge (n = 123) |
|---|---|---|---|---|---|
| No stent | 198 (4.1) | 19 (7.5) | 18 (5.9) | 219 (4.3) | 13 (10.6) |
| Drug-eluting | 2032 (42.4) | 78 (30.7) | 107 (35.2) | 2113 (41.8) | 39 (31.7) |
| Bare-metal | 2560 (53.4) | 157 (61.8) | 179 (58.9) | 2723 (53.9) | 71 (57.7) |
AF, atrial fibrillation.
Patients with new onset AF more often underwent coronary artery bypass graft (CABG) surgery (44/342, 12.8%) when compared with those without new onset AF (155/5400, 2.9%). Among patients having CABG surgery, new onset AF occurred mostly after CABG surgery (35/44, 79.5%). Accounting for the timing of AF, patients with new onset AF were more likely to undergo subsequent CABG surgery [adjusted hazard ratio (HR) 1.94; 95% CI 1.01–3.75; P = 0.04].
Antithrombotic therapy
The antithrombotic therapy at discharge according to the timing of AF is shown in Table 3. Patients with new onset AF received more warfarin, less aspirin, and less clopidogrel when compared with patients without new onset AF. Patients with new onset AF received more aspirin alone, clopidogrel alone, and warfarin alone when compared with those patients without new onset AF. Patients with new onset AF also received more triple therapy with aspirin, clopidogrel, and warfarin. Of all patients with AF at discharge, only 43.4% (59/136) received warfarin and 27.2% (37/136) received triple therapy.
Table 3.
Antithrombotic therapy according to timing of atrial fibrillation
| Parameter, n (%) | No AF (n = 5110) | AF at baseline (n = 274) | New onset AF (n = 341) | AF at discharge (n = 136) | AF during hospitalization (n = 627) |
|---|---|---|---|---|---|
| Aspirin | 4899 (95.9) | 233 (85) | 277 (81.2) | 117 (86.0) | 522 (83.3) |
| Warfarin | 244 (4.8) | 63 (23) | 59 (17.3) | 59 (43.4) | 126 (20.1) |
| Clopidogrel | 4545 (88.9) | 222 (81) | 251 (73.6) | 110 (80.9) | 482 (76.9) |
| Aspirin alone | 383 (7.5) | 15 (5.5) | 29 (8.5) | 11 (8.1) | 45 (7.2) |
| Warfarin alone | 4 (0.1) | 7 (2.6) | 5 (1.5) | 5 (3.7) | 12 (1.9) |
| Clopidogrel alone | 34 (0.7) | 5 (1.8) | 3 (0.9) | 3 (2.2) | 8 (1.3) |
| Aspirin plus warfarin | 23 (0.5) | 9 (3.3) | 9 (2.6) | 8 (5.9) | 20 (3.2) |
| Aspirin plus clopidogrel | 4294 (84) | 170 (62) | 203 (59.5) | 61 (44.9) | 380 (60.6) |
| Triple therapy | 199 (3.9) | 39 (14.2) | 36 (10.6) | 37 (27.2) | 77 (12.3) |
AF, atrial fibrillation.
Patients with drug-eluting stents received more warfarin (7.7%) and more triple therapy (6.7%) when compared with patients who were treated with bare-metal stents (4.7 and 4.0%, respectively).
Among all patients with coronary stents, 5.1% were discharged on triple therapy (aspirin, clopidogrel, and warfarin). Patients with AF at baseline and drug-eluting stents received more triple therapy (22%) when compared with patients with drug-eluting stents without AF at baseline (6.0%). A similar pattern of more triple therapy was seen for patients with AF at baseline treated with bare-metal stents. The same pattern was seen in patients who developed new onset AF.
The use of antithrombotic therapy according to AF at discharge and type of stent is shown in Table 4. Among patients treated with drug-eluting stents, 48.7% of patients with AF at discharge received triple therapy compared with only 6.0% of patients without AF at discharge. Patients with AF and bare-metal stents received more triple therapy (19.7%) than those without AF (3.6%), but less than those with AF and drug-eluting stents (48.7%).
Table 4.
Antithrombotic therapy according to type of stents in patients with atrial fibrillation at discharge
| Antithrombotic therapy, n (%) | No AF/no stent (n = 193) | No AF/DE stent (n = 2130) | No AF/BM stent (n = 2724) | AF/no stent (n = 13) | AF/DE stent (n = 39) | AF/BM stent (n = 71) |
|---|---|---|---|---|---|---|
| Aspirin | 188 (97.4) | 2089 (98.1) | 2686 (98.6) | 11 (84.6) | 33 (84.6) | 64 (90.1) |
| Warfarin | 15 (7.8) | 146 (6.9) | 116 (4.3) | 8 (61.5) | 25 (64.1) | 19 (26.8) |
| Clopidogrel | 125 (64.8) | 2089 (98.1) | 2619 (96.1) | 5 (38.50 | 38 (97.4) | 64 (90.1) |
| Aspirin alone | 56 (29.0) | 84 (3.9) | 92 (3.4) | 2 (15.4) | 1 (2.6) | 3 (4.2) |
| Warfarin alone | 2 (1.0) | 2 (0.1) | 1 (0) | 1 (7.7) | 0 (0) | 1 (1.4) |
| Clopidogrel alone | 0 (0) | 18 (0.8) | 17 (0.6) | 0 (0) | 0 (0) | 3 (4.2) |
| Aspirin plus warfarin | 7 (3.6) | 4 (0.2) | 4 (0.1) | 4 (30.8) | 0 (0) | 1 (1.4) |
| Aspirin plus clopidogrel | 119 (61.7) | 1873 (87.9) | 2491 (91.4) | 2 (15.4) | 13(33.3) | 46 (64.8) |
| Triple therapy | 6 (3.1) | 128 (6) | 99 (3.6) | 3 (23.1) | 19 (48.7) | 14 (19.7) |
AF, atrial fibrillation; BM, bare-metal; DE, drug-eluting.
Antithrombotic therapy by CHADS2 score
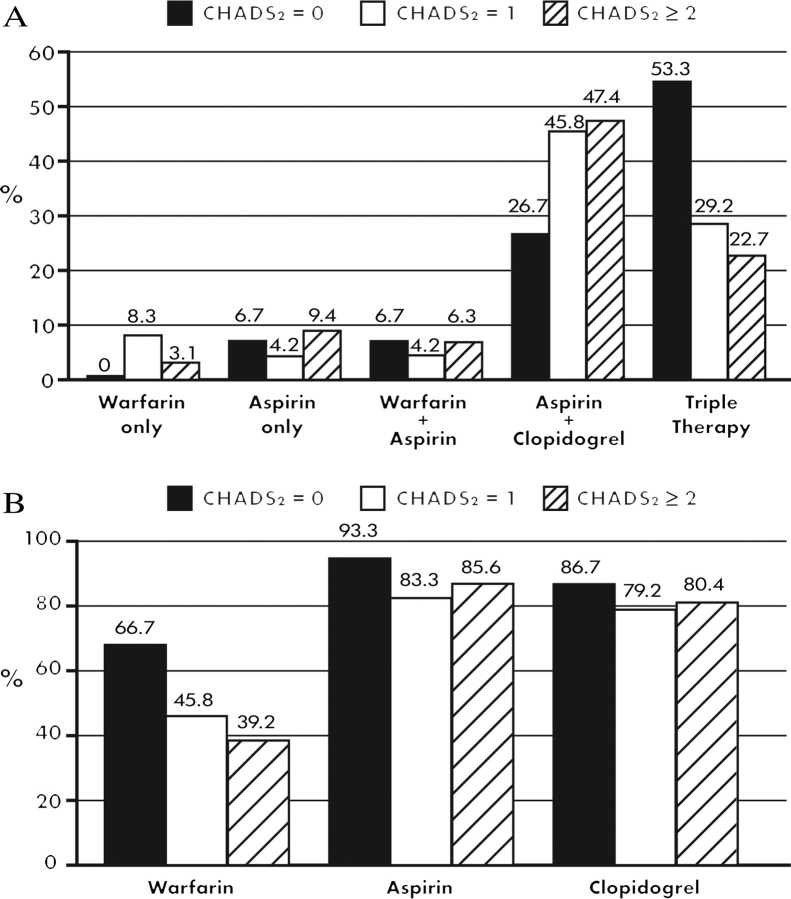
The use of antithrombotic therapy in patients with AF at discharge according to the CHADS2 score assessed at discharge is shown in Figure 3. According to the CHADS2 score, the use of triple therapy decreases with increasing risk of stroke. Likewise, the use of any warfarin, aspirin, or clopidogrel decreases with increasing CHADS2 score. These results were similar even after excluding patients from the analyses who had any in-hospital bleeding, and including patients who had any AF (baseline, new onset, or discharge) during the hospitalization.
Figure 3.
Antithrombotic therapy in patients with atrial fibrillation (AF) at discharge according to CHADS2 score. (A) Percentage of patients with AF who received warfarin only, aspirin only, warfarin plus aspirin, aspirin plus clopidogrel, or triple therapy at discharge according to CHADS2 score. All medication categories are mutually exclusive. (B) Percentage of patients with AF who received any warfarin, any aspirin, or any clopidogrel at discharge according to CHADS2 score.
Outcomes
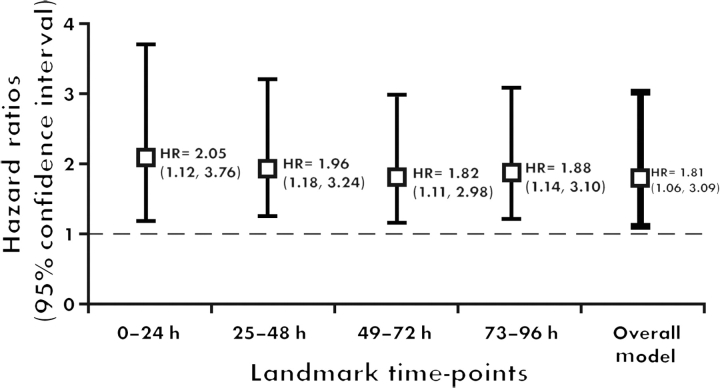
After adjusting for all baseline variables, in-hospital complications, and procedures, new onset AF was associated with higher 90 day mortality at all time points in the landmark analyses (Figure 4). The overall 90 day mortality adjusted HR is 1.81 (95% CI 1.06–3.09; P = 0.029). Using the alternative GUSTO-I covariates, the overall 90 day mortality adjusted HR is 1.84 (95% CI 1.13–3.02; P = 0.016).
Figure 4.
Landmark analysis: new onset atrial fibrillation is an independent predictor of 90 day mortality.
After adjusting for baseline covariates, new onset AF was also associated with other outcomes at 90 days such as death or stroke, congestive heart failure, death or heart failure, shock, and death or shock (Table 5).
Table 5.
Impact of new onset atrial fibrillation (vs. no new onset atrial fibrillation) on 90 day outcomes, adjusted for baseline covariates
| 90 day clinical outcomes | χ2 | HR (95% CI) | P-value |
|---|---|---|---|
| Death or congestive heart failure | 44.5 | 2.77 (2.05–3.74) | <0.0001 |
| Death or stroke | 29 | 2.67 (1.87–3.83) | <0.0001 |
| Congestive heart failure | 20 | 2.66 (1.74–4.06) | <0.0001 |
| Death or shock | 18 | 2.53 (1.65–3.90) | <0.0001 |
| Shock | 14 | 3.81 (1.88–7.70) | 0.0002 |
| Stroke | 9.2 | 2.98 (1.47–6.04) | 0.0024 |
CI, confidence interval; HR, hazard ratio.
Other in-hospital complications
Patients with new onset AF experienced more moderate or severe in-hospital bleeding (13.8%) when compared with patients without AF (4.6%). Of those patients with new onset AF who bled in-hospital, 7.9% (27/340) bled after developing AF and 5.9% (20/340) bled before developing AF. Accounting for the timing of AF, patients with new onset AF were more likely to experience in-hospital bleeding (adjusted HR 2.47; 95% CI 1.45–4.20; P = 0.0008). However, after excluding patients who underwent CABG surgery from the analyses, the higher risk of bleeding with new onset AF was no longer statistically significant (adjusted HR 1.95; 95% CI 0.93–4.09; P = 0.07).
New onset AF was associated with more in-hospital complications such as ventricular tachycardia (HR 6.4; 95% CI 3.5–11.5), ventricular fibrillation (HR 3.7; 95% CI 1.88–7.58), asystole (HR 8.40; 95% CI 5.2–13.5), cardiac arrest (HR 5.7; 95% CI 3.2–10.1), atrioventricular block (HR 3.2; 95% CI 1.2–8.1), cardiac tamponade (HR 6.3; 95% CI 1.7–23.06), and symptomatic hypotension (HR 3.5; 95% CI 2.3–5.3). For each of these complications, we analysed the relationship using AF as a time-dependent covariate, such that we excluded AF occurring after the complication.
Antithrombotic therapy and outcomes
The use of warfarin in patients with AF at discharge corresponded with slightly lower rates of 90 day mortality and stroke when compared with patients who did not receive warfarin. The rate of 90 day mortality was 3.4% (95% CI 0.4–11.7) in patients who received warfarin at discharge compared with 3.9% (95% CI 0.8–11.0) in those who did not receive warfarin. The same pattern was observed for stroke at 90 days, with rates of 3.4% (95% CI 0.4–11.7) and 5.2% (95% CI 1.4–12.7), respectively. A stronger trend was observed between treatment with triple therapy and 90 day mortality and stroke. The rate of 90 day mortality was 0.0% (95% CI 0.0–9.5) in those patients who received triple therapy at discharge compared with 5.1% (95% CI 1.7–11.4) in those who did not receive this therapy. The same pattern was observed for stroke at 90 days, the rates were 2.7% (95% CI 0.1–14.1) and 5.1% (95% CI 1.7–11.4), respectively.
Discussion
This is one of the largest studies to examine AF and its timing (baseline, new onset, and discharge) and to describe the associated use of antithrombotic therapy in patients with acute MI treated with primary PCI. An important feature of this study is that we performed a comprehensive analysis, adjusting for all possible baseline characteristics, in-hospital complications, and procedures, to assess the independent relationship of new onset AF with 90 day outcomes.
We found that the proportion of STEMI patients who developed new onset AF was 6.3%; this is similar to previous reports.1–4 In both prior reports and the current study, of those patients who had AF at some time during the hospitalization (i.e. at baseline or new onset), most had new onset AF. In our study, 14% of the patients who were noted to have AF as a complication also had AF at baseline; thus, this was not new onset AF, but a worsening status of a pre-existing AF. A previous study has shown that the overlap between AF as a complication and AF at baseline was about 18%.4 This underscores that when AF is noted as a clinical complication, it is generally, but not always, new onset AF. In addition, new onset AF occurs early in the hospital course with 88% occurring within 4 days of admission (Figure 1).
Patients with AF are usually sicker, have more risk factors, more comorbidities, and experience more in-hospital complications. Therefore, there are important confounding factors that must be accounted for when assessing the association of new onset AF with short- and long-term outcomes. For example, while new onset AF is associated with higher risk of bleeding even after accounting for timing of bleeding and after excluding patients undergoing CABG surgery, the relationship is weaker and no longer statistically significant after excluding patients undergoing CABG surgery.
The fact that most prior studies do not have the timing of AF is another limitation to precise determination of the association of AF and outcomes.7,18 Thus, it has been unclear to what extent AF after MI is a marker of risk for worse outcomes vs. a predictor of subsequent worse outcomes. Our study allowed us to dissect this further with landmark analyses to account for the biases due to the timing of the AF, survival bias, and potential confounders including in-hospital complications and procedures. We have been able to show that even after this type of adjustment, new onset AF remains associated with 90 day mortality.
In this study, patients with new onset AF received more antiarrhythmic drugs when compared with patients without AF. In patients who developed new onset AF and underwent CABG surgery, the AF occurred after CABG surgery approximately 80% of the time. This accounted for 10% of overall new onset AF.
Antithrombotic therapy is indicated for the treatment of both STEMI and AF.19–21 However, for patients with STEMI and AF, the use of combinations of these drugs may significantly increase the risk of bleeding.21 In a meta-analysis, Rothberg et al.8 showed that the combination of aspirin and warfarin for patients with MI reduces mortality compared with the use of aspirin alone; however, bleeding rates are higher.
Stenestrand et al.10 showed in an observational study that patients with AF and MI who received warfarin and a platelet inhibitor had lower 1 year mortality compared with those who received aspirin alone. They also showed that only 30% of the patients with AF and acute MI received oral anticoagulation therapy at discharge. Our results are similar, 43.4% of patients with AF at discharge received oral anticoagulation, and extend these observations by describing the use of several different combinations of antithrombotic therapy according to the presence or absence of AF and presence and type of stent. Among patients with AF at discharge who received bare-metal stents, 27% received warfarin compared with 64% of those with drug-eluting stents and 61% of those with no stents. Of interest, only 49% of the patients with AF at the time of discharge and drug-eluting stents received triple therapy; 33% were treated with aspirin and clopidogrel only. Approximately 20% of the patients with AF and bare-metal stents received triple therapy at discharge and 65% were on aspirin and clopidogrel alone.
In a small study, Ruiz-Nodar et al.22 have shown that of the patients with chronic AF who undergo PCI, 55% are treated with triple therapy, and of patients with paroxysmal AF, 41% were discharged on triple therapy. Lip and Karpha23 also demonstrated that among patients with AF undergoing PCI, the rates of aspirin plus clopidogrel and of triple therapy at discharge were around 70 and 20%, respectively. Similarly, our study of primary PCI shows heterogeneity of practice regarding the use of antithrombotic therapy at discharge in this high-risk AF population.
The current AF guidelines recommend the use of warfarin for patients with a CHADS2 score ≥2 as a class IA recommendation. We showed that among patients with AF at discharge, warfarin use was paradoxically inversely related to the CHADS2 score. We similarly showed that the use of triple therapy at discharge decreases as the CHADS2 score increases. The use of triple therapy was still less with higher CHADS2 score, excluding patients with in-hospital bleeding, so higher risk of bleeding with higher CHADS2 may not completely explain the lower use.
Importantly, the observed practice of patients at higher risk of stroke being less likely to receive warfarin is contrary to current ACC/AHA/ESC AF guidelines recommendations.20 The current guidelines recommend triple therapy for patients with STEMI and AF, but for the shortest duration possible, with a lower target INR, and with clinical judgment of assessing risk and benefit.20 The treatment-risk paradox we observed regarding warfarin use highlights the need for better understanding of antithrombotic therapies in patients with STEMI and AF, including the balance of risk of bleeding and risk of stroke with combination therapies. Finally, we observed a pattern of lower rates of 90 day mortality and stroke among patients who received warfarin and triple therapy at discharge, although this is also a lower risk population and the number of events was small.
Study limitations
One limitation of this study is that it was an observational study in which one cannot prove cause and effect between AF, its treatment, and worse outcomes. Although we have adjusted for all available candidate variables, we cannot account for unmeasured variables. The duration of hospitalization varied across patients, such that the observed period during which a patient could have developed new onset AF also varied. The duration of use of antiplatelet agents as well as warfarin after discharge is of great interest. Unfortunately, we did not collect this information. We also did not collect information on post-discharge bleeding, post-acute phase blood pressure control, and we do not have information about specific in-hospital treatment of new onset AF. In addition, NT-proBNP measures were missing in most patients. Thus, we could not include BNP as a covariate in the mortality models. Finally, we did not collect information on concomitant drugs other than antithrombotic, antiarrhythmic, and rate control medications.
Conclusions
In STEMI patients, AF prevalence at admission and at discharge was 4.8 and 2.5%, respectively. The proportion of patients who developed new onset AF was 6.3% and was independently associated with 90 day mortality and other long-term outcomes such as shock and congestive heart failure. Most of the patients who had AF at discharge did not receive oral anticoagulation therapy. In a large contemporary STEMI population treated with primary PCI, our study shows that new onset AF is an independent marker of 90 day mortality and is associated with other adverse outcomes. This illustrates the need for a randomized trial of antithrombotic therapy in patients with MI complicated by AF.
Funding
Funding was provided by Duke Clinical Research Institute. R.D.L. also received financial support from Brazil's Ministry of Education's Coordenacao de Aperfeicoamento de Pessoal de Nivel Superior (CAPES) as a research grant for a postdoctoral fellowship.
Conflict of interest: J.S.H. received modest honoraria from Procter & Gamble for serving on the steering committee. Other authors: none declared.
References
- 1.Goldberg RJ, Seeley D, Becker RC, Brady P, Chen ZY, Osganian V, Gore JM, Alpert JS, Dalen JE. Impact of atrial fibrillation on the in-hospital and long-term survival of patients with acute myocardial infarction: a community-wide perspective. Am Heart J. 1990;119:996–1001. doi: 10.1016/s0002-8703(05)80227-3. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen OD, Baggar H, Køber L, Torp-Perdersen C. The occurrence and prognostic significance of atrial fibrillation/-flutter following acute myocardial infarction. Eur Heart J. 1999;20:748–754. doi: 10.1053/euhj.1998.1352. [DOI] [PubMed] [Google Scholar]
- 3.Pizzetti F, Turazza FM, Franzosi MG, Barlera S, Ledda A, Maggioni AP, Santoro L, Tognoni G. Incidence and prognostic significance of atrial fibrillation in acute myocardial infarction: the GISSI-3 data. Heart. 2001;86:527–532. doi: 10.1136/heart.86.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopes RD, Pieper KS, Horton JR, Al-Khatib SM, Newby LK, Mehta RH, Van de Werf F, Armstrong PW, Mahaffey KW, Harrington RA, Ohman EM, White HD, Wallentin L, Granger CB. Short- and long-term outcomes following atrial fibrillation in patients with acute coronary syndromes with or without ST-segment elevation. Heart. 2008;94:867–873. doi: 10.1136/hrt.2007.134486. [DOI] [PubMed] [Google Scholar]
- 5.Crenshaw BS, Ward SR, Granger CB, Stebbins AL, Topol EJ, Califf RM. Atrial fibrillation in the setting of acute myocardial infarction: the GUSTO-I experience. J Am Coll Cardiol. 1997;30:406–413. doi: 10.1016/s0735-1097(97)00194-0. [DOI] [PubMed] [Google Scholar]
- 6.Rathore SS, Berger AK, Weinfurt KP, Schulman KA, Oetgen WJ, Gersh BJ, Solomon AJ. Acute myocardial infarction complicated by atrial fibrillation in the elderly: prevalence and outcomes. Circulation. 2000;101:969–974. doi: 10.1161/01.cir.101.9.969. [DOI] [PubMed] [Google Scholar]
- 7.Al-Khatib SM, Pieper KS, Lee KL, Mahaffey KW, Hochman JS, Pepine CJ, Kopecky SL, Akkerhuis M, Stepinska J, Simoons ML, Topol EJ, Califf RM, Harrington RA. Atrial fibrillation and mortality among patients with acute coronary syndromes without ST-segment elevation: results from the PURSUIT trial. Am J Cardiol. 2001;88:76–79. doi: 10.1016/s0002-9149(01)01593-4. [DOI] [PubMed] [Google Scholar]
- 8.Rothberg MB, Celestin C, Fiore LD, Lawler E, Cook JR. Warfarin plus aspirin after myocardial infarction or the acute coronary syndrome: meta-analysis with estimates of risk and benefit. Ann Intern Med. 2005;143:241–250. doi: 10.7326/0003-4819-143-4-200508160-00005. [DOI] [PubMed] [Google Scholar]
- 9.Testa L, Zoccai GB, Porto I, Trotta G, Agostoni P, Andreotti F, Crea F. Adjusted indirect meta-analysis of aspirin plus warfarin at international normalized ratios 2 to 3 versus aspirin plus clopidogrel after acute coronary syndromes. Am J Cardiol. 2007;99:1637–1642. doi: 10.1016/j.amjcard.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 10.Stenestrand U, Lindbäck J, Wallentin L. Anticoagulation therapy in atrial fibrillation in combination with acute myocardial infarction influences long-term outcome: a prospective cohort study from the Register of Information and Knowledge About Swedish Heart Intensive Care Admissions (RIKS-HIA) Circulation. 2005;112:3225–3231. doi: 10.1161/CIRCULATIONAHA.105.552984. [DOI] [PubMed] [Google Scholar]
- 11.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, II, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC, Jr, Jacobs AK, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:e1–e157. doi: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernandez-Aviles F, Fox KA, Hasdai D, Ohman EM, Wallentin L, Wijns W Task Force for Diagnosis Treatment of Non-ST-Segment Elevation Acute Coronary Syndrome of European Society of Cardiology. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007;28:1598–1660. doi: 10.1093/eurheartj/ehm161. [DOI] [PubMed] [Google Scholar]
- 13.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong PW, Adams PX, Al-Khalidi HR, Hamm C, Holmes D, O'Neill W, Todaro TG, Vahanian A, Van de Werf F, Granger CB. Assessment of Pexelizumab in Acute Myocardial Infarction (APEX-AMI): a multicenter, randomized, double-blind, parallel-group, placebo-controlled study of pexelizumab in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J. 2005;149:402–407. doi: 10.1016/j.ahj.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong PW, Granger CB, Adams PX, Hamm C, Holmes D, Jr, O'Neill WW, Todaro TG, Vahanian A, Van de Werf F. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA. 2007;297:43–51. doi: 10.1001/jama.297.1.43. [DOI] [PubMed] [Google Scholar]
- 16.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 17.Califf RM, Pieper KS, Lee KL, Van de Werf F, Simes RJ, Armstrong PW, Topol EJ. Prediction of 1-year survival after thrombolysis for acute myocardial infarction in the Global Utilization of Streptokinase and TPA for Occluded coronary arteries trial. Circulation. 2000;101:2231–2238. doi: 10.1161/01.cir.101.19.2231. [DOI] [PubMed] [Google Scholar]
- 18.Mehta RH, Dabbous OH, Granger CB, Kuznetsova P, Kline-Rogers EM, Anderson FA, Jr, Fox KA, Gore JM, Goldberg RJ, Eagle KA. Comparison of outcomes of patients with acute coronary syndromes with and without atrial fibrillation. Am J Cardiol. 2003;92:1031–1036. doi: 10.1016/j.amjcard.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, Hochman JS, Krumholz HM, Lamas GA, Mullany CJ, Pearle DL, Sloan MA, Smith SC., Jr 2007 Focused Update of the ACC/AHA 2004 Guidelines for the Management of Patients with ST-Elevation Myocardial Infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration With the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients with ST-Elevation Myocardial Infarction, Writing on behalf of the 2004 Writing Committee. Circulation. 2008;117:296–329. doi: 10.1161/CIRCULATIONAHA.107.188209. [DOI] [PubMed] [Google Scholar]
- 20.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 21.Rubboli A, Halperin JL, Airaksinen KE, Buerke M, Eeckhout E, Freedman SB, Gershlick AH, Schlitt A, Tse HF, Verheugt FW, Lip GY. Antithrombotic therapy in patients treated with oral anticoagulation undergoing coronary artery stenting. An expert consensus document with focus on atrial fibrillation. Ann Med. 2008;40:428–436. doi: 10.1080/07853890802089786. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz-Nodar JM, Marín F, Hurtado JA, Valencia J, Pinar E, Pineda J, Gimeno JR, Sogorb F, Valdés M, Lip GY. Anticoagulant and antiplatelet therapy use in 426 patients with atrial fibrillation undergoing percutaneous coronary intervention and stent implantation implications for bleeding risk and prognosis. J Am Coll Cardiol. 2008;51:818–825. doi: 10.1016/j.jacc.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 23.Lip GY, Karpha M. Anticoagulant and antiplatelet therapy use in patients with atrial fibrillation undergoing percutaneous coronary intervention: the need for consensus and a management guideline. Chest. 2006;130:1823–1827. doi: 10.1378/chest.130.6.1823. [DOI] [PubMed] [Google Scholar]