Abstract
Several investigations have demonstrated that higher body weight, as assessed by body mass index (BMI), is associated with improved prognosis in patients with heart failure (HF). The purpose of the present investigation was to assess the influence of HF etiology on the prognostic ability of BMI in a cohort undergoing cardiopulmonary exercise testing (CPX). One thousand one hundred and sixty subjects were included in the analysis. All subjects underwent CPX where the minute ventilation/carbon dioxide production (VE/VCO2) slope and peak oxygen consumption (VO2) were determined. There were 193 cardiac deaths in the overall group during a mean follow-up of 30.7 ±25.6 months (annual event rate: 6.0%). Subjects classified as obese consistently had improved survival compared to normal weight subjects (overall survival 88.0% vs. ≤81.1%, p<0.001). Differences in survival according to HF etiology were observed for subjects classified as overweight. In the ischemic subgroup, survival characteristics for overweight subjects (75.5%) were similar to individuals classified as normal weight (81.1%). The converse was true for the non-ischemic subgroup where survival trends for obese (86.4%) and overweight subjects (88.4%) were similar. The VE/VCO2 slope was the strongest prognostic marker (Chi-square: ≥43.4, p<0.001) for both etiologies while BMI added prognostic value (Residual Chi-square: ≥4.7, p<0.05). In conclusion, these results further support the notion that obesity confers improved prognosis in patients with HF, irrespective of HF etiology. Moreover, BMI appears to add predictive value during CPX assessment. However, survival appears to differ according to HF etiology in subjects classified as overweight.
Keywords: Mortality, Prognosis, Cardiopulmonary exercise test, body weight
Introduction
Several recent investigations have demonstrated that higher body weight, as assessed by body mass index (BMI), is associated with improved prognosis in patients with HF. This phenomenon has aptly been named the “obesity paradox”.1 In a recent meta-analysis, Oreopolis et al.2 reported on nine studies, collectively including over 25,000 patients, that found subjects who were overweight (BMI = 25.0-29.9 kg/m2) and obese (BMI ≥ 30 kg/m2) had a more favorable prognosis compared to subjects who were both underweight and normal weight. Given the differences in clinical characteristics and prognosis between patients with non-ischemic and ischemic heart failure (HF)3-5, it is plausible to hypothesize the prognostic interaction this cardiac condition has with body weight may likewise differ according to etiology. We are unaware of any previous investigation that has examined: 1) the cardiopulmonary exercise testing (CPX) response according to both body weight and HF etiology; 2) the prognostic value of bodyweight according to HF etiology; and 3) the prognostic value of including body weight to a multivariate regression analysis according to HF etiology. The purpose of the present investigation was to address these issues in a large multicenter HF cohort referred for CPX.
Methods
This study was a multi-center analysis including HF patients from the exercise testing laboratories at San Paolo Hospital, Milan, Italy, Wake Forrest University Baptist Medical Center, Winston-Salem, North Carolina, USA, LeBauer Cardiovascular Research Foundation, Greensboro, North Carolina, USA, VA Palo Alto Health Care System, Palo Alto, California, USA and Virginia Commonwealth University, Richmond, Virginia, USA. A total of 1,160 patients diagnosed with chronic HF were included. Inclusion criteria consisted of a diagnosis of HF6 and evidence of left ventricular dysfunction by two-dimensional echocardiography obtained within one month of data collection. All subjects completed a written informed consent and institutional review board approval was obtained at each institution.
Ventilatory expired gas analysis was performed using a metabolic cart at all five centers (Medgraphics CPX-D and Ultima, Minneapolis, MN, Sensormedics Vmax29, Yorba Linda, CA or Parvomedics TrueOne 2400, Sandy, UT). Before each test, the equipment was calibrated in standard fashion using reference gases and a 3-liter syringe. All subjects then underwent a symptom-limited exercise test using a conservative ramping protocol. The height and weight used to determine BMI were measured the day of CPX. Peak oxygen consumption (peak VO2), peak respiratory exchange ratio (RER) and the minute ventilation (VE)/carbon dioxide production (VCO2) slope were determined for this analysis. Peak VO2 and RER were expressed as 10-second averaged samples obtained during the exercise test. VE and VCO2 values, acquired from the initiation of exercise to peak, were input into spreadsheet software (Microsoft Excel, Microsoft Corp., Bellevue, WA) to calculate the VE/VCO2 slope via least squares linear regression (y = mx + b, m=slope).
Subjects were followed for cardiac mortality via hospital and outpatient medical chart review. Subjects were followed by the HF programs at their respective institution providing a high likelihood that all events were captured. Any death with a cardiac-related discharge diagnosis was considered an event. Subjects suffering a non-cardiac death or undergoing a heart transplant or left ventricular assist device implantation were treated as censored cases at the time of the event.
All continuous data are reported as mean values ± standard deviation (SD). Dichotomous categorical data are reported as percentages. Differences in continuous variables between ischemic and non-ischemic HF subjects within BMI subgroups (Normal weight: 18.5-24.9 kg/m2/ Overweight: 25.0-29.9 kg/m2/ Obese: ≥30.0 kg/m2) were tested by two-way analysis of variance (ANOVA). Both main (ischemic vs. non-ischemic and BMI subgroup) and interaction (HF etiology*BMI subgroup) effects were assessed. The chi-square test was used to determine differences in categorical variables between the HF etiology/BMI subgroups. Kaplan-Meier analysis assessed differences in survival between subjects according to BMI categories in the overall cohort as well as ischemic and non-ischemic HF subgroups. The log-rank test determined statistical significance between BMI categories for all Kaplan-Meier analyses. A contingency table was used to determine relative risk ratios for cardiac mortality according to BMI categories in the overall cohort as well as ischemic and non-ischemic HF subgroups. Univariate and multivariate (forward stepwise method, entry and removal values 0.05 and 0.10, respectively) Cox regression analysis was used to assess the prognostic ability of baseline (including BMI) and CPX variables in the overall cohort as well as ischemic and non-ischemic HF subgroups. A statistical software package was used for all calculations (SPSS 16.0, Chicago, IL). Statistical differences with a p-value <0.05 were considered significant.
Results
Baseline and CPX characteristics for the study group subdivided according to HF etiology and BMI are listed in Table 1. The ischemic HF subgroup was significantly older and was comprised of more male subjects compared to subjects with non-ischemic HF, irrespective of BMI classification. Within both HF etiology subgroups, subjects classified as obese were significantly younger. A significantly lower percentage of normal weight subjects were prescribed a beta-blocking agent at the time of testing, irrespective of HF etiology. Relative peak VO2 was similar in each subgroup although, as expected, absolute peak VO2 increased significantly with BMI. Absolute peak VO2 was also significantly higher in the non-ischemic subgroup. While peak RER surpassed 1.0 in all subgroups, it was significantly higher among the overweight vs. the normal weight and obese subjects. Lastly, the VE/VCO2 slope was significantly higher in normal weight subjects compared to the other BMI subgroups.
Table 1.
Differences in Baseline, Pharmacologic and Cardiopulmonary Exercise Test Characteristics According to Heart Failure Etiology and Body Mass Index
| Ischemic Heart Failure | Non-Ischemic Heart Failure | |||||
|---|---|---|---|---|---|---|
| Normal Weight (n=164) |
Overweight (n=216) |
Obese (n=144) |
Normal Weight (n=156) |
Overweight (n=213) |
Obese (n=267) |
|
| Baseline Characteristics | ||||||
| Age (years)* | 64.4 ±10.4 | 62.3 ±10.0 | 58.8 ±9.4 | 56.6 ±17.0 | 55.4 ±14.1 | 52.4 ±14.0 |
| Percent Male£ | 76 (%) | 89 (%) | 87 (%) | 60 (%) | 67 (%) | 59 (%) |
|
Body Mass Index (kg/m2)€ |
22.9 ±1.6 | 27.3 ±1.5 | 33.8 ±3.5 | 22.1 ±1.8 | 27.3 ±1.4 | 35.7 ±5.2 |
|
Left Ventricular Ejection Fraction |
31.9 ±12.6 (%) | 33.4 ±12.4 (%) | 30.6 ±10.7 (%) | 31.0 ±14.1 (%) | 32.3 ±15.5 (%) | 32.8 ±15.6 (%) |
|
New York Heart Association Class |
2.2 ±0.73 | 2.2 ±0.79 | 2.4 ±0.83 | 2.4 ±0.80 | 2.3 ±0.83 | 2.4 ±0.82 |
| Pharmacologic Management | ||||||
| Beta Blockerλ | 61 (%) | 65 (%) | 70 (%) | 58 (%) | 67 (%) | 69 (%) |
|
Angiotensin Converting Enzyme Inhibitor |
74 (%) | 70 (%) | 76 (%) | 77 (%) | 73 (%) | 71 (%) |
| Cardiopulmonary Exercise Test Response | ||||||
|
Peak oxygen consumption (mlO2•kg−1•min−1) |
15.4 ±5.3 | 15.6 ±5.9 | 15.1 ±6.0 | 15.7 ±6.4 | 16.1 ±5.2 | 14.8 ±5.6 |
|
Peak oxygen consumption (L/min)γ |
0.97 ±0.54 | 1.09 ±0.65 | 1.28 ±0.78 | 1.00 ±0.65 | 1.21 ±0.53 | 1.40 ±0.72 |
|
Peak Respiratory Exchange RatioΩ |
1.07 ±0.15 | 1.10 ±0.17 | 1.09 ±0.14 | 1.07 ±0.15 | 1.10 ±0.14 | 1.07 ±0.13 |
|
Minute Ventilation /Carbon Dioxide Production slopeβ |
38.0 ±11.0 | 35.0 ±8.6 | 34.2 ±9.3 | 37.6 ±9.7 | 34.8 ±7.8 | 33.7 ±8.8 |
a: Ischemic HF group significantly older than non-ischemic group (p<0.001).
b: Obese subjects significantly younger than normal and overweight subjects (p<0.001).
a: Normal Weight Ischemic Heart Failure: male percentage significantly lower than other ischemic heart failure groups and significantly higher than all non-ischemic heart failure groups (p<0.01).
b: Overweight and Obese Ischemic Heart Failure: male percentage significantly greater than all other groups (p<0.001)
c: Overweight Non-Ischemic Heart Failure: male percentage significantly greater than normal weight and obese ischemic heart failure (p<0.05)
a: BMI significantly different amongst normal weight, overweight and obese groups (p<0.05)
b: BMI significantly higher in non-ischemic HF group (p<0.001)
c: Significant interaction between HF etiology and body weight: BMI of non-ischemic obese subjects significantly greater than ischemic obese subjects (p<0.05)
a: Percent of normal weight ischemic and non-ischemic HF groups prescribed a beta-blocker significantly less than non-ischemic overweight and ischemic and non-ischemic obese subjects (p<0.05)
a: Absolute peak VO2 significantly different amongst all three BMI groups (p<0.01)
b: Absolute peak VO2 significantly higher in non-ischemic HF compared to ischemic HF group (p<0.05)
a: Overweight subjects: Significantly higher peak RER than normal weight and obese subjects (p<0.05)
a: Normal weight subjects: Significantly higher VE/VCO2 slope compared to overweight and obese subjects (p<0.001)
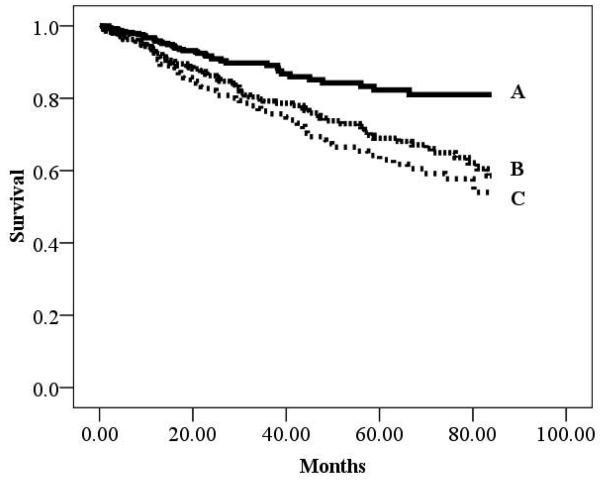
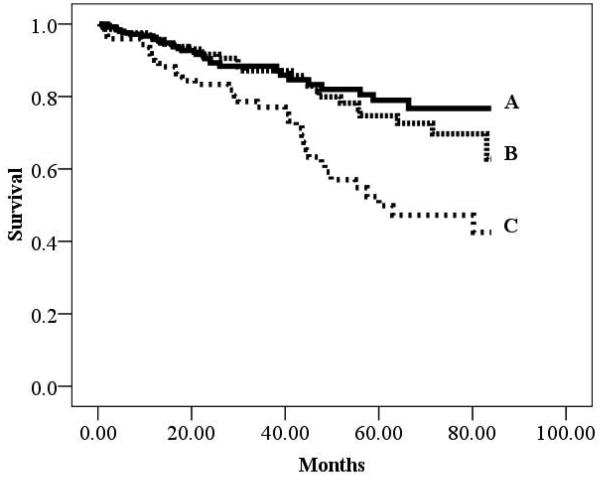
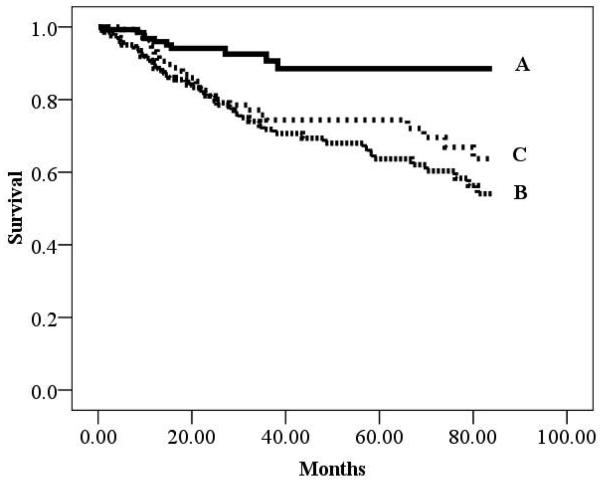
There were 193 cardiac deaths in the overall group during a mean follow-up of 30.7 ±25.6 months (annual event rate: 6.0%). There were 94 (annual event rate: 6.4%) and 99 (annual event rate: 5.8%) cardiac deaths in the ischemic and non-ischemic subgroups, respectively. Kaplan-Meier analyses according to BMI for the overall cohort as well as HF etiology-based subgroups are illustrated in Figures 1-3. All three Kaplan-Meier analyses were prognostically significant. Subjects classified as obese consistently had improved survival compared to normal weight subjects. Differences in survival according to HF-etiology became apparent for subjects classified as overweight. In the ischemic subgroup, survival characteristics for overweight subjects were similar to individuals classified as normal weight. The converse was true for the non-ischemic subgroup where survival trends for obese and overweight subjects were similar.
Figure 1.
Kaplan-Meier analysis for Body Mass Index in the Overall Group
Figure 3.
Kaplan-Meier analysis for Body Mass Index in the Non-ischemic Subgroup
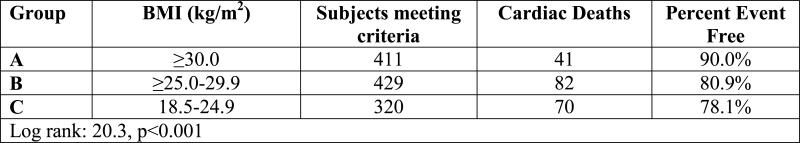
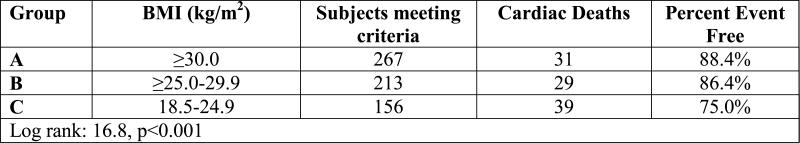
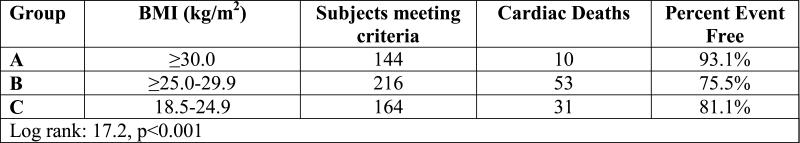
Kaplan-Meier analysis results for HF-etiology subgroups when the definition for normal weight was changed to 22.0-24.9 kg/m2 is listed in Table 2. Survival trends for the normal weight BMI class remained consistent in both ischemic and non-ischemic HF subgroups when the lower threshold used to defined normal weight was increased from 18.5 to 22 kg/m2.
Table 2.
Survival Results According to BMI when Low End of Normal Weight Group Increased to 22.0 kg/m2
| Subgroup | Kaplan-Meier Analysis | |||
|---|---|---|---|---|
| Ischemic Heart Failure Subgroup |
Body Mass Index | n | events | % survival |
| ≥30.0 kg/m2 | 144 | 10 | 93.1% | |
| ≥25.0-29.9 kg/m2 | 216 | 53 | 75.5% | |
| 22.0-24.9 kg/m2 | 114 | 19 | 83.3% | |
| Log-rank: 17.5, p<0.001 | ||||
| Non-ischemic Heart Failure Subgroup |
Body Mass Index | n | events | % survival |
| ≥30.0 kg/m2 | 267 | 31 | 88.4% | |
| ≥25.0-29.9 kg/m2 | 213 | 29 | 86.4% | |
| 22.0-24.9 kg/m2 | 88 | 25 | 71.6% | |
| Log-rank: 13.4, p<0.01 | ||||
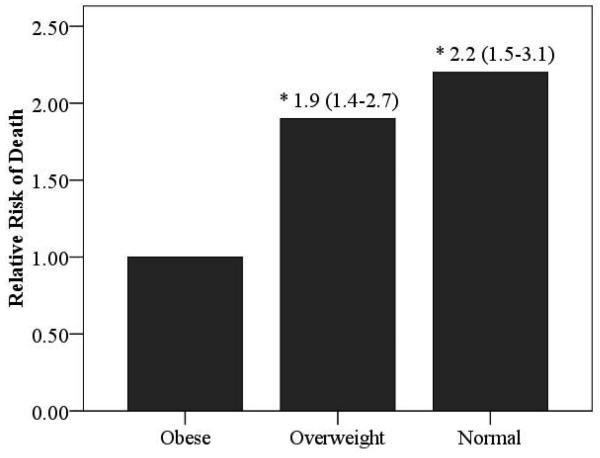
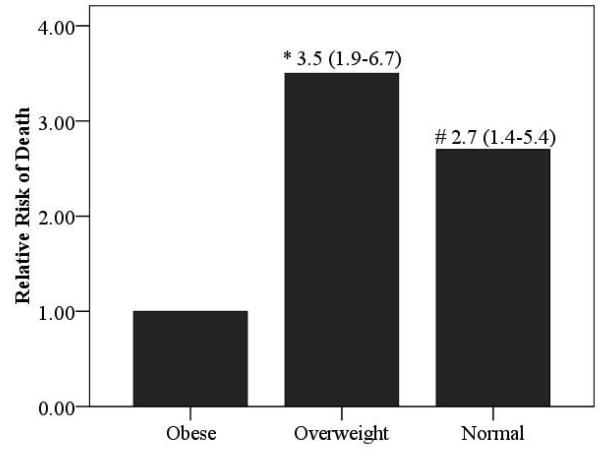
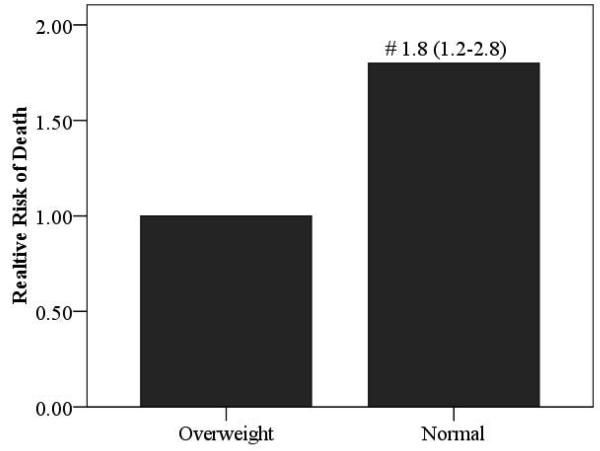
Relative risk ratios according to BMI for the overall cohort as well as HF etiology-based subgroups are illustrated in Figures 4 and 5. Obese subjects (referent group) had the lowest mortality risk. The relative risk of cardiac mortality was significantly higher in both overweight and normal weight subjects in the ischemic subgroup. In subjects with non-ischemic HF, only normal weight subjects were at a significantly higher risk compared to obese subjects. Moreover, the relative risk of death was also significantly higher in normal weight subjects with a non-ischemic etiology when compared to subjects who were classified as overweight.
Figure 4.
Relative Risk of Death According to BMI Class in the Overall Group and Ischemic and Non-Ischemic Subgroups
(Figure 4a: Overall Group, Figure 4b: Ischemic Heart Failure, Figure 4c: Non-Ischemic Heart Failure)
Significantly different compared to obese group; *p<0.001, # p<0.01
Figure 5.
Relative Risk of Death According to BMI Class in Normal Weight and Overweight Non-Ischemic Subjects
Significantly different compared to overweight group; # p<0.01
Univariate and multivariate Cox regression analyses are listed in Table 3. For the Cox regression analysis, BMI level was dichotomously classified according to the results of the Kaplan-Meier and relative risk analysis (Ischemic etiology: Obese vs. Overweight/normal weight, Non-ischemic etiology: Obese/Overweight vs. normal weight). The VE/VCO2 slope was the strongest predictor of cardiac mortality in both the ischemic and non-ischemic subgroups. Body mass index and left ventricular ejection fraction were both significant univariate predictors in each subgroup and retained in both multivariate regression analyses. BMI however, added stronger prognostic value to the multivariate analysis in the ischemic subgroup. While peak VO2 was a significant univariate predictor of cardiac death in both subgroups, it was only retained in the multivariate regression for subjects with ischemic HF. Age was not a significant univariate predictor in either subgroup but did add prognostic value to the multivariate regression performed in the non-ischemic subgroup.
Table 3.
Cox Regression Analysis Results
| Ischemic Heart Failure Subgroup | ||
|---|---|---|
| Variable | Chi-square | p-value |
| Minute Ventilation/Carbon Dioxide Production Slope |
48.3 | <0.001 |
| Variable | Residual Chi-square | p-value |
| Body Mass Index (Obese vs. Overweight/normal weight)* |
15.6 (Univariate chi square: 15.9, p<0.001) |
<0.001 |
| Peak Oxygen Consumption* | 9.0 (Univariate chi square: 24.8, p<0.001) |
0.003 |
| Left Ventricular Ejection Fraction | 1.1 (Univariate chi square: 7.1, p=0.008) |
0.29 |
| Age | 0.02 (Univariate chi square: 2.4, p=0.12) |
0.88 |
| Non-Ischemic Heart Failure Subgroup | ||
| Variable | Chi-square | p-value |
| Minute Ventilation/Carbon Dioxide Production Slope |
43.4 | <0.001 |
| Variable | Residual Chi-square | p-value |
| Left Ventricular Ejection Fraction* | 30.0 (Univariate chi square: 30.0, p<0.001) |
<0.001 |
| Age* | 6.8 (Univariate chi square: 3.6, p=0.06) |
0.009 |
| Body Mass Index (Obese vs. Overweight/normal weight)* |
4.7 (Univariate chi square: 17.2, p<0.001) |
0.03 |
| Peak Oxygen Consumption | 0.53 (Univariate chi square: 12.3, p<0.001) |
0.47 |
Retained in multivariate regression
Discussion
The results of the present investigation are consistent with previous trials2,7 demonstrating that a higher BMI, although it increases the risk for the development of HF8, improves survival once a patient is diagnosed with this condition. Previous investigations have used various low-end thresholds to define normal weight including ≥18.5 kg/m2 9 and ≥22.0 kg/m2 10. Our findings are consistent with previous investigations by demonstrating prognosis was poor in normal weight individuals using both of these low-end thresholds to define this BMI class. Recognizing the limitations of BMI in accurately reflecting true body composition in all instances, using a threshold of ≥22.0 kg/m2 to define the beginning of the normal weight classification may indicate the protective effect of a higher BMI in HF is not entirely explained by malnourishment and cardiac cachexia.11 Our findings indicate that, despite differences in age, gender distribution, BMI, and pharmacologic management, both ischemic and non-ischemic HF patients classified as obese have the most favorable survival trends.
Several physiologic mechanisms for the protective effect of a higher BMI in HF have been proposed, including the ability of adipose tissue to counteract higher levels of circulating inflammatory markers such as tumor necrosis factor.2 Previous research has also demonstrated inflammatory cytokines are significantly lower in patients with non-ischemic HF compared to those with an ischemic etiology.12 Perhaps the lower adipose tissue levels in overweight patients with ischemic HF are not sufficient to provide a favorable prognostic effect. Conversely, although higher than that in apparently healthy individuals, the generally lower levels of inflammatory cytokines in patients with non-ischemic HF may be better counteracted with the adipose tissue levels associated with a BMI between 25.0 and 29.0 kg/m2. B-type natriuretic peptide has also been found to decrease as BMI increases in patients with HF.13 As with inflammatory cytokines, this important neurohormone has also been found to be lower in patients with non-ischemic HF compared to those with an ischemic etiology.14 These findings may lend further support to the hypothesis that BMI levels in the obese range are needed for improved outcomes in patients with ischemic HF while overweight BMI levels may be sufficient for the non-ischemic population. The present data do not allow for assessment of this hypothesis, but suggest further research in this area.
A limitation of our study in common with prior reports is that we cannot exclude a potential contribution of non-intentional weight loss, which may comprise a substantial portion of sustained weight loss in general patient populations. Also like other studies, there were relatively few patients with morbid obesity (BMI ≥ 40) and thus there is uncertainty whether the trends in prognosis with BMI extend to such patients. It is possible that exercise-induced weight loss, which has been shown to favorably impact inflammatory cytokines15 and neurohormonal levels16,17 in other populations, may allow for achievement of optimal body composition without an increased risk for adverse events in patients with HF. Ultimately, randomized intervention studies will be required to fully understand the relationship of BMI with HF outcomes and its mechanisms.
The VE/VCO2 slope has been shown to be related to cardiovascular function.18,19 The fact that this CPX variable is less dependent upon subject effort, and thus potentially a better reflection of cardiovascular health in many patients, may be a primary reason significant differences between normal weight and overweight/obese subjects were apparent in both HF-etiology based subgroups. However, even with a mean VE/VCO2 slope similar to those classified as obese, an unfavorable prognosis persisted in patients with ischemic HF who were overweight. This finding may have important clinical implications when using this CPX variable to assess prognosis in this HF subgroup (i.e. a lower VE/VCO2 slope does not necessarily equate to improved prognosis in overweight patients with ischemic HF).
A large body of evidence supports the prognostic application of CPX in patients with HF.20-24 While peak VO2 is well established from a clinical assessment perspective, there is now strong evidence supporting the VE/VCO2 slope as the CPX variable with the highest prognostic value.25 The results of the present study support findings from previous investigations in this area in the following ways: 1) the VE/VCO2 slope provides superior prognostic value in both patients with ischemic and non-ischemic HF; and 2) while peak VO2 was a significant univariate predictor of mortality in both etiology-based subgroups, it was only retained in the multivariate analysis conducted in patients with ischemic HF. Novel findings from this component of the present study include the fact that BMI was retained in the multivariate prognostic analysis for both patients with ischemic and non-ischemic HF, although its additive value was stronger in the former subgroup. Moreover, the dichotomous BMI threshold used in the Cox multivariate regression analysis differed in the ischemic (obese vs. overweight/normal weight) and non-ischemic (obese/overweight vs. normal weight) subgroups.
Previous investigations examining the prognostic value of BMI in HF have done so in non-select HF cohorts.2 The present investigation confirms the prognostic value of BMI and introduces the possibility on an etiology-based influence. The present cohort was clinically referred for CPX, introducing the possibility of selection bias. In addition, assessing the prognostic value of more accurate assessments of lean and fat mass in a prospective fashion may be fruitful as BMI has inherent limitations in this respect. For the time being, our findings should exclusively be used to support the clinical consideration of BMI for patients with HF undergoing CPX.
Figure 2.
Kaplan-Meier analysis for Body Mass Index in the Ischemic Subgroup
Acknowledgments
Supported in part by NIH grants R37AG18915 and P60AG10484
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, Kosiborod M, Portnay EL, Sokol SI, Bader F, Krumholz HM. The Obesity Paradox: Body Mass Index and Outcomes in Patients With Heart Failure. Archives of Internal Medicine. 2005;165:55–61. doi: 10.1001/archinte.165.1.55. [DOI] [PubMed] [Google Scholar]
- 2.Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008;156:13–22. doi: 10.1016/j.ahj.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 3.Clark AL, Harrington D, Chua TP, Coats AJ. Exercise capacity in chronic heart failure is related to the aetiology of heart disease. Heart. 1997;78:569–571. doi: 10.1136/hrt.78.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Feo S, Franceschini L, Brighetti G, Cicoira M, Zanolla L, Rossi A, Golia G, Zardini P. Ischemic etiology of heart failure identifies patients with more severely impaired exercise capacity. International Journal of Cardiology. 2005;104:292–297. doi: 10.1016/j.ijcard.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 5.Follath F. Ischemic versus non-ischemic heart failure: should the etiology be determined? Heart Fail Monit. 2001;1:122–125. [PubMed] [Google Scholar]
- 6.Radford MJ, Arnold JM, Bennett SJ, Cinquegrani MP, Cleland JGF, Havranek EP, Heidenreich PA, Rutherford JD, Spertus JA, Stevenson LW, Heidenreich PA, Goff DC, Grover FL, Malenka DJ, Peterson ED, Radford MJ, Redberg RF. ACC/AHA Key Data Elements and Definitions for Measuring the Clinical Management and Outcomes of Patients With Chronic Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Heart Failure Clinical Data Standards): Developed in Collaboration With the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Failure Society of America. Circulation. 2005;112:1888–1916. doi: 10.1161/CIRCULATIONAHA.105.170073. [DOI] [PubMed] [Google Scholar]
- 7.Hall JA, French TK, Rasmusson KD, Vesty JC, Roberts CA, Rimmasch HL, Kfoury AG, Renlund DG. The paradox of obesity in patients with heart failure. J Am Acad Nurse Pract. 2005;17:542–546. doi: 10.1111/j.1745-7599.2005.00084.x. [DOI] [PubMed] [Google Scholar]
- 8.Nicklas BJ, Cesari M, Penninx BW, Kritchevsky SB, Ding J, Newman A, Kitzman DW, Kanaya AM, Pahor M, Harris TB. Abdominal obesity is an independent risk factor for chronic heart failure in older people. J Am Geriatr Soc. 2006;54:413–420. doi: 10.1111/j.1532-5415.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- 9.Bozkurt B, Deswal A. Obesity as a prognostic factor in chronic symptomatic heart failure. Am Heart J. 2005;150:1233–1239. doi: 10.1016/j.ahj.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Cicoira M, Maggioni AP, Latini R, Barlera S, Carretta E, Janosi A, Soler Soler J, Anand I, Cohn JN. Body mass index, prognosis and mode of death in chronic heart failure: Results from the Valsartan Heart Failure Trial. European Journal of Heart Failure. 2007;9:397–402. doi: 10.1016/j.ejheart.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Anker SD, Coats AJS. Cardiac Cachexia: A Syndrome With Impaired Survival and Immune and Neuroendocrine Activation. Chest. 1999;115:836–847. doi: 10.1378/chest.115.3.836. [DOI] [PubMed] [Google Scholar]
- 12.Lindberg E, Magnusson Y, Karason K, Andersson B. Lower levels of the host protective IL-10 in DCM--a feature of autoimmune pathogenesis? Autoimmunity. 2008;41:478–483. doi: 10.1080/08916930802031645. [DOI] [PubMed] [Google Scholar]
- 13.Horwich TB, Hamilton MA, Fonarow GC. B-Type Natriuretic Peptide Levels in Obese Patients With Advanced Heart Failure. Journal of the American College of Cardiology. 2006;47:85–90. doi: 10.1016/j.jacc.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 14.Miller WL, Hartman KA, Burritt MF, Burnett JC, Jr., Jaffe AS. Troponin, B-type natriuretic peptides and outcomes in severe heart failure: differences between ischemic and dilated cardiomyopathies. Clin Cardiol. 2007;30:245–250. doi: 10.1002/clc.20075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gielen S, Adams V, M÷bius-Winkler S, Linke A, Erbs S, Yu J, Kempf W, Schubert A, Schuler G, Hambrecht R. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. Journal of the American College of Cardiology. 2003;42:861–868. doi: 10.1016/s0735-1097(03)00848-9. [DOI] [PubMed] [Google Scholar]
- 16.Passino C, Ry SD, Severino S, Gabutti A, Prontera C, Clerico A, Giannessi D, Emdin M. C-type natriuretic peptide expression in patients with chronic heart failure: effects of aerobic training. Eur J Cardiovasc Prev Rehabil. 2008;15:168–172. doi: 10.1097/HJR.0b013e3282f10e9b. [DOI] [PubMed] [Google Scholar]
- 17.Passino C, Severino S, Poletti R, Piepoli MF, Mammini C, Clerico A, Gabutti A, Nassi G, Emdin M. Aerobic training decreases B-type natriuretic peptide expression and adrenergic activation in patients with heart failure. J Am Coll Cardiol. 2006;47:1835–1839. doi: 10.1016/j.jacc.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 18.Myers J, Dziekan G, Goebbels U, Dubach P. Influence of high-intensity exercise training on the ventilatory response to exercise in patients with reduced ventricular function. Med Sci Sports Exerc. 1999;31:929–937. doi: 10.1097/00005768-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Reindl I, Wernecke KD, Opitz C, Wensel R, Konig D, Dengler T, Schimke I, Kleber FX. Impaired ventilatory efficiency in chronic heart failure: possible role of pulmonary vasoconstriction. Am Heart J. 1998;136:778–785. doi: 10.1016/s0002-8703(98)70121-8. [DOI] [PubMed] [Google Scholar]
- 20.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Jr., Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 21.O'Neill JO, Young JB, Pothier CE, Lauer MS. Peak Oxygen Consumption as a Predictor of Death in Patients With Heart Failure Receiving {beta}-Blockers. Circulation. 2005;111:2313–2318. doi: 10.1161/01.CIR.0000164270.72123.18. [DOI] [PubMed] [Google Scholar]
- 22.Myers J, Gullestad L, Vagelos R, Do D, Bellin D, Ross H, Fowler MB. Cardiopulmonary exercise testing and prognosis in severe heart failure: 14 mL/kg/min revisited. Am Heart J. 2000;139:78–84. doi: 10.1016/s0002-8703(00)90312-0. [DOI] [PubMed] [Google Scholar]
- 23.Francis DP, Shamim W, Davies LC, Piepoli MF, Ponikowski P, Anker SD, Coats AJ. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2)slope and peak VO(2) Eur Heart J. 2000;21:154–161. doi: 10.1053/euhj.1999.1863. [DOI] [PubMed] [Google Scholar]
- 24.Arena R, Myers J, Abella J, Peberdy MA, Bensimhon D, Chase P, Guazzi M. Development of a Ventilatory Classification System in Patients With Heart Failure. Circulation. 2007;115:2410–2417. doi: 10.1161/CIRCULATIONAHA.107.686576. [DOI] [PubMed] [Google Scholar]
- 25.Arena R, Myers J, Guazzi M. The clinical and research applications of aerobic capacity and ventilatory efficiency in heart failure: an evidence-based review. Heart Fail Rev. 2008;13:245–269. doi: 10.1007/s10741-007-9067-5. [DOI] [PubMed] [Google Scholar]