Abstract
Background
Urotensin-II (U-II) is an endogenous peptide upregulated in failing hearts. To date, insights into the myocardial actions of U-II have been obscured by its potent vasoconstrictor effects and interspecies differences in physiological responses to U-II.
Methods and Results
We examined the direct effects of exogenous U-II on in vitro contractility in nonfailing and failing human myocardial trabeculae (n=47). Rapid cooling contractures (RCC) were used to examine SR Ca2+ load. In nonfailing myocardium, exogenous U-II increased developed force (DF), rates of force generation and decline and RCC amplitude suggesting increased SR Ca2+ load. In isolated myocyte suspensions from nonfailing hearts, U-II increased phospholamban phosphorylation. In failing myocardium, exogenous U-II reduced DF and rates of force generation and decline without a significant change in RCC amplitude in trabeculae or a change in phospholamban phosphorylation in myocytes. To examine the effects of endogenous U-II, we administered the peptidic U-II receptor antagonist (UT-A) GSK248451A to isolated trabeculae. UT-A induced a decrease in DF in nonfailing myocardium and an increase in DF in failing myocardium. UT-A increased RCC amplitude slightly in both nonfailing and failing myocardium. During ongoing UT-A, exogenous U-II had little effect on DF and RCC amplitude, confirming effective receptor blockade.
Conclusions
U-II modulates contractility independent of vasoconstriction with opposite effects in failing and nonfailing hearts. Positive inotropic responses to UT-A alone suggests that increased endogenous U-II constrains contractility in failing hearts via an autocrine and/or paracrine mechanism. These findings support a potential therapeutic role for UT-A in heart failure.
Keywords: contractility, calcium, sarcoplasmic reticulum, pharmacology
Introduction
Numerous studies have demonstrated activation of multiple endogenous neurohormonal systems in the setting of congestive heart failure,1, 2 and many of these systems have become targets for therapeutic interventions. In this context, recent studies have identified urotensin-II (U-II), a potent endogenous vasoconstrictor peptide, as a potential contributor to the pathogenesis and progression of human heart failure. In addition to the vasculature, U-II and its specific G-protein coupled receptor (UT) are expressed within the heart as well a variety of other organs (i.e. kidneys, adrenal, pancreas, thyroid and pituitary).
Circulating plasma levels of U-II are increased in humans with heart failure3-5, and these increases have been inversely correlated with ejection fraction6 and directly associated with increases in left ventricular filling pressures,3 dimension,6 and prognostic markers such as BNP3, 4 and endothelin-1.7 Moreover, several different studies have demonstrated an upregulation of the U-II system (both ligand and receptor) within the failing heart.6, 8, 9 For example, Douglas et al. observed strong expression of the U-II system in the cardiac myocytes and nonmyocytes of failing human hearts compared with lesser expression in the myocardium of patients with mild heart failure and little to no expression in nonfailing hearts.6
While studies have indicated that exogenous U-II can acutely modulate cardiac function, the pathophysiologic implications of increased U-II remains uncertain. Specifically, in vivo vasoconstriction and species-related differences in myocardial responses to U-II have impeded studies aimed at defining the functional role of U-II in heart failure. For example, in isolated-perfused rat hearts, exogenous U-II administration was associated with increases in coronary resistance, negative inotropy and increases in biomarkers indicative of acute cardiac injury.10 In this study, detrimental effects of exogenous U-II were even greater in the setting of ischemia-reperfusion injury where upregulation of UT was observed. However, in isolated human right atrial tissue, U-II was found to be a potent positive inotrope with responses implicating a PKC-dependent mechanism and increased phosphorylation of myosin light chain-2 (MLC-2).11 In ventricular trabeculae obtained from humans, the positive inotropic action of U-II was more potent than endothelin-1 or norepinephrine.12 Interestingly, while endothelin-1 has known differential effects in failing and nonfailing myocardium,13 there have been no direct comparisons of myocardial responses to U-II in the presence or absence of heart failure.
Accordingly, we investigated the direct effects of exogenous U-II on in vitro contractile performance in freshly isolated myocardial trabeculae from severely failing human hearts and from nonfailing control hearts. To examine potential autocrine/paracrine effects of endogenous U-II, we also examined the myocardial actions of acute U-II receptor antagonism (UT-A) on in vitro contractile performance. To provide insight into the mechanisms through which U-II is modulating contractility, we used rapid cooling contractures to examine the actions of U-II on sarcoplasmic reticulum Ca2+ load. In addition, we investigated the effects of U-II and UT-A on phospholamban phosphorylation in freshly isolated myocyte suspensions. Our findings indicate that the direct inotropic actions of U-II are qualitatively and quantitatively different in nonfailing and failing human myocardium and support a role for endogenous U-II in the regulation of myocardial contractility.
Materials and Methods
Tissue Procurement
Human myocardium was obtained from patients with end-stage heart failure at the time of transplantation and from nonfailing donors whose hearts were deemed unsuitable for transplant. IRB-approved prospective informed consent routines for transplant recipients at the Hospital of the University Pennsylvania, and similar prospective consent for research use of nonfailing hearts through our local organ procurement organization (Gift of Life, Inc.) ensured ethical use of all tissue. Relevant clinical information including patient demographics, clinical history, medications and heart failure etiology was collected from all subjects who provided heart tissue. Myocardial perfusion with cold, 4:1 blood cardioplegia and prompt transport to the laboratory was performed as previously described.14 All in vitro physiologic evaluations were performed immediately and were concluded no more than 8 hours following tissue procurement.
Isolated Trabeculae Studies
In vitro muscle mechanics were assessed using previously described methods15 without modification. Briefly, human myocardial trabeculae were isolated from the right ventricular free wall and mounted in a slackened position in a custom designed isometric muscle chamber (Scientific Instruments, Heidelberg, Germany). The Ca2+ concentration of physiological buffer solution within the muscle chamber incremented gradually to a concentration of 1.75 mmol/L CaCl2. Following a 60 min. equilibration period, acute studies were conducted at 37°C and at a stimulation frequency of 0.5 Hz. Trabeculae were continuously stimulated by 3.0 ms asymmetric pulses with an energy that was 20% above threshold (typically 3-7 V). Experimental trabeculae length (L) was set to 80% of the difference between Lmax and L0 as previously described.15 Average muscle dimensions were 0.35 ± 0.01 mm wide, 0.32 ± 0.01 mm thick and 3.19 ± 0.10 mm long. Consistent with previous studies,16 we employed only trabeculae that had a maximal thickness of < 0.50 mm to ensure adequate tissue oxygenation. All experimental solutions were continuously bubbled with 95%O2-5%CO2 gas mixture to maintain a pH of 7.4.
U-II Concentration-Response Experiments
The purpose of these studies was to determine the direct effects of exogenous U-II on in vitro contractile performance in failing and nonfailing human myocardium. Once stabilized at 0.5 Hz, steady-state (determined when developed force fluctuated less than 5% over a 3 minute period) isometric twitches were recorded (at 0.5 Hz) before and after bath application of the following U-II concentrations: 0.1, 1.0, 10, 100, 1000 nM. For these studies, we employed human U-II from GlaxoSmithKline (King of Prussia, PA).
U-II Receptor Antagonist Experiments
To examine the effects of Urotensin-II receptor antagonism (UT-A) alone, steady-state isometric twitches were recorded (at 0.5 Hz) before and after administration of a UT antagonist (UT-A, GSK248451A, Cin-c[DCys-Pal-DTrp-Orn-Val-Cys]-His-amide)17 in failing and nonfailing trabeculae. Pilot studies demonstrated that 1 μM of this agent produces a maximal contractile response in human myocardial preparations (data not shown). To determine the ability of UT-A to block the contractile effects of exogenous U-II, isometric twitches were also recorded following application of U-II (1000 nM) during ongoing UT-A.
Rapid Cooling Contracture (RCC) Experiments
Trabeculae were mounted in a specialized muscle chamber conditioned to determine sarcoplasmic reticulum (SR) Ca2+ load by rapid cooling as previously described.18, 19 Under steady-state isometric conditions, electrical stimulation was stopped and trabeculae were rapidly cooled from 37°C to less than 1°C within 1 s. Rapid cooling releases all SR Ca2+ while simultaneously inhibiting SR Ca2+ uptake and sarcolemmal Ca2+ transport, resulting in the development of a tension contracture. Once the contracture reached a steady state, trabeculae were re-warmed and electrical stimulation was resumed. The amplitude of the tension contracture was measured as an index of SR Ca2+ load.
U-II and UT-A Treaments of Isolated Cardiac Myocytes
Left ventricular myocytes were isolated from both failing and nonfailing human hearts as previously described.20 A small aliquot of cells were removed from the cell suspension solution, and the viable cell number was counted using a hemacytometer (Advent Genetics, Ann Arbor, MI). Cells within the cell suspension solution were centrifuged, the supernatant was discarded and the cell pellet was resuspended in 1% BSA. Identical aliquots of cells (1.0 ml) were exposed to U-II (0.1, 10, 1000 nM), UT-A (1000 nM) or no drug at all (Baseline) for 20 minutes on a shaker (Biotech, Philadelphia, PA) at 25°C. Cells were centrifuged for 3 minutes, the BSA supernatant was removed and the samples were immediately frozen in liquid nitrogen for subsequent molecular analysis.
Western Blot Analysis
Phospholamban levels of phosphorylation were analyzed in isolated myocytes exposed to either U-II or UT-A using Western blot analysis as previously reported.21 The following antibodies were used: phospholamban (PLB; Upstate, Lake Placid NY) serine 16 and threonine 17 (phosphorylation site specific; PLB) (Badrilla, UK).
Statistical Analysis
A Fisher’s Exact Test was performed to examine potential differences in subject gender between groups. For continuous variables, results are presented as the mean ± S.E.M. For all hypothesis testing, a p-value of less than 0.05 was considered statistically significant. Differences in the absolute values of all contractile parameters as well as the values obtained through Western blot analysis were analyzed using a two-way repeated measures ANOVA. When the group by condition interaction was significant, pairwise within- and between-group comparisons were made using the Hochberg adjustment. Differences in the absolute change from baseline were determined between and within groups using two sample and paired t-tests, respectively. In these analyses, a Bonferroni adjustment was used to account for multiple pairwise comparisons and assure that values considered statistically significant achieved an experiment-wise p-value of <0.05.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Patient Characteristics
Clinical characteristics of subjects providing myocardial tissue are presented in Table 1. There were no significant differences between age and body weight between patients supplying nonfailing and failing myocardium. Among the failing hearts, 5 were from individuals with ischemic cardiomyopathy and 10 were from individuals with non-ischemic cardiomyopathy. Nonfailing hearts were rejected for clinical transplantation due to chronic hypertension (n=7), donors being over the age of 60 (n=6) or prior viral hepatitis exposure (n=1). The average ejection fraction was 17 ± 2% among failing hearts compared to 65 ± 6% among nonfailing hearts (# P<0.05).
Table 1. Patient Characterization.
| Group | Age (years) |
Gender | BW (kg) |
Etiology | LVEF (%) |
Medications |
|---|---|---|---|---|---|---|
| Failing (n=15) |
50 ± 2 | 10 male 5 female |
89 ± 4 | 5 ischemic 10 non-ischemic |
17 ± 2 # | BB 15/15, ACEI 7/15, ARB 4/15, Mil 6/15, Dig 7/15, Diu 11/15, N 5/15, Amio 6/15, Hyd 7/15, W 7/15 |
| Nonfailing (n=14) |
58 ± 4 | 8 male 6 female |
78 ± 4 | NA | 65 ± 6 | BB 4/14, ARB 1/14, N 1/14, W 1/14, CCB 1/14 |
BW, body weight; LVEF, left ventricular ejection fraction; BB, beta-blocker; ACEI, ACE inhibitor; ARB, angiotensin receptor blocker; Mil, milrinone; Dig, digoxin; Diu, diuretic; N, nitrates; Amio, amiodarone; Hyd, hydralazine; W-warfarin; CCB, calcium channel-blocker.
denotes P<0.05 compared to nonfailing group.
U-II Concentration-Response
Representative steady-state twitch tracings from U-II concentration-response experiments are shown in Figures 1A and 1B and absolute values of all contractile parameters are presented in Table 2A. U-II induced increases in developed force in nonfailing myocardium, but decreases in developed force in failing myocardium (Figure 1C). In addition, U-II increased the rates of force generation and relaxation (+dF/dt and −dF/dt) in nonfailing myocardium, but decreased the rates of force generation and relaxation in failing myocardium (Figures 1D and 1E). Though not precisely defined by these experiments, our findings suggest an EC50 between 0.1 and 1.0 nM for exogenous U-II in human ventricular myocardium. To examine whether intergroup differences in contractility are due to U-II-dependent alterations in SR Ca2+ load, RCC experiments were performed. A representative RCC tracing is shown in Figure 2A. In nonfailing myocardium, RCC amplitude increased significantly during graded increases in U-II but remained stable in failing myocardium (Figure 2B).
Figure 1.
Representative twitch tracings in (A) nonfailing and (B) failing trabeculae before and after bath application of urotensin-II (U-II). (C) Mean absolute change in steady-state developed twitch force from baseline before and after administration of U-II in nonfailing and failing human trabeculae. (D) Mean absolute change in maximal rate of force generation and (E) relaxation from baseline before and after application of U-II in nonfailing and failing human trabeculae. † denotes p<0.01 within groups compared to Baseline. # denotes p<0.01 between groups under the same condition.
Table 2. Contractile Responses.
(A) Responses to graded doses of exogenous Urotensin-II (U-II) in nonfailing and failing myocardium. (B) Responses to U-II receptor antagonism (UT-A) with GSK248451A at 1 μM with and without coadministration of U-II (1 μM).
| A | ||||||
|---|---|---|---|---|---|---|
| Group | Condition | Dev F (mN/mm2) |
+dF/dt (mN/s/mm2) |
TPF (ms) |
Dia F (mN/mm2) |
−dF/dt (mN/s/mm2) |
|
Nonfailing (n=13) |
Baseline | 10.5 ± 0.4 | 91.0 ± 5.9 | 191 ± 9.3 | 3.85 ± 0.5 | 66.9 ± 5.0 |
| 0.1 nM U-II | 14.6 ± 0.7 † | 121 ± 9.2 † | 198 ± 9.2 | 2.85 ± 0.4 | 97.9 ± 7.5 † | |
| 1.0 nM U-II | 16.5 ± 1.2 † | 136 ± 10 † | 196 ± 9.7 | 2.94 ± 0.4 | 102 ± 8.5 † | |
| 10 nM U-II | 15.0 ± 1.3 * | 132 ± 12 † | 188 ± 8.8 | 2.97 ± 0.3 | 93.6 ± 8.5 * | |
| 100 nM U-II | 14.6 ± 1.3 * | 130 ± 13 † | 185 ± 9.0 | 2.63 ± 0.4 | 88.1 ± 8.5 | |
| 1000 nM U-II | 14.1 ± 1.2 | 126 ± 12 * | 183 ± 15 | 3.06 ± 0.5 | 84.6 ± 7.1 | |
|
Failing (n=11) |
Baseline | 17.2 ± 2.4 | 122 ± 19 | 235 ± 9.8 | 4.65 ± 0.7 | 89.6 ± 18 |
| 0.1 nM U-II | 16.3 ± 2.4 | 128 ± 22 | 223 ± 13 | 4.18 ± 0.9 | 94.2 ± 18 | |
| 1.0 nM U-II | 14.8 ± 2.0 | 120 ± 21 | 224 ± 16 | 4.53 ± 1.0 | 79.9 ± 15 | |
| 10 nM U-II | 13.7 ± 1.7 | 113 ± 17 | 224 ± 18 | 4.49 ± 1.0 | 76.8 ± 13 | |
| 100 nM U-II | 12.8 ± 1.5 * | 109 ± 16 | 210 ± 12 | 5.57 ± 1.2 | 69.0 ± 11 | |
| 1000 nM U-II | 12.5 ± 1.6 * | 104 ± 17 | 216 ± 14 | 5.93 ± 1.4 | 60.5 ± 8.5 | |
| B | ||||||
|---|---|---|---|---|---|---|
| Group | Condition | Dev F (mN/mm2) |
+dF/dt (mN/s/mm2) |
TPF (ms) |
Dia F (mN/mm2) |
−dF/dt (mN/s/mm2) |
|
Nonfailing (n=9) |
Baseline | 13.8 ± 1.0 # | 104 ± 7 | 220 ± 21 | 8.3 ± 2.7 | 75 ± 5 # |
| UT-A | 10.8 ± 1.2 # | 91 ± 12 | 209 ± 19 | 6.2 ± 1.9 | 65 ± 8 | |
| UT-A w/ U-II | 13.2 ± 1.4 | 101 ± 14 | 231 ± 26 | 7.0 ± 2.0 | 75 ± 8 | |
|
Failing (n=14) |
Baseline | 10.6 ± 0.4 | 82 ± 6 | 236 ± 18 | 5.2 ± 1.0 | 53 ± 3 |
| UT-A | 14.8 ± 0.8 † | 105 ± 7 † | 243 ± 20 | 4.9 ± 1.1 | 74. ± 4 † | |
| UT-A w/ U-II | 14.4 ± 1.2 † | 107 ± 9 † | 229 ± 15 | 4.8 ± 1.2 | 69 ± 5 * | |
Abbreviations: Dev F, developed force; +dF/dt, maximal rate of force generation; TPF, time to peak force; Dia F, diastolic force; −dF/dt, maximal rate of force relaxation.
denotes p<0.05
denotes p<0.01 compared with Baseline within group compared to Baseline.
denotes p<0.05 between groups under the same condition.
Figure 2.
(A) Representative rapid cooling contracture (RCC) tracing to indirectly assess SR Ca2+ load. (B) Mean absolute change in RCC amplitude from baseline before and after administration of exogenous U-II in nonfailing and failing human trabeculae. † denotes p<0.01 within groups compared to Baseline.
U-II Receptor Antagonism (UT-A)
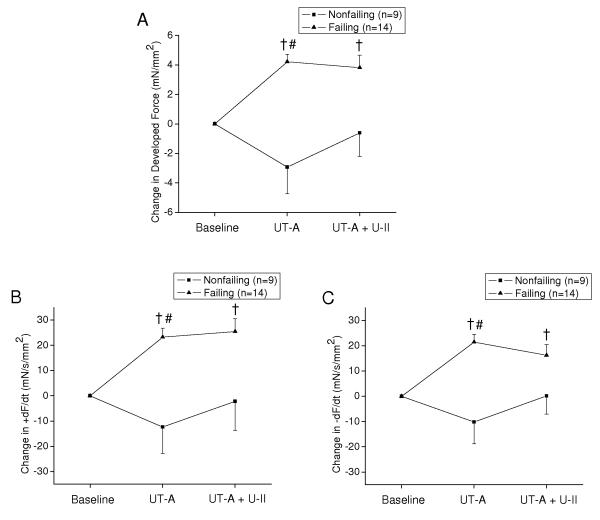
Absolute values of all contractile parameters are shown in Table 2B for both nonfailing and failing myocardium before and after bath application of UT-A (1000 nM) alone and upon subsequent administration of U-II (1000 nM). UT-A induced a mild decrease in developed force in nonfailing myocardium, but a pronounced increase in developed force in failing myocardium (Figure 3A). UT-A also increased both the rates of force generation and relaxation in failing myocardium, but had minimal effect on myocardium from nonfailing hearts (Figure 3B and 3C). RCC amplitude increased slightly following UT-A in both failing and nonfailing myocardium (Figure 4). Subsequent administration of U-II during ongoing UT-A had little effect on developed force, the rates of force generation and relaxation and RCC amplitude (Figure 3A, 3B, 3C and Figure 4).
Figure 3.
(A) Mean absolute change in steady-state developed twitch force from baseline before and after administration of UT-A alone as well as in the presence of U-II in failing and nonfailing human trabeculae. (B) Mean absolute change in maximal rate of force generation and (C) relaxation from baseline before and after administration of UT-A and U-II in failing and nonfailing human trabeculae. For all of these experiments, the concentration of both UT-A and U-II was 1000 nm. † denotes p<0.01 within groups compared to Baseline. # denotes p<0.025 between groups under the same condition.
Figure 4.
Mean absolute change in RCC amplitude from baseline before and after administration of UT-A alone as well as in the presence of U-II in failing and nonfailing human trabeculae.
Western Blot Analysis
Western blots were performed on freshly isolated human myocyte suspensions to determine the effects of U-II and UT-A on levels of phospholamban phosphorylation. Representative Western blots are shown in Figure 5A for PLB (serine 16 and threonine 17). PLB phosphorylation levels at serine 16 were virtually undetectable in both nonfailing and failing myocytes and were not altered by exposure to U-II or UT-A (Figure 5A). In nonfailing myocytes, U-II induced an inverse dosage-dependent phosphorylation of PLB at threonine 17 above baseline (Figure 5B) and had no effect on failing myocytes (Figure 5C) when the data was normalized by total PLB abundance. In addition, UT-A had no effect on the phosphorylation level of PLB at threonine-17 in both nonfailing and failing myocytes (Figure 5B and 5C).
Figure 5.
(A) Western blots for total phospholamban (PLB), phospholamban phosphorylation at Serine 16 (PLB-PS16) and phospholamban phosphorylation at Threonine-17 (PLB-PT17) in nonfailing and failing human myocytes before and after exposure to U-II and UT-A. Mean PLB-PT17/Total PLB levels for (B) nonfailing and (C) failing human myocytes before and following exposure to U-II and UT-A. † denotes p<0.01 within groups compared to Baseline.
Discussion
The present studies demonstrate striking differences in the direct myocardial actions of exogenous and endogenous U-II in failing and nonfailing human myocardium. By using high quality isolated myocardial preparations under controlled in vitro conditions, our methods permitted fair assessment of pathology-related differences, independent of potentially confounding effects of in vivo vasoconstriction, loading conditions and/or other neurohormonal influences. In nonfailing myocardium, exogenous U-II increased developed force and the rates of force generation and decline in association with increases in the phosphorylation level of PLB at threonine-17 and RCC amplitude suggesting increases in SR Ca2+ load. However, in failing myocardium exogenous U-II did not increase developed force or the rates of force generation and decline in the absence of changes in PLB phosphorylation or RCC amplitude. Opposite positive inotropic responses to acute UT-A in failing myocardium suggest that endogenous myocardial or vascular U-II is modulating contractility via an autocrine or paracrine effect in the failing heart. These findings indicate modulation of myocardial contractility by endogenous U-II and suggest a potential therapeutic application of UT-A in patients with advanced heart failure.
The syndrome of heart failure is characterized by the chronic activation of several neurohormonal systems.22-24 Recent studies have identified the endogenous U-II system as a potential contributor to cardiovascular pathology, including heart failure. While U-II is present throughout the cardiovascular system and is upregulated in heart failure,3-6, 8, 9 the myocardial actions of U-II have been obscured by species- and disease-related differences.6, 10, 12, 25 In vivo myocardial responses to U-II may also be influenced by alterations in coronary vascular tone.26, 27 In this context, our use of isolated myocardial preparations from both failing and nonfailing human hearts to examine the actions of both exogenous and endogenous U-II on in vitro contractile performance, independent of alterations in blood flow, loading conditions and other neurohormonal influences, is unique among published studies to date.
In nonfailing myocardium, exogenous U-II increased contractility in a concentration-dependent manner. These findings are consistent with previous studies in which U-II increased contractile force generation in isolated human right atrial and ventricular trabeculae.11, 12 However, in nonfailing ventricular trabeculae we observed a greater positive inotropic response to U-II than previously reported results derived from a small number of heterogeneous human hearts.12 Beyond our larger sample size and segregation of failing and nonfailing hearts, our use of perfusion-based cardioplegia and very thin trabeculae without adrenergic-receptor pretreatment also may have contributed to the higher levels of developed force we observed under basal conditions and following U-II exposure. We also observed that U-II increased the rates of force generation and relaxation in nonfailing myocardium. These findings are consistent with increases in the efficiency of intracellular Ca2+ cycling, and the associated increases in the phosphorylation of PLB at threonine-17 and RCCs indicate that SR Ca2+ activity and load is indeed increasing in response to exogenous U-II in nonfailing myocardium. Our observations of an increase in PLB phosphorylation at threonine-17 suggests that the increase in SR Ca2+ load and developed force in response to exogenous U-II in nonfailing myocardium is mediated, at least in part, through CaMKII signaling.
In contrast, studies in failing myocardium revealed that exogenous U-II did not increase contractility and exhibited dose-dependent decreases in developed force and rates of force generation and relaxation. These divergent myocardial responses to U-II in the presence and absence of heart failure are analogous to the divergent vascular responses to U-II reported by Lim et al. in which vasodilation was observed in normal subjects and vasoconstriction was observed in heart failure.27 The lack of positive inotropic responses to U-II in failing myocardium were associated with an inability to augment RCC amplitude, suggesting an inability to augment Ca2+ cycling or SR Ca2+ load. In this regard, U-II had no effect on the phosphorylation levels of PLB at threonine 17 in failing hearts. Thus, the CaMKII-dependent PLB phosphorylation implicated in the ability of U-II to increase SR load and contractility in the nonfailing heart does not appear to occur with exogenous U-II in the failing heart. Though additional experiments with skinned fiber preparations (data not shown) did not indicate that U-II affected the force-Ca2+ relationship in nonfailing or failing myocardium, concerns about disruption of UT receptors and downstream signaling by cell permeabilization in these experiments do not permit us to exclude a direct action of U-II on myofilament Ca2+ sensitivity.
Our study indicates that UT-A induces a pronounced increase in contractility which is accompanied by an increase in the rates of force generation and relaxation in failing myocardium. Given that these responses were observed in an isolated tissue preparation, our findings suggest that elevated endogenous myocardial U-II exerts a negative inotropic action via an autocrine and/or paracrine mechanism that is independent of changes in tissue perfusion, loading conditions or circulating neurohormonal mediators in the nonfailing human heart. In addition, positive inotropic responses to UT-A in failing myocardium suggest that the limited response to exogenous UII may be due to previous receptor occupancy. Several factors support the conclusion that responses to UT-A are consequent to inhibition of endogenous tissue U-II activity. First, recent studies demonstrate that GSK248451A is a potent peptidic antagonist with minimal intrinsic activity and high specificity for the U-II receptor.17 Indeed, in the present study, administration of high-concentration exogenous U-II during ongoing UT-A exposure, induced virtually no physiological changes in both failing and nonfailing myocardium. The fact that contractile responses to UT-A were different in failing and nonfailing myocardium also argues against nonspecific responses. Finally, the observations that contractile responses to UT-A tended to be the inverse of the responses to exogenous U-II in the failing myocardium suggests that UT-A modulated the effects of endogenous U-II within the myocardial tissue.
Overall, the present studies suggest that endogenous U-II is a negative regulator of cardiac contractility in patients with advanced heart failure. This conclusion is based on previous studies demonstrating increases in myocardial U-II expression and content in failing human hearts,6 our demonstration that exogenous U-II has negative inotropic actions in failing myocardium that are independent of perfusion or loading conditions, and the demonstration that UT-A increases contractility in an isolated tissue model. The positive inotropic effect of UT-A in failing myocardium occurs as a result of an inhibition of endogenous U-II to negatively regulate myocardial contractility. Though the present studies do not address whether the contractility modulating actions of U-II and UT-A will be sustained or attenuated during longer time frames, preclinical data indicate that chronic UT-A treatment reduced adverse cardiac remodeling and lung congestion at eight weeks following experimental myocardial infarction in rats.28 Recent studies also implicate U-II in calcineurin-mediated hypertrophic signaling in human hearts.29 Thus, our findings further support an emerging therapeutic role for UT-A in the treatment of patients with advanced heart failure.
Clinical Perspective.
Effective evidence-based pharmacotherapy for congestive heart failure (HF) consists primarily of agents that modulate endogenous vasoactive substances to exert favorable effects on the myocardium and/or peripheral vasculature. Some agents, like angiotensin antagonists, have little or no direct effects on myocardial systolic performance, yet produce favorable effects on the HF syndrome. Though beta-adrenergic receptor blockers may induce acute negative inotropic actions necessitating gradual dose titration, they ultimately achieve beneficial effects on myocardial performance. Interestingly, virtually all drugs with acute positive inotropic actions have proven ineffective as disease modifying therapies for heart failure. In this context, the present studies indicate that endogenous U-II is a negative regulator of cardiac contractility and that acute UT-A induces moderate positive inotropic actions in failing human myocardium. Given prior studies showing reduced adverse cardiac remodeling in animals, our results provide support for clinical trials examining the effects of UT-A on cardiac contractility and clinical outcomes in heart failure patients. Recognizing that many promising new therapies were ultimately found lacking when examined in patients already treated with angiotensin antagonists and beta blockers, trials of UT-A should be performed in patients who are already being treated with these agents. However, if a beneficial effect of sustained UT-A treatment is ultimately observed, it would counter the concept that all agents with acute positive inotropic actions are detrimental in favor of a paradigm that makes distinctions based on the mechanism through which contractility is supported.
Acknowledgements
The authors would like to thank the Heart Failure and Transplant nurses at Penn, Cardiothoracic surgeons and operating room staff at Penn, and the Gift-of-Life donor program (Philadelphia, PA) for their assistance with heart tissue procurement.
Funding Sources These studies were supported by funding from GlaxoSmithKline, Inc. through an industry-academic alliance (ADDI) with the University of Pennsylvania School of Medicine (Penn) and by funding from the National Institutes of Health, Bethesda, MD (AG17022 to K.B.M.).
Footnotes
Conflict of Interest Disclosures Michael P. Quaile, PhD, Stephen A. Douglas, PhD and Carie L. Kimbrough, MPH currently are and/or were employees of GlaxoSmithKline.
References
- 1.Francis GS. Pathophysiology of chronic heart failure. Am J Med. 2001;110:37S–46S. doi: 10.1016/s0002-9343(98)00385-4. [DOI] [PubMed] [Google Scholar]
- 2.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341:577–585. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 3.Lapp H, Boerrigter G, Costello-Boerrigter LC, Jaekel K, Scheffold T, Krakau I, Schramm M, Guelker H, Stasch JP. Elevated plasma human urotensin-II-like immunoreactivity in ischemic cardiomyopathy. Int J Cardiol. 2004;94:93–97. doi: 10.1016/j.ijcard.2003.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Ng LL, Loke I, O’Brien RJ, Squire IB, Davies JE. Plasma urotensin in human systolic heart failure. Circulation. 2002;106:2877–2880. doi: 10.1161/01.cir.0000044388.19119.02. [DOI] [PubMed] [Google Scholar]
- 5.Russell FD, Meyers D, Galbraith AJ, Bett N, Toth I, Kearns P, Molenaar P. Elevated plasma levels of human urotensin-II immunoreactivity in congestive heart failure. Am J Physiol Heart Circ Physiol. 2003;285:H1576–1581. doi: 10.1152/ajpheart.00217.2003. [DOI] [PubMed] [Google Scholar]
- 6.Douglas SA, Tayara L, Ohlstein EH, Halawa N, Giaid A. Congestive heart failure and expression of myocardial urotensin II. Lancet. 2002;359:1990–1997. doi: 10.1016/S0140-6736(02)08831-1. [DOI] [PubMed] [Google Scholar]
- 7.Richards AM, Nicholls MG, Lainchbury JG, Fisher S, Yandle TG. Plasma urotensin II in heart failure. Lancet. 2002;360:545–546. doi: 10.1016/s0140-6736(02)09709-x. [DOI] [PubMed] [Google Scholar]
- 8.Johns DG, Ao Z, Naselsky D, Herold CL, Maniscalco K, Sarov-Blat L, Steplewski K, Aiyar N, Douglas SA. Urotensin-II-mediated cardiomyocyte hypertrophy: effect of receptor antagonism and role of inflammatory mediators. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:238–250. doi: 10.1007/s00210-004-0980-z. [DOI] [PubMed] [Google Scholar]
- 9.Tzanidis A, Hannan RD, Thomas WG, Onan D, Autelitano DJ, See F, Kelly DJ, Gilbert RE, Krum H. Direct actions of urotensin II on the heart: implications for cardiac fibrosis and hypertrophy. Circ Res. 2003;93:246–253. doi: 10.1161/01.RES.0000084382.64418.BC. [DOI] [PubMed] [Google Scholar]
- 10.Zhou P, Wu SY, Yu CF, Wang H, Tang CS, Lin L, Yuan WJ. Effects of urotensin II on isolated rat hearts under normal perfusion and ischemia reperfusion. Sheng Li Xue Bao. 2003;55:442–448. [PubMed] [Google Scholar]
- 11.Russell FD, Molenaar P. Investigation of signaling pathways that mediate the inotropic effect of urotensin-II in human heart. Cardiovasc Res. 2004;63:673–681. doi: 10.1016/j.cardiores.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Russell FD, Molenaar P, O’Brien DM. Cardiostimulant effects of urotensin-II in human heart in vitro. Br J Pharmacol. 2001;132:5–9. doi: 10.1038/sj.bjp.0703811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penna C, Rastaldo R, Mancardi D, Cappello S, Pagliaro P, Westerhof N, Losano G. Effect of endothelins on the cardiovascular system. J Cardiovasc Med (Hagerstown) 2006;7:645–652. doi: 10.2459/01.JCM.0000242996.19077.ba. [DOI] [PubMed] [Google Scholar]
- 14.Dipla K, Mattiello JA, Jeevanandam V, Houser SR, Margulies KB. Myocyte recovery after mechanical circulatory support in humans with end-stage heart failure. Circulation. 1998;97:2316–2322. doi: 10.1161/01.cir.97.23.2316. [DOI] [PubMed] [Google Scholar]
- 15.Rossman EI, Petre RE, Chaudhary KW, Piacentino V, 3rd, Janssen PM, Gaughan JP, Houser SR, Margulies KB. Abnormal frequency-dependent responses represent the pathophysiologic signature of contractile failure in human myocardium. J Mol Cell Cardiol. 2004;36:33–42. doi: 10.1016/j.yjmcc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Janssen PM, Lehnart SE, Prestle J, Lynker JC, Salfeld P, Just H, Hasenfuss G. The trabecula culture system: a novel technique to study contractile parameters over a multiday time period. Am J Physiol. 1998;274:H1481–1488. doi: 10.1152/ajpheart.1998.274.5.H1481. [DOI] [PubMed] [Google Scholar]
- 17.Behm DJ, Stankus G, Doe CP, Willette RN, Sarau HM, Foley JJ, Schmidt DB, Nuthulaganti P, Fornwald JA, Ames RS, Lambert DG, Calo G, Camarda V, Aiyar NV, Douglas SA. The peptidic urotensin-II receptor ligand GSK248451 possesses less intrinsic activity than the low-efficacy partial agonists SB-710411 and urantide in native mammalian tissues and recombinant cell systems. Br J Pharmacol. 2006;148:173–190. doi: 10.1038/sj.bjp.0706716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quaile MP, Rossman EI, Berretta RM, Bratinov G, Kubo H, Houser SR, Margulies KB. Reduced sarcoplasmic reticulum Ca(2+) load mediates impaired contractile reserve in right ventricular pressure overload. J Mol Cell Cardiol. 2007;43:552–563. doi: 10.1016/j.yjmcc.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Petre RE, Quaile MP, Rossman EI, Ratcliffe SJ, Bailey BA, Houser SR, Margulies KB. Sex-based differences in myocardial contractile reserve. Am J Physiol Regul Integr Comp Physiol. 2007;292:R810–818. doi: 10.1152/ajpregu.00377.2006. [DOI] [PubMed] [Google Scholar]
- 20.Mattiello JA, Margulies KB, Jeevanandam V, Houser SR. Contribution of reverse-mode sodium-calcium exchange to contractions in failing human left ventricular myocytes. Cardiovasc Res. 1998;37:424–431. doi: 10.1016/s0008-6363(97)00271-x. [DOI] [PubMed] [Google Scholar]
- 21.Kubo H, Margulies KB, Piacentino V, 3rd, Gaughan JP, Houser SR. Patients with end-stage congestive heart failure treated with beta-adrenergic receptor antagonists have improved ventricular myocyte calcium regulatory protein abundance. Circulation. 2001;104:1012–1018. doi: 10.1161/hc3401.095073. [DOI] [PubMed] [Google Scholar]
- 22.Bolger AP, Sharma R, Li W, Leenarts M, Kalra PR, Kemp M, Coats AJ, Anker SD, Gatzoulis MA. Neurohormonal activation and the chronic heart failure syndrome in adults with congenital heart disease. Circulation. 2002;106:92–99. doi: 10.1161/01.cir.0000020009.30736.3f. [DOI] [PubMed] [Google Scholar]
- 23.Davila DF, Nunez TJ, Odreman R, de Davila CA. Mechanisms of neurohormonal activation in chronic congestive heart failure: pathophysiology and therapeutic implications. Int J Cardiol. 2005;101:343–346. doi: 10.1016/j.ijcard.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 24.Chatterjee K. Neurohormonal activation in congestive heart failure and the role of vasopressin. Am J Cardiol. 2005;95:8B–13B. doi: 10.1016/j.amjcard.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Ames RS, Sarau HM, Chambers JK, Willette RN, Aiyar NV, Romanic AM, Louden CS, Foley JJ, Sauermelch CF, Coatney RW, Ao Z, Disa J, Holmes SD, Stadel JM, Martin JD, Liu WS, Glover GI, Wilson S, McNulty DE, Ellis CE, Elshourbagy NA, Shabon U, Trill JJ, Hay DW, Ohlstein EH, Bergsma DJ, Douglas SA. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282–286. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- 26.Douglas SA, Sulpizio AC, Piercy V, Sarau HM, Ames RS, Aiyar NV, Ohlstein EH, Willette RN. Differential vasoconstrictor activity of human urotensin-II in vascular tissue isolated from the rat, mouse, dog, pig, marmoset and cynomolgus monkey. Br J Pharmacol. 2000;131:1262–1274. doi: 10.1038/sj.bjp.0703690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim M, Honisett S, Sparkes CD, Komesaroff P, Kompa A, Krum H. Differential effect of urotensin II on vascular tone in normal subjects and patients with chronic heart failure. Circulation. 2004;109:1212–1214. doi: 10.1161/01.CIR.0000121326.69153.98. [DOI] [PubMed] [Google Scholar]
- 28.Bousette N, Hu F, Ohlstein EH, Dhanak D, Douglas SA, Giaid A. Urotensin-II blockade with SB-611812 attenuates cardiac dysfunction in a rat model of coronary artery ligation. J Mol Cell Cardiol. 2006;41:285–295. doi: 10.1016/j.yjmcc.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Wang J, Russell FD, Molenaar P. Activation of calcineurin in human failing heart ventricle by endothelin-1, angiotensin II and urotensin II. Br J Pharmacol. 2005;145:432–440. doi: 10.1038/sj.bjp.0706217. [DOI] [PMC free article] [PubMed] [Google Scholar]