Abstract
Constipation is a common gastrointestinal complaint in clinical practice, affecting an estimated 27% of the population. Many patients are disappointed by current conventional treatments and, therefore, seek help from complementary and alternative medicine (CAM). Traditional Chinese medicine, is the most important part of CAM and has been practiced for treating diseases and promoting the health of humans for thousands of years, and has become a popular alternative choice. Although there are many Chinese herbal medicine (CHM) interventions available, and some have been verified by clinical trials, their efficacy and safety are still questioned by both patients and health care providers worldwide. The purposes of this review are, first, to appraise the qualities of individual study designs in the new Cochrane approach. Second, the benefits of individual CHM interventions or individual types of CHM intervention for the treatment of functional constipation are analyzed. Finally, valid and comprehensive conclusions are drawn, if applicable, in order to make clinical recommendations.
Keywords: Chinese herbal medicine, Functional constipation, Systematic review
INTRODUCTION
Constipation is a common gastrointestinal complaint in clinical practice. It affects an estimated 12%-19% of Americans[1], 14% of Asian[2], and up to 27% of the general population depending on demographic factors, sampling, and definition[3]. A variety of over-the-counter medications are available. It is estimated that in the US alone, more than $800 million is spent annually on laxatives[4], with each constipated patient spending approximately $7900, accounting for 6.5% of the total medical expenditure on lower gastrointestinal diseases[5]. However, many patients are disappointed by current conventional treatments[6,7] and, therefore, seek help from complementary and alternative medicine (CAM)[8].
Many traditional Chinese medicine (TCM) interventions have been used for the treatment of constipation. A recent review listed the current clinical research findings of TCM interventions on treating functional constipation (FC)[9]. However, an analysis on the benefits of individual interventions or individual types of interventions, and the qualities of individual study designs has not been undertaken. To draw valid and comprehensive conclusions and make clinical recommendations, a systematic review of Chinese herbal medicine (CHM) for FC is necessary.
This review aimed to determine the efficacy and safety of CHM for the treatment of FC by summarizing current available randomized controlled trials (RCTs) according to the Cochrane approach, newly revised in 2008.
CRITERIA AND METHODS FOR LITERTURE SEARCH
Criteria for considering studies for this review
The criteria for considering studies for this review are as follows. (1) Types of studies. Only RCTs without restriction on language and publication types were included; pseudo-RCTs were not considered; (2) Types of participants. Patients of both sexes and of any age or any ethnic group with diagnosed FC according to the Rome criteria (Rome I, II or III) were included while those with secondary constipation due to medication and/or other diseases were excluded; (3) Types of interventions. Any form of CHM in any dose or as add-on combination treatment was considered, including oral and external preparations. Comparisons could include placebo, no intervention, acupuncture, massage, Western conventional medication (WCM) or any other interventions. Studies comparing one kind of CHM to another CHM were also included; (4) Types of outcomes. The responder rate of patients with a mean increase of ≥ 1 complete spontaneous bowel movement (CSBM) per week was considered a primary outcome. This outcome by combining a subjective measure of the completeness of defecation with an objective measure of stool frequency was considered to be clinically meaningful[10]. If this outcome measure was not used in the study, the overall effectiveness assessment according to the references of Criteria of Diagnosis and Therapeutic Effect of Diseases and Syndromes in Traditional Chinese Medicine[11], Guidelines for Clinical Research on New Chinese Herbal Medication[12], Guidelines for Clinical Research on New Chinese Herbal Medication (Draft)[13] or criteria made by the authors with details and comparable definitions were also considered. Based on the above criteria, interventions which resulted in improvement in general constipated symptoms and/or objective examination indices, for general improvement ≥ 30% compared to their baselines, were counted as effective. Secondary outcomes including (a) Changes in individual symptoms, such as stool frequency, straining, completeness of defecation; (b) Changes in examination indices, such as blood nitric oxide (NO) and substance P (SP) levels, total colon transit test (TCTT) and anorectal pressure; (c) Changes in quality of life assessment as assessed with the Health Related Quality of Life (HRQOL) or other validated scales; (d) Adverse events (AEs), such as functional injury of liver or kidney, nausea, vomiting, diarrhea and allergic reaction.
Search methods for identification of studies
All relevant studies regardless of language or publication status were identified by searching the following databases from 1994, the year of the establishment of Rome criteria, up to the May 18 of 2009. (1) Ovid SP, which included the databases of Cochrane DSR (Cochrane Database of Systematic Reviews), ACP Journal Club, DARE (Database of Abstracts of Reviews of Effects), CCTR (Cochrane Central Register of Controlled Trials), CMR (Cochrane Methodology Register), HTA (Health Technology Assessment), and NHSEED (NHS Economic Evaluation Database), AMED, BIOSIS Previews (2001-2006), Biological Abstracts (1994-2000), Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations (1950 to Present), Ovid MEDLINE(R) (1950 to Present), Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations Present, and Ovid MEDLINE(R) Daily Update Present. Detailed search strategy presented in Table 1. (2) VIP Citation Databases (VIP); (3) Traditional Chinese Medical Database System (TCM Database System); (4) China Journal Net (CJN). The common search strategy for VIP, TCM Database System and CJN is listed in Table 2. The Chinese wordings were presented in Pinyin.
Table 1.
Search strategy for Ovid SP (advanced Ovid search)
| No. | Searches |
| 1 | case*.m_titl |
| 2 | clinical observation.mp |
| 3 | clinical trial.mp |
| 4 | clinical study.mp |
| 5 | efficacy.mp |
| 6 | effectiveness.mp |
| 7 | 1 OR 2 OR 3 OR 4 OR 5 OR 6 |
| 8 | random.mp |
| 9 | randomi*ed.mp |
| 10 | randomi*zation.mp |
| 11 | 8 OR 9 OR 10 |
| 12 | functional constipation.mp |
| 13 | irritable bowel syndrome.m_titl |
| 14 | ibs.m_titl |
| 15 | 13 OR 14 |
| 16 | 12 NOT 15 |
| 17 | rome.mp |
| 18 | 16 AND 17 |
| 19 | Chinese medicine*.mp |
| 20 | herbal medicine*.mp |
| 21 | herb*.mp. |
| 22 | Chinese adj10 oriental medicine*.mp |
| 23 | 19 OR 20 OR 21 OR 22 |
| 24 | 7 AND 11 AND 18 AND 23 |
"*"was used for truncation.
Table 2.
Common search strategy for VIP, TCM Database System and CJN
| Title contains “case (Li)” OR Title/Abstract/Keyword contains [“efficacy observation (LiaoXiao GuanCha)” OR “efficacy comparison (LiaoXiao BiJiao)” OR “efficacy analysis (LiaoXiao FenXi)” OR “clinical observation (LinChuang GuanCha)” OR “clinical research (LinChuang YanJiu)” OR “clinical trial (LinChuang ShiYan)”] |
| AND |
| Text word contains “random (SuiJi)” |
| AND |
| Title/Abstract/Keyword contains “functional constipation (GongNengXing BianMi)” NOT Title contains [“irritable bowel syndrome (ChangYiJi ZongHeZheng)” OR “ibs”] |
| AND |
| Text word contains [“rome (LuoMa)” OR “rome”] |
| AND |
| Text word contains [“Chinese medicine (ZhongYao/ZhongYiYao)” OR “herbs (CaoYao)” OR Chinese herbal medicine (ZhongCaoYao) OR “Chinese proprietary medicine (ZhongChengYao)”] |
TCM: Traditional Chinese medicine; CJN: China Journal Net.
Data collection and analysis
The title and abstract of the search results were scanned and full articles for all potentially relevant trials were retrieved. A data extraction form was used to extract data on study characteristics including methods, participants, interventions and outcomes. The reasons for the exclusion of studies were recorded accordingly.
The treatment effects of all CHM interventions were analyzed using Review Manager (Version 5.0). Mean difference with 95% confidence interval was used for continuous data while relative risks with 95% confidence interval was used for binary data. The risk of bias on sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other potential threats to validity were assessed as “YES” (low risk of bias), “NO” (high risk of bias) and “UNCLEAR” (uncertain risk of bias) according to the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions 5.0.1[14].
SUMMARY OF LITERATURE
Description of studies
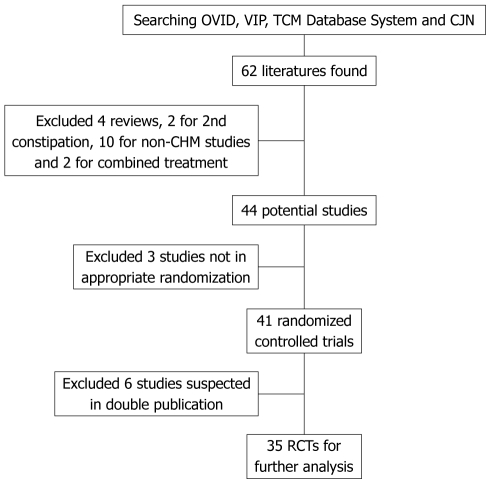
A total of 62 articles were identified. Of these, four articles were excluded because they were reviews[9,15-17], two articles were excluded because they dealt with secondary constipation[18,19], ten articles were excluded because they did not include CHM[20-29], and two articles were excluded because they evaluated combination treatment of WCM and CHM by comparing with massage or WCM[30,31]. This left 44 studies which claimed to be “randomized controlled” trials for FC.
Of these studies, three were not real RCTs because they used the admission sequence for treatment allocation, and thus were excluded[32-34]. Six studies were suspected of being published more than once by the authors or publishers, and were excluded[35-40]. This further screening left 35 studies for review. The screening process is summarized in a flow diagram (Figure 1).
Figure 1.
Flow diagram for literature search. TCM: Traditional Chinese medicine; CJN: China Journal Net; CHM: Chinese herbal medicine; RCTs: Randomized controlled trials.
Characteristics of included studies
A total of 3571 participants (ranging in age from 1 mo to 93 years) were included in these 35 studies. With the exception of two[41,42] in 3 parallel groups, all studies used a 2 parallel group design. Thirty six CHM interventions, including add-on with WCM treatment, were investigated by comparing with another CHM and/or WCM. The details of CHM interventions are listed in Table 3.
Table 3.
Details of CHM interventions in the included studies
| Intervention | Preparation form | n |
| LiuWei Auxiliary | Capsule | 4 |
| LiuWeiAnXiao Capsule/ LiuWeiNengXiao Capsule | ||
| MaRen Auxiliary | 7 | |
| MaRen Pill/MaZiRen Pill | Pill | 2 |
| MaRenRunChang Wan | Pill | 1 |
| Modified MaRenRunChang Wan | Pill | 1 |
| MaRen Capsule | Capsule | 1 |
| MaRen Soft Capsule | Capsule | 2 |
| RunChangTongBianNongSuo Pill | Pill | 2 |
| Others (in single investigation) | Decoction (w/o modification) | 16 |
| Decoction (w/modification) | 4 | |
| Capsule | 4 | |
| Pill | 3 | |
| Solution | 1 | |
| Granule | 1 |
CHM: Chinese herbal medicine.
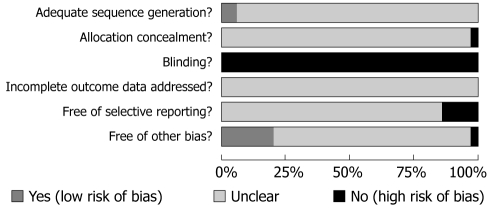
Risk of bias: The methodological quality of each study’s randomization sequence, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and potential threats are summarized in Figures 2 and 3.
Figure 2.
Methodological quality graph: judgments about each methodological quality item presented as percentages across all included studies.
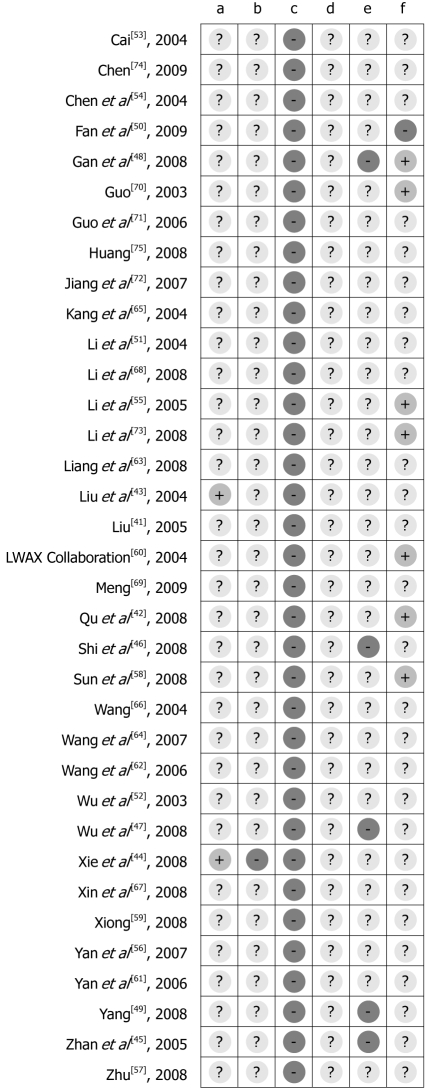
Figure 3.
Methodological quality summary: judgments about each methodological quality item for each included study. a: Adequate sequence generation? b: Allocation concealment? c: Blinding? d: Incomplete outcome data addressed? e: Free of selective reporting? f: Free of other bias?
Randomization & allocation concealment: Only two studies clearly stated a random component in the sequence generation process, Liu et al[43] used randomization software while Xie et al[44] used an open random allocation schedule in sequence generated with a random number table. For the others, the words “random allocation” were cited in abstracts and/or main texts but without description.
Blinding: None of the participants, personnel or outcome assessors were blinded in any of these studies. Although minority outcome measures were based on the objective examination results, such as blood NO and SP levels, total colon transit time and anorectal pressure, the risk of both performance bias and detection bias with regard to general symptom improvement and safety issues were deemed very high.
Flow of participants and intention-to-treat: None of the trials reported the withdrawal, drop-out and/or loss to follow up rates. The method of handling missing data regarding intention-to-treat or per protocol analysis was not addressed.
Selective outcome reporting: Five studies had a high risk of bias with regard to selective outcome reporting[45-49] because the data on individual symptoms, overall improvement and colon transit test pre-specified were reported incompletely in the results. Thus further meta-analysis could not be implemented.
Other potential threats to validity: MaRen Capsule, which is derived from the ancient formula MaZiRenWan, was used as the control for treating FC in the Syndrome of Qi and Yin Deficiency in the study by Fan et al[50]. As MaZiRenWan is the representative formula for treating heat constipation (excessive constipation), it is therefore, not suitable for patients suffering from FC with Qi and Yin deficiency, and such a study design was a potential source of bias in assessing the efficacy and safety of MaRen Capsule.
Effects of interventions
None of the trials reported the responder rate on complete spontaneous bowel movement; instead overall effectiveness in which patients with improvement in general constipation symptoms and/or objective examination indices, was commonly used as a primary outcome measure.
CHINESE HERBAL MEDICINE VS PLACEBO/NO TREATMENT (COMPARISON 01)
None of the included trials used a placebo control, but one used no treatment as a control[51]. General treatment was allowed for all participants in both groups, such as increased fibre and liquid intake, physical exercise and defecation habit training. The total effectiveness rates of Modified MaRenRunChang Pill and the control were 91.4% and 59.6%, respectively (P < 0.01). Reported AEs included two cases of diarrhea and one case of nausea and loss of appetite.
CHINESE HERBAL MEDICINE VS WESTERN CONVENTIONAL MEDICINE (COMPARISON 02)
Twenty-six studies, including two with three parallel groups[41,42], tested 24 different CHM interventions compared with cisapride, polyethylene glycol 4000 (PEG), mosapride, phenolphthalein, itopride and bifidobacterium.
CHM vs cisapride
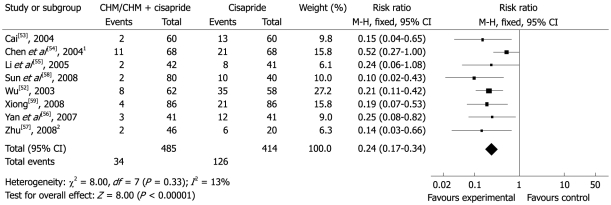
Nine studies compared nine different CHM interventions with cisapride or add-on with cisapride and/or lactulose[45,52-59]. Eight studies reported total effectiveness rates in the group using CHM which varied from 83.3%-96.7%, while these rates varied from 39.6%-80.5% in the cisapride group (RR 0.24, 95% CI 0.17 to 0.34). The difference suggested that CHM was more effective than cisapride (Figure 4).
Figure 4.
Comparison of CHM vs cisapride, failure to respond at endpoint. 1Add-on treatment: CHM + cisapride vs cisapride; 2Add-on combined treatment: CHM + (cisapride + lactulose) vs cisapride + lactulose. CHM: Chinese herbal medicine.
The study by Li et al[55] showed that 92.6% of the CHM group and 68.3% of the cisapride group had normal stool consistency on the fifteenth day of treatment (half of treatment course) (P < 0.01). Sustainable improvement was noted on the seventh day of the follow-up period when 95.2% and 80.3% of participants reported normal stool consistency, respectively (P < 0.05). The stool interval was shortened significantly from 4.4 ± 1.4 d to 2.2 ± 1.3 d during treatment and 2.1 ± 1.1 d during the post-treatment follow-up period for the CHM treatment group, but not for the cisapride control group. Both groups had shown a significant increase in barium strips excretion in the total colon transit test from 30.13% ± 9.2% (before treatment) to 69.45% ± 11.32% (during treatment) and 73.2% ± 12.16% (after treatment) in the treatment group and from 29.86% ± 11.34% to 41.43% ± 12.05% and 48.01% ± 12.76%, respectively, for the controlled group (P < 0.05). In the study by Zhan et al[45], patients showed a significant improvement in stool type, ease of defecation, stool frequency and total colon transit test in both groups (P < 0.05). Statistically significant differences between the groups were found only for stool type and difficulty of defecation (P < 0.01).
Six out of nine studies did mention safety measures. All adverse events were reported among patients receiving cisapride in two studies. 43 (43/68) cases in the study by Chen et al[54] reported diarrhea, gas or abdominal pain and four needed to reduce the dose by half due to adverse reactions. In the study by Cai[53], one (1/60) case reported headache and two (2/60) cases reported lassitude after taking cisapride. No cases were withdrawn due to adverse events.
CHM vs PEG
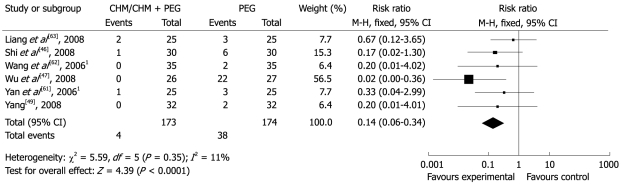
Eight studies compared seven different CHM interventions with PEG or add-on with PEG treatment[43,46,47,49,60-63]. Six studies reported that the total effectiveness rates in the group using CHM or add-on with PEG varied from 92%-100%, while these rates were 18.5%-94% in the PEG group (RR 0.14; 95% CI 0.06-0.34). This finding suggested that CHM or add-on with PEG was more effective than PEG alone (Figure 5).
Figure 5.
Comparison of CHM/CHM + PEG vs PEG, failure to respond at endpoint. 1Add-on combined treatment: CHM + PEG vs PEG. PEG: Polyethylene glycol.
The study by Liang et al[63] comparing CHM with PEG showed a statistically significant improvement with regard to abdominal bloating and TCM symptoms (P < 0.05), but not stool frequency, hardness of stool, straining and abdominal pain (P > 0.05). With the exception of time to defecation, the study by Wu et al[47], showed that CHM, when compared with PEG significantly improved all symptoms, including stool frequency, sensation of urge to defecate, straining, dry stool, use of rescue drug and total symptom score (P < 0.01). The study by Yang[49] comparing CHM with PEG control, reported that CHM resulted in significant benefit with regard to stool frequency, stool type, difficulty and time of defecation during treatment (P < 0.05). Liu et al[43] showed that the effectiveness of the CHM intervention was equivalent to PEG with regard to stool frequency, stool type, straining, abdominal bloating, abdominal pain and loss of appetite and excretion rate of the total colon transit test. The LiuWeiAnXiao Capsule Collaboration Group[60] reported that those who took CHM showed statistically significant improvement in QoL for components on general feeling, vitality, and daily activities (P < 0.05) and difficulty of defecation during follow-up (P = 0.026), but not on stool frequency, stool type, and excretion rate of the total colon transit test (P > 0.05). Since the outcome measures among these five studies were on different scales, further meta-analysis was not implemented.
Three studies mentioned the issue of safety[43,60,61]. Only one AE (i.e. abnormal facial muscle tone) was reported by a patient receiving PEG from the study of LiuWeiAnXiao Capsule.
CHM vs mosapride
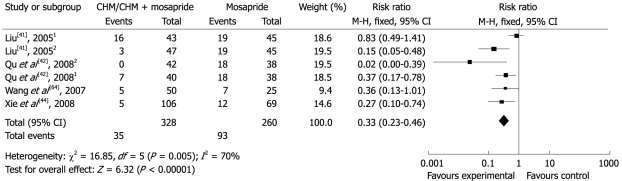
Four studies compared four different CHM interventions with mosapride[41,42,44,64]. Two of them in three parallel groups included a CHM arm, a mosapride arm and a CHM plus mosapride treatment arm[41,42]. All studies reported total effectiveness rates in the group using CHM or add-on with mosapride which varied from 65.2%-100%, while the effectiveness rate was 54.4%-82.6% in the mosapride group (RR 0.33; 95% CI 0.23 to 0.46). This suggested that CHM or add-on with mosapride was more effective than mosapride alone (Figure 6).
Figure 6.
Comparison of CHM/CHM + mosapride vs mosapride, failure to respond at endpoint. 13 arms study: CHM vs mosapride; 23 arms study: CHM + mosapride vs mosapride.
The study by Xie et al[44] comparing CHM with mosapride showed a statistically significant improvement in the bothersome of constipation, straining and TCM Qi deficient symptoms (P < 0.01). The combined treatment group in Liu’s study[41] showed symptom relief with regard to abdominal pain, abdominal bloating and loss of appetite which was significantly better than both CHM and mosapride alone (P < 0.01).
Two studies evaluated the safety of CHM interventions. Two patients (2/38) with abdominal pain from the CHM arm, two (2/40) with diarrhea from the mosapride arm and one (1/42) with diarrhea from the combined treatment arm were reported in the study by Qu et al[42]. Liu[41] reported 17 AEs, nine patients with abdominal pain (two from the CHM arm, two from the mosapride arm and three from the combined treatment arm), five with diarrhea (two from the CHM arm and three from the combined treatment arm), two with active bowel sounds and one with dry mouth (both of the latter from the mosapride arm).
CHM vs phenolphthalein
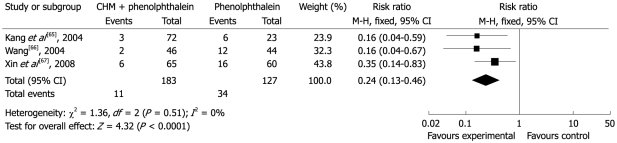
Three studies compared three different CHM interventions with phenolphthalein[65-67]. The total effectiveness rates in the group treated with CHM were 90.8%-95.8%, while the comparable rates for phenolphthalein were 72.7%-73.9% (RR 0.24; 95% CI 0.13-0.46). Thus the results suggested that CHM was more effective than phenolphthalein (Figure 7). Only Kang et al[65] mentioned that no AEs were observed.
Figure 7.
Comparison of CHM vs phenolphthalein, failure to respond at endpoint.
Other
The study by Li et al[68] showed that the total effectiveness of the combined treatment (a TCM intervention add-on with itopride) and itopride alone were 92% and 76%, respectively (P < 0.05). In total three cases of mild abdominal pain and two cases of loose stool were reported in the combined treatment arm while two cases of mild abdominal pain were reported in the itopride arm.
The study by Meng[69] showed that the total effectiveness of the combined treatment (a TCM intervention add-on with live bifidobacterium) and live bifidobacterium alone were 94% and 64%, respectively (P < 0.01). No studies reported safety issues.
CHINESE HERBAL MEDICINE VS CHINESE HERBAL MEDICINE (COMPARISON 03)
Ten different CHM interventions were tested in seven trials[48,50,70-74]. Six of them used MaZiRenWan/MaRenWan or its modifications as control (MaRen auxiliary) while one used LuiWeiNengXiao capsules as control (LuiWei auxiliary).
CHM vs MaRen auxiliary
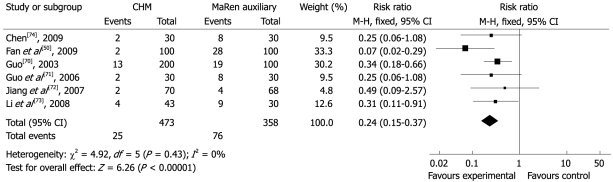
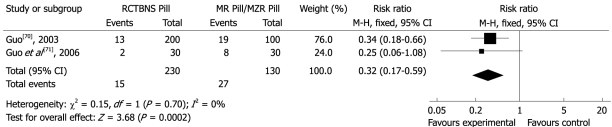
The total effectiveness rates in the group treated with CHM varied from 90.7%-98%, and was 70%-94.1% in the MaRen auxiliary (RR 0.24, 95% CI 0.15 to 0.37) (Figure 8). RunChangTongBian NongSuo Pill was the only intervention compared with the same control in two studies[70,71] (RR 0.32, 95% CI 0.17 to 0.59) (Figure 9). These results suggested that the CHM interventions developed by the study authors were more effective than the MaRen auxiliary.
Figure 8.
Comparison of CHM vs MaRen auxiliary, failure to respond at endpoint.
Figure 9.
Comparison of RunChangTongBianNongSuo Pill vs MaRen Pill/MaZiRen Pill, failure to respond at endpoint.
Li et al[73] found that CHM resulted in a statistically significant improvement in time of defecation, abdominal bloating and pain, incompleteness of defection, and total symptoms score by comparing QiLang Mixture with MaRenRuan Capusle (P < 0.05), but not on stool frequency, straining and stool type (P > 0.05). From the studies by Guo et al[70,71] published in 2003 and 2006, RunChangTongBian NongSuoWan resulted in significant improvement in the main constipation related symptoms, such as incompleteness and difficulty of defecation, when compared with MaRen Pill/MaZiRen Pill (P < 0.05), but it did not improve the minor symptoms of dry mouth, dizziness and palpitation, and blood NO and SP levels (P > 0.05).
Only two studies reported adverse effects[50,70]. Three cases treated with YiQiRunChang Capsule reported diarrhea or abdominal pain while no AEs were observed in Guo’s study.
CHM vs LiuWei auxiliary
The study by Gan et al[48] compared TiaoChang Decoction with LuiWeiNengXiao capsules. The total effectiveness rates were 90% and 83.3%, respectively. Patients taking TiaoChang Decoction showed a significant improvement in constipation-related symptoms compared with LiuWeiNengXiao Capsule, and both were safe for consumption without any prominent AEs reported.
CHINESE HERBAL MEDICINE VS NON-PHARMACEUTICAL INTERVENTIONS (COMPARISON 04)
The study by Huang[75] compared massage with FuFangLuHui capsules. The total effectiveness rates were 97.8% and 53.3%, respectively (P < 0.05). Massage was more effective in improving stool frequency, straining, lumpy or hard stool, time to defecation, sensation of anorectal blockage, manual maneuvers to facilitate the process , sensation of incomplete evacuation and stool weight for each defecation. No AEs were reported.
SUMMARY
This review analyzed 35 randomized trials that were conducted in China and published in Chinese medical journals. The results favored the tested CHM interventions in comparison with controls, WCM and some CHM interventions, but not when compared with massage; however, there was not enough replicable evidence to conclude that any specific CHM intervention is effective for FC.
Furthermore, the results of these trials should be interpreted with caution due to the generally low methodological quality of the included studies. First, all studies provided insufficient information on how the random allocation was generated and/or concealed, which is necessary to avoid selection bias. It has been shown that trials with inadequate concealment of allocation or unclear reporting of the technique used were on average 18% more “beneficial” than effect estimates from trials with adequate concealment (95% CI 5% to 29%)[14]. Second, none of the studies used any blinding method. Lack of blinding to participants, healthcare providers and assessors can introduce performance bias and detection bias. Lack of blinding can also be associated with exaggerated estimated intervention effects-by 9% on average, measured as odds ratio[14]. Third, none of the included studies addressed incomplete outcome data, such as missing data due to attrition or exclusions. Inadequate handling of missing data can compromise statistical analysis. Fourth, none of studies had been registered, despite a statement issued in 2004 by the International Committee of Medical Journal Editors (ICMJE) requiring that all clinical trials must be registered in order to be considered for publication[76]. Therefore, protocols were not available to confirm free of selective reporting, especially for those trials which tended to address statistical conclusions instead of listing the details of individual outcomes[46-48]. Fifth, the majority of experimental CHM interventions were prepared by the investigators without detailed information describing underlying rationales on formulation, dosage, manufacturing process, etc. The quality control processes of their tested interventions are unknown. For all these reasons, independent validation of the findings of these trials is necessary.
With regard to selection of an active control in the trials, it is necessary to consider whether there is evidence to support the efficacy of an active control. If no evidence supports the selection of a control, it may bias the trial results. Among all the active controls selected, only PEG had good supporting evidence for the treatment of constipation[6]. Cisapride, mosapride and itopride have been used as gastroprokinetic agents for the symptomatic treatment of functional dyspepsia. They were thought to be useful for the treatment of constipation due to their stimulating effect on gastrointestinal motility as reported in recent research findings. However, cisapride was suspended by the US Food and Drug Administration in 2000 because of its side effects of heart rhythm disturbances, including QT prolongation[77,78]. More clinical data, especially evidence from systematic reviews, are urged to confirm the efficacy and safety of mosapride, itopride, phenolphthalein and bifidobacterium for the treatment of FC. Therefore, the effectiveness of tested CHM interventions is not conclusive, despite beneficial findings from meta-analyses.
The herbal medicines evaluated in this review generally appeared to be safe and well tolerated by patients. However, the safety of their use for FC could not be confirmed because only 48.6% studies (17/35) mentioned the safety of interventions or investigated AEs as one of the secondary outcome measures. It is recommended that more attention should be given to both recording and reporting the harmful effects of these interventions.
This systematic review has several methodological limitations. First, all the data were collected from the reports without directly contacting the trial authors. Therefore, many items of the “Risk of bias” assessment tool could only be classified as “unclear”. Second, most of the included studies were small and without formal sample size calculation. The results were likely to be underpowered. Third, in some cases, different CHM interventions were grouped together for analysis. The results might have been compromised by the heterogeneity within each CHM intervention and by the study design. Fourth, in general, the concept of TCM syndrome was not considered when analyzing the data, as some studies only targeted a WCM disease in a particular type of TCM symptom. Therefore, the actual therapeutic effect might not have been fully captured.
CONCLUSION
CHM interventions or CHM combined treatments showed benefit in the treatment of FC when compared with cisapride, PEG, mosapride, phenolphthalein, itopride and bifidobacterium alone, but not when compared with massage. However, the evidence and reliability of these conclusions are compromised by methodological flaws and lack of replicable validation. Further well-designed, randomized, double-blind, placebo-controlled trials need to be carried out and reported in detail according to the Consolidated Standards of Reporting Trials (CONSORT) and/or CONSORT for TCM Statements.
Footnotes
Supported by Health and Health Services Research Fund of Hong Kong Health, Welfare and Food Bureau, No. 05060161
Peer reviewer: Mohammad Abdollahi, Professor, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran 1417614411, Iran
S- Editor Tian L L- Editor Webster JR E- Editor Lin YP
References
- 1.Eoff JC. Optimal treatment of chronic constipation in managed care: review and roundtable discussion. J Manag Care Pharm. 2008;14:1–15. doi: 10.18553/jmcp.2008.14.S8-A.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng C, Chan AO, Hui WM, Lam SK. Coping strategies, illness perception, anxiety and depression of patients with idiopathic constipation: a population-based study. Aliment Pharmacol Ther. 2003;18:319–326. doi: 10.1046/j.1365-2036.2003.01663.x. [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA, Corazziari E, Delvaux M, Spiller RC, Talley NJ, Thompson WG, Whitehead WE. Rome III: The Functional Gastrointestinal Disorders. 3rd ed. McLean, VA: Degnon Assoc; 2006. [Google Scholar]
- 4.Yamada T, Alpers DH, Kaplowitz N, Laine L, Owyang C, Powell DW. Textbook of gastroenterology. 4th ed. Volume 1. Philadelphia, USA: Lippincott Williams & Wilkins; 2003. pp. 894–910. [Google Scholar]
- 5.Nyrop KA, Palsson OS, Levy RL, Korff MV, Feld AD, Turner MJ, Whitehead WE. Costs of health care for irritable bowel syndrome, chronic constipation, functional diarrhoea and functional abdominal pain. Aliment Pharmacol Ther. 2007;26:237–248. doi: 10.1111/j.1365-2036.2007.03370.x. [DOI] [PubMed] [Google Scholar]
- 6.Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100:936–971. doi: 10.1111/j.1572-0241.2005.40925.x. [DOI] [PubMed] [Google Scholar]
- 7.Youssef NN, Sanders L, Di Lorenzo C. Adolescent constipation: evaluation and management. Adolesc Med Clin. 2004;15:37–52. doi: 10.1016/j.admecli.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 8.van Tilburg MA, Palsson OS, Levy RL, Feld AD, Turner MJ, Drossman DA, Whitehead WE. Complementary and alternative medicine use and cost in functional bowel disorders: a six month prospective study in a large HMO. BMC Complement Altern Med. 2008;8:46. doi: 10.1186/1472-6882-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang FL, Li P. Current clinical researches and viewpoints of Chinese medicine on functional constipation. Huanqiu Zhongyiyao. 2008;1:56–60. [Google Scholar]
- 10.Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med. 2008;358:2344–2354. doi: 10.1056/NEJMoa0800670. [DOI] [PubMed] [Google Scholar]
- 11.The State Administration of traditional Chinese Medicine of the People’s Republic of China. Criteria of diagnosis and therapeutic effect of diseases and syndromes in traditional Chinese medicine. Beijing: Nanjing University Press; 1994. p. 11. [Google Scholar]
- 12.Ministry of Health of the People’s Republic of China. Guidelines for clinical research on new Chinese herbal medicine. Volume 1. Beijing: Ministry of Health of the People’s Republic of China; 1993. pp. 131–133. [Google Scholar]
- 13.Zheng XY. Guidelines for Clinical Research on New Chinese Herbal Medication (Draft) Beijing: China Medico-Pharmaceutical Science & Technology Puhlishig House; 2002. p. 123. [Google Scholar]
- 14.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1, Updated September 2008. The Cochrane Collaboration, 2008. Available from: URL: http://www.cochrane-handbook.org. [Google Scholar]
- 15.Jiang M, Xiong NN, Zhou XH, Shen H. Design of clinical trials of TCM new drugs in treatment of patients with chronic idiopathic constipation. Zhongguo Linchang Yaolixue Yu Zhiliao. 2005;10:594–597. [Google Scholar]
- 16.Huo LX, Zhang J, Jiang GP. Progression of Chinese and western medical treatment on slow transit constipation. Sichuan Zhongyi. 2006;24:35–37. [Google Scholar]
- 17.Jiang YW, Wang LL. Analyzing status of treatment of acupuncture and moxibustion on chronic functional constipation. Liaoning Zhongyiyao Daxue Xuebao. 2008;10:45–46. [Google Scholar]
- 18.Zhang L, Li N, Di ZL, Liu TL. A clinical study of LuiWeiAnXiao capsules (BangXiaoAn) for the treatment of post-stroke functional constipation in 160 cases. Shiyong Yiji Zazhi. 2006;13:2251–2252. [Google Scholar]
- 19.Kong LX. A clinical observation of modified WenPi decoction for functional constipation on patients blood dialysis. Beijing Zhongyiyao. 2008;27:442–444. [Google Scholar]
- 20.Zhan CE, Wang FJ. A clinical observation of acupuncture for the treatment of functional constipation. Zhenjiu Linchuang Zazhi. 2005;21:24–25. [Google Scholar]
- 21.Yan J, Liu YX. Cisapride combined with lactulose for the treatment of senile functional constipation in 32 cases. Youjiang Minzu Yixueyuan Xuebao. 2005;27:817. [Google Scholar]
- 22.Huang CF, Jin H. An efficacy observation of mosapride combined with birid triple viable for the treatment of functional constipation. Linchuang Xiaohuabing Zazhi. 2007;19:321–323. [Google Scholar]
- 23.Yu Y. A clinical observation of probiotics and acupuncture for the treatment of slow transit constipation in elderly. Shiyong Laonian Yixue. 2007;21:353–354. [Google Scholar]
- 24.Jin Z, Yang BL, Wang CY. Efficacy analysis of polysthylene glycol 4000 combined with mosapride for the treatment of senile functional constipation. Shiyong Zhongxiyi Jiehe Linchuang. 2008;8:5–6. [Google Scholar]
- 25.Sun GY, Li JQ, Cai JH. An efficacy observation of birid triple viable combined with polysthylene glycol 4000 for the treatment of senile functional constipation. Guangdong Yixue. 2008;29:1029–1031. [Google Scholar]
- 26.Qiu B, Tang XY, Wang YY. An analysis on the effect of treating senile chronic functional constipation with polysthylene glycol 4000 and bifico. Zhongguo Bingan. 2008:9: F0003–F0004. [Google Scholar]
- 27.Song SF. An efficacy observation of bifidobacterium combined with mosapride for the treatment of functional constipation. Yixue Lilun Yu Shijian. 2008;21:676–677. [Google Scholar]
- 28.Zheng HG. An efficacy observation of clebopride combined with bifidobacterium for the treatment of functional constipation. Shiyong Yiji Zazhi. 2008;15:1140–1141. [Google Scholar]
- 29.Shi ZH, Xu W, Chen DL, Luo L, Ge YC, Wang H. Clinical research of functional constipation with far-infrared thermy and heat instrument. Zhongguo Yixue Wulixue Zazhi. 2009;26:1118–1119, 1123. [Google Scholar]
- 30.Lin ZW, Li S. XiMo decoction and Deanxit orally combined with electricity pulse for chronic functional constipation in 90 cases. Haixia Yaoxue. 2007;19:85. [Google Scholar]
- 31.Tang TY, Qin JJ, Wang YK, Gao PJ, Piao YF. A clinical controlled study of medilac-S and LiuWei-AnXiao capsules in patients with functional constipation. Zhongguo Yiyao Zhinan. 2009;7:20–21. [Google Scholar]
- 32.Li F, Chen QS. Modified PiYue Pill for the treatment of senile functional constipation in 30 cases. Guangxi Zhongyi Xueyuan Xuebao. 2008;11:21–22. [Google Scholar]
- 33.Fan DM, Ou ZS, Liu YZ. A clinical study of method for harmonizing the Intestine and nourishing the Spleen for the treatment of chronic functional constipation in the Syndrome of Qi Deficiency in 35 cases. Xin Zhongyi. 2007;39:33–35. [Google Scholar]
- 34.Wang HB, Jin HW. Chinese medicine for the treatment of senile functional constipation in 40 cases. Xiandai Yiyao Weisheng. 2004;20:52. [Google Scholar]
- 35.Guan RJ, Zhao JN, Zhao J. A clinical study of HuangShi decoction for the treatment of senile chronic functional constipation. Zhongguo Zhongyao Zazhi. 2008;33:2968–2970. [Google Scholar]
- 36.Guan RJ, Zhao JN, Zhao J. A clinical study of HuangShi decoction for the treatment of senile chronic functional constipation. Zhongguo Shiyong Yiyao. 2008;3:127–128. [Google Scholar]
- 37.Wu WZ. A clinical observation on birid triple viable for the treatment of functional bowel disorders in elderly for 60 cases. Zhejiang Linchuang Yixue. 2005;3:270. [Google Scholar]
- 38.Wu WZ. A clinical observation on birid triple viable for the treatment of functional bowel disorders in the elderly. Zhongguo Xiangcun Yiyao Zazhi. 2005;12:12–13. [Google Scholar]
- 39.Sun Y, Cui Z, Lin LM, Zhao Y. A clinically controlled study of LiuWei-NengXiao capsules for functional constipation in old patients. Zhongguo Xinyao Zazhi. 2008;17:602–604. [Google Scholar]
- 40.Xie YK, Tang XH, Li M. A clinical controlled study of LiuWei-Neng Xiao capsules for functional constipation in old patients. Jiefangjun Baojian Yixue Zazhi. 2007;9:18–20. [Google Scholar]
- 41.Liu ZX. A clinical observation of integrated therapy for prevention and treatment of functional constipation in adolescent. Guangdong Yaoxue. 2005;15:71–73. [Google Scholar]
- 42.Qu QF, Bai XR. To investigated the efficacy of treating chronic functional constipation with LiuWeiAnXiao capsule and mosapride citrate tablets. Neimenggu Yixue Zazhi. 2008;40:1048–1049. [Google Scholar]
- 43.Liu XL, Li L. A clinical observation of WuRong decoction for the treatment of functional constipation. Sichuan Zhongyi. 2004;22:41–42. [Google Scholar]
- 44.Xie S, Feng JJ. YiQiXiaoMi decoction for the treatment of function constipation in the Syndrome of Qi Deficiency in 106 cases. Gansu Zhongyi. 2008;21:24–25. [Google Scholar]
- 45.Zhan CE, Chen JY. A clinical observation of LiQiTongBian solution for the treatment of functional constipation in 20 cases: by comparing with mosapride in 20 cases. Zhejiang Zhongyi Zazhi. 2005;21:18–19. [Google Scholar]
- 46.Shi C, He Y, Zhou JH. A clinical observation of Nourishing Qi and Yin for the treatment of functional constipation in 30 cases. Xinglin Zhongyiyao Zhongyiyao. 2008;28:26–27. [Google Scholar]
- 47.Wu SL, Zhou JB. A clinical observation of Nourishing Qi and Yin Formulation for the treatment of senile functional constipation. Jiangsu Zhongyiyao. 2008;40:54–55. [Google Scholar]
- 48.Gan AP, Zhang F. A clinical observation of TiaoChang decoction for the treatment of functional constipation. Zhongwai Jiankang Wenzhai. 2008;5:83–84. [Google Scholar]
- 49.Yang TZ. A clinical observation of the method of ZengShuiXingZhou for the treatment of senile functional constipation in 64 cases. Zhongguo Laonianxue Zazhi. 2008;10:1025–1026. [Google Scholar]
- 50.Fan DB, Qin XB, Xu JZ, Bai HH, Zeng YH, Zeng GQ, Yin HY. YiQiRunChang capsules for the treatment of QiYin deficiency constipation in 100 cases. Yunnan Zhongyi Zhongyao Zazhi. 2009;30:33. [Google Scholar]
- 51.Li FZ, Shen JL, Yi QL. Modified MaRen RunChang pills for treating functional constipation in 58 cases. Henan Zhongyi Xueyuan Zazhi. 2004;19:62–63. [Google Scholar]
- 52.Wu RM. A clinical observation of the method of GuShen SuoNiao for the treatment of chronic functional constipation in 62 cases. Sichuan Zhongyi. 2003;21:33–34. [Google Scholar]
- 53.Cai HQ. TongBian granules for the treatment of chronic functional constipation in 60 cases. Zhongguo Minjian Liaofa. 2004;12:45–46. [Google Scholar]
- 54.Chen ZH, Zeng EM. BuZhongYiQi pills combined with cisapride for the treatment of senile functional constipation in 68 cases. Zhongguo Zhongxiyi Jiehe Xiaohua Zazhi. 2004;12:243–244. [Google Scholar]
- 55.Li SJ, Song CH. A clinical observation of benefiting Qi, warming Yang, nourishing blood and Jin for the treatment of chronic functional constipation. Zhongyiyao Xuekan. 2005;23:1913–1914. [Google Scholar]
- 56.Yan LX, Cao YQ, Huang H, Lu L. A clinical efficacy observation of self designated SanYiXingChang decoction for chronic functional constipation in the elderly. Zhongguo Xiandai Yaowu Yingyong. 2007;1:41–42. [Google Scholar]
- 57.Zhu QH. A clinical observation of integrated therapy for the treatment of functional constipation. Zhongguo Zhongyiyao Xiandai Yuancheng Jiaoyu. 2008;6:1375. [Google Scholar]
- 58.Sun HP, Qiao YZ. ZengYi RunChang pills for the treatment of chronic functional constipation in 86 cases. Guangming Zhongyi. 2008;23:1954–1955. [Google Scholar]
- 59.Xiong GH. ZengYi TongBian formulation for the treatment of chronic functional constipation in 86 cases. Zhongwai Yiliao. 2008;35:67. [Google Scholar]
- 60.LiuWeiAnXiao Collaboration. A multi-centers clinical study of LiuWeiAnXiao capsules for the treatment of chronic functional constipation. Zhonghua Xiaohua Zazhi. 2004;24:297–298. [Google Scholar]
- 61.Yan X, Guo MY. Low dose of LiuWeiAnXiao capsules combined with polyethylene glycol 4000 for the treatment of senile functional constipation in 25 cases. Zhongguo Zhongxiyi Jiehe Xiaohua Zazhi. 2006;14:56–57. [Google Scholar]
- 62.Wang WW, Li X. Two stages Integrated therapy for chronic functional constipation in 35 cases. Jiangsu Zhongyiyao. 2006;27:33. [Google Scholar]
- 63.Liang C, Wu XB. A clinical study of method promoting the function of Spleen and Stomach, circulation of Qi and removing the stasis blood for the treatment of senile functional constipation. Sichuan Zhongyi. 2008;26:82–84. [Google Scholar]
- 64.Wang XP, Zhu RH. An efficacy observation of YiQiJianPiRunChang decoction for the treatment of slow transit constipation in 50 cases. Yunnan Zhongyi Zhongyao Zazhi. 2007;28:8–9. [Google Scholar]
- 65.Kang YL, Li NX. A clinical observation of RunChangJian for the treatment of functional constipation. Zhongyuan Yikan. 2004;31:58. [Google Scholar]
- 66.Wang QC. ErBai decoction for the treatment of senile functional constipation in 46 cases. Shandong Zhongyi Zazhi. 2004;23:696. [Google Scholar]
- 67.Xin H, Zhang JQ. An efficacy observation of modified ZengYiChengQi decoction for the treatment of senile functional constipation. Sichuan Zhongyi. 2008;26:58–59. [Google Scholar]
- 68.Li L, Wang YZ, Zhu CT, Yang H, Li J. AnZhongTongBian capsules combined with western medicine for the treatment of senile functional constipation in 50 cases. Anhui Zhongyi Xueyuan Xuebao. 2008;27:8–9. [Google Scholar]
- 69.Meng LJ. YiNianJin combined with live bifidobacterium preparation for the treatment of functional constipation in childhood. Hebei Yike Daxue Xuebao. 2009;30:188–189. [Google Scholar]
- 70.Guo SY. An efficacy observation of RunChangTongBian NongSuo pills for the treatment of chronic functional constipation in 200 cases. Zhongguo Zhongyiyao Xinxi Zazhi. 2003;10:48–49. [Google Scholar]
- 71.Guo SY, Xu JY, Gao LY. A clinical observation of RunChangTongBian NongSuo pills for the treatment of chronic functional constipation in 30 cases and its effect on blood SP and NO levels. Zhongyi Yanjiu. 2006;19:26–28. [Google Scholar]
- 72.Jiang XD, Zhang Q, Liu D. Clinical study on the purge decoction for 70 patients with the senile functional constipation. Zhongguo Minkang Yixue. 2007;19:1071, 1145. [Google Scholar]
- 73.Li YP, Wang J, Li Y, Yu LF. Observations about curative effect of QiLang mixture on chronic functional constipation. Liaoning Zhongyi Zazhi. 2008;35:1043–1045. [Google Scholar]
- 74.Chen Y. Clinical observation of “ChangBi Decoction” in treating slow transit constipation. Shanghai Zhongyiyao Zazhi. 2009;43:36–37. [Google Scholar]
- 75.Huang MB. A clinical observation of massage for the treatment of functional constipation. Beijing Zhongyiyao. 2008;27:42–43. [Google Scholar]
- 76.Sekeres M, Gold JL, Chan AW, Lexchin J, Moher D, Van Laethem ML, Maskalyk J, Ferris L, Taback N, Rochon PA. Poor reporting of scientific leadership information in clinical trial registers. PLoS One. 2008;3:e1610. doi: 10.1371/journal.pone.0001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsubouchi T, Saito T, Mizutani F, Yamauchi T, Iwanaga Y. Stimulatory action of itopride hydrochloride on colonic motor activity in vitro and in vivo. J Pharmacol Exp Ther. 2003;306:787–793. doi: 10.1124/jpet.102.048603. [DOI] [PubMed] [Google Scholar]
- 78.Wu WT, Yang Y, Deng W. Review on clinical researches on prokinetics. Zhongguo Yaoye. 2008;17:77–78. [Google Scholar]