Abstract
Adenocarcinoma of the prostate remains a significant public health problem and a prevalent cancer in men. Prostate-specific antigen used as a biomarker has established a clear migration of patients towards earlier-stage disease at presentation. However, in spite of process improvements in traditional therapies including surgery, radiation therapy, and hormone management, there remains a significant cohort of patients with intermediate- to high-risk features for poor outcome in spite of optimal use of traditional management. This paper focuses on future treatment strategies integrating new therapeutic options with traditional management, specifically to pinpoint new radiation therapy strategies.
Keywords: Casodex®, IGF-IR, integrin, prostate cancer, radiation therapy
Excluding carcinoma of the skin, adenocarcinoma (ADC) of the prostate is the most common cancer affecting men in the USA [1]. Owing to the high incidence of the disease, process improvements in both disease detection and treatment are important issues in public health. Technical improvements in both surgery and radiation management have improved clinical outcome for patients. Extended use of prostate-specific antigen (PSA) as a biomarker has also led to clinical-stage migration in a favorable manner, thus promoting improved clinical outcome as more patients are identified with early-stage disease. However, improvements in translational science are the next required step to improve the outcome of patients who remain at high risk for failure in spite of optimal clinical management and treatment.
The development of the PSA assay has greatly improved clinical strategies for patients with this disease. There has been a favorable stage migration because of the assay and many more patients with early-stage disease and favorable Gleason grade are now identified at disease presentation. Because of the success of both surgery and advanced technology radiation therapy in early-stage patients with limited tumor burden, these favorable patients have become an excellent cohort to evaluate quality-of-life index, since their outcome with treatment has been outstanding. Hormone and advanced technology radiation therapy have good success rates in patients with intermediate-risk factors for recurrence, including patients with less favorable features on pretreatment imaging and those with Gleason-7 disease. Patients with more advanced tumor burdens and those with Gleason 8–10 disease score comprise a measurable patient population and require continued process improvements in treatment strategy, as their disease can recur in spite of optimal hormone and radiotherapy management strategies [1,2]. In this review, we examine possible strategies for future direction for the care of patients with ADC of the prostate, with a focus on process improvements for the application of radiation therapy with translational science.
Challenges for radiation therapy
Classic radiation biology matured under the watchful eye of many outstanding investigators, whose primary interest was in identifying DNA damage associated with radiation exposure. These pioneer experiments were based in cell culture and animal studies. These important studies focused on identifying DNA-damage-repair mechanisms, including the speed of repair. Downstream consequence of damage could not be assessed due to historical limitations in scientific technique. Radiation therapy is thought to deliver lethal, potentially lethal, and sublethal damage to the tumor. The balance of the therapeutic index is to ablate the tumor compartment with as limited impact as possible to normal tissue. Lethal delivery of radiation therapy to the tumor, however, cannot come at the expense of normal tissue function. Thus, therapies that either make the tumor more sensitive to radiation treatment or protect normal tissue from radiation therapy can be of significant benefit to the patient population. Strategies that convert sublethal events into lethal events sensitize the tumor to radiation therapy. Potentially lethal damage occurs when the post-treatment environment is altered to facilitate cell death. Conversely, the environment can also be modified to limit cell death (hypoxia), which in turn, can facilitate tumor repair. Therefore, treatment protocols that modify the environment for repair could convert potentially sublethal treatment into lethal treatment. Sublethal damage can be intrinsically repaired by cells. Many cell systems, including bone marrow, have limited to no capacity to repair sublethal events, while other compartments, including solid tumor systems, have significant capability of sublethal repair. Prostate cancer is thought to have measurable capacity to repair sublethal events. Classic experiments have shown that the intrinsic ability to repair is restored in cells in a short period of time after radiation therapy (minutes to hours) [3]. Therapies designed to inhibit repair processes during this posttreatment phase may be successful in making the tumor more sensitive to radiation therapy.
Linear–quadratic platforms are often the best mathematical models to describe normal tissue and tumor cell death with radiation therapy. Therefore, cell death with radiation therapy is best demonstrated using a semi-log Y-axis and increasing dose on the X-axis, as cell death is initially linear at low dose, then becomes exponential in function as the radiation dose increases. Certain tissues are extremely sensitive to radiation therapy and enter exponential cell death with low doses of radiation therapy. These are thought to have a high α component to the cell-survival model. Bone marrow progenitors including granulocytes, macrophages and sperm are examples of cells sensitive to radiation therapy as these enter exponential cell death at low doses of radiation therapy. Most epithelial normal tissue and tumor demonstrate the described two phases of cell death in response to radiation exposure. In developed models, the α component of cell death is the linear component and equals the log cells killed/dose. The α component is the response to low-dose radiation therapy. The β component is the exponential phase and equals the log cell death/dose squared. This is best exhibited at higher doses of radiation therapy. The linear–quadratic model best describes variances in normal tissue and tumor response to radiation therapy. The ratio of α to β is often used to describe the response of cells to radiation exposure. Prostate cancer cells are thought to have a low α:β ratio, specifically they have a more protracted response to radiation therapy and require a relatively high dose over an extended period of time for complete cell death. The linear–quadratic model will permit evaluation of targeted therapies both in vitro and in vivo and determine which phase of cell death targeted therapies may influence. Sequential or combination targeted therapies may be used that influence either the α (linear) and/or β (exponential) phase of cell death, or perhaps both components simultaneously [3].
Thus, both downstream intracellular biological mechanisms and tumor environment likely share importance with DNA damage repair as key factors when identifying mechanisms of cell injury owing to radiation therapy [2].
Cellular & molecular responses during ionizing radiation
Biological processes beyond intrinsic cellular repair response likewise play an important role in identifying strategies for additive treatment with radiation therapy. Oxygen is known to sensitize the tumor to radiation therapy. Conversely, tumor residing in a hypoxic environment requires much more radiation therapy to achieve the same degree of cell death. Hypoxia may play an important role in prostate cancer. Facilitating reoxygenation of the tumor target with biological modifiers may prove useful. Radiation therapy is clearly more effective in the G2/M phase of the cell cycle and more resistant in the DNA-synthesis phase of the cell cycle. Strategies enhancing cell death during DNA synthesis, or compounds that accelerate cell-cycle kinetics during fractionated radiation therapy may prove very effective as a therapy copartner to radiation therapy. This, in part, may explain the historical success of low-dose rate brachytherapy in the treatment of prostate cancer since, in this case, treatment is delivered in a continuous manner throughout the cell cycle and the tumor is killed during the reoxygenation phase of tumor recovery [2,3].
The science of radiation therapy has expanded into the identification of molecular products expressed after radiation therapy. Within a short period of time after exposure to radiation therapy, expression of FOS, JUN, and EGR1 and other products occurs [4,5]. This is thought to be due to transcriptional activation and protein synthesis. Radiation therapy induces TNF, PDGF and FGF. These molecules are likely released from the stroma and vascular endothelium as a by-product of radiation therapy. Now that we can identify specific molecular products of treatment, the next step in the process is to determine how they function with respect to tumor proliferation and normal tissue recovery. This would permit investigators to exploit therapeutic advantages and prevent tumor recovery after radiation therapy [2,3].
Recent advances in radiation science have demonstrated a measurable impact on several tumor cell expression products. Radiation therapy has an impact on cell signaling pathways, tumor angiogenesis and tumor cell adhesion [2]. Targeted therapies are beginning to mature in clinical use and it will be important to vet these therapies in the context of radiation management in order to potentially improve therapeutic outcome for patients treated with radiation. This is an important consideration for prostate carcinoma as many patients are treated with radiation therapy with curative intent. As stated, there exists a cohort of patients who would benefit from continued process improvement in therapeutic interventions. The role of chemotherapy is not yet fully established in this disease. Multiple clinical trials have not yet established benefit from the cytotoxic effect of chemotherapy. There may be an advantage of Taxol (Taxotere®) in this disease, however, the benefits of taxanes may be related to their use as antiangiogenesis agents as well as facilitating more rapid cell-cycle kinetics, thus placing the tumor into a more vulnerable phase of the cell cycle. Angiogenesis and cell-cycle kinetics therapies may both interdigitate with radiation therapy; hence these may become important targets for facilitating cell death with radiation therapy. Signaling-pathway inhibitors and cell-adhesion modulators may also become important copartners for radiation therapy moving forward as evidence grows that radiation therapy has a relationship with these important agents [2].
Cell adhesion
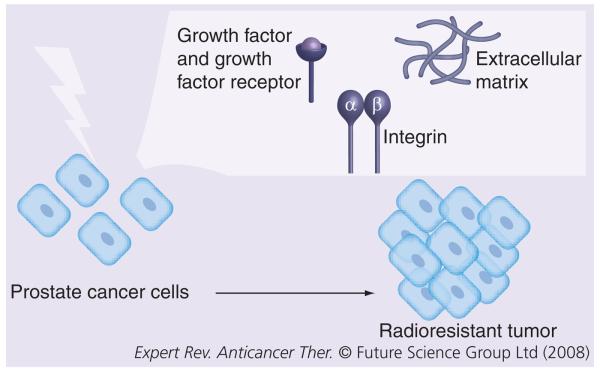
Tumor cell adhesion is evolving as an important target area for radiation therapy. Radiation appears to have a clear impact on integrin biology. Likewise, integrins appear to facilitate and promote prostate cancer cell survival and growth and may accelerate resistance to radiation therapy (Figure 1). Copartnering inhibition of integrin-mediated cell adhesion with radiation therapy may become integral to improve patient outcome, especially for those patients with intermediate- and high-risk features for tumor recurrence.
Figure 1. Treatment of prostate cancer cells with radiation controls tumor progression.
Aberrant expression or activation of either integrins or growth factor receptors stimulates cell survival or antiapoptotic pathways, which results in escape from irradiation-induced cell death and tumor formation.
There is increasing evidence that cell adhesion molecules influence oncogenesis, tumor aggression and resistance to treatment. Interactions between cells and extracellular matrix (ECM) are known to modulate sensitivity to radiation and drugs. Adhesion of small-cell lung cancer to the ECM enhances tumorigenicity and confers resistance to chemotherapy, perhaps through amplification of integrin-stimulated and tyrosine-kinase-mediated suppression of chemotherapy-induced apoptosis [6]. Human lung carcinoma cells grown on fibronectin significantly elevated clonogenic survival as well as prolonged G2 arrest after radiation therapy, yet this effect was not nearly as pronounced when grown on polystyrene [7]. Ionizing radiation is also known to upregulate the expression of integrins in a dose-dependent manner; the effect being most notable at low doses [8]. Combination therapy with αvβ3 integrin antagonists appears beneficial in local tumor therapy [9]. Similarly, Cao et al. have demonstrated that the combination of αV siRNA and irradiation leads to increased radiosensitivity [10]. Investigators have demonstrated that β1-integrin-mediated signaling through cytoplasmic domains is critical for efficient cell survival after radiation therapy [11]. Similarly, integrin expression appears to be clinically relevant for glottic and cervix cancers [12,13]. Therefore, cell adhesion influences cell survival and appears to affect the response to radiotherapy across many disease sites.
Data generated in our laboratory demonstrated that only high doses of radiation inhibit integrin expression in prostate cancer cells lines [14]. In prostate cancer cell line LNCaP stably transfected with αvβ3 integrin, increased radiation resistance of LNCaP cells after clinically relevant doses of 2–10 Gy and enhanced anchorage-independent growth after 5 Gy irradiation [15]. The data revealed that integrin expression enhanced resistance to radiation therapy in LNCaP cells exposed to radiation as evaluated in clonogenic assays and anchorage-independent growth assays. In addition, treatment of cells with cRGDfV integrin antagonist increased sensitivity of cells to radiation [15]. Integrin expression prevented down-regulation of two downstream effectors, survivin and cdc2, in irradiated LNCaP cells [16]. The data suggest that integrin expression is associated with resistance to radiation therapy in prostate cancer cell lines.
The biology of radiation therapy is revealing a provocative and developing relationship with cell adhesion. In a series of experiments performed by our group, LNCaP cells were irradiated at doses of 500, 1000 and 1500 cGy, in the presence or absence of androgen. Tumor cell adhesion to fibronectin was increased in the presence of androgen at radiation doses of 1000 and 1500 cGy. Casodex® (bicalutamide) is a non-steroidal antiandrogen chemical that binds to the androgen receptor in target tissues and competitively inhibits the action of androgen in that tissue, by assembly of a transcriptionally inactive receptor [17]. Casodex had minimal influence on adhesion modulation in nonirradiated tumors in the presence of androgen; however, it significantly inhibited the adhesion response in irradiated cells, thus reversing the effect of androgen [18]. It implies that Casodex may play a key role in adhesion modulation and possible tumor cell death during radiation therapy and, interestingly, may have little to no adhesion modulation effect in untreated tissue in the presence of androgen. This further implies that patients may benefit from adhesion modulation during radiation therapy with very limited impact of Casodex prior to treatment in the presence of androgen. This is an important concept in clinical trials since biological therapies are tested in a Phase I environment that may or may not identify the appropriate environment for treatment application. Casodex is used in varied formats in patient care in patients treated for prostate cancer, however, the data imply that it may play an important role for patients undergoing radiation management. Identifying the optimal environmental strategy for targeted therapies will be crucial in validating their utility and enabling treatment sequence strategies to move forward.
As noted, we now know that integrin expression is only negatively affected by relatively high-dose radiation therapy. Simon et al. demonstrated that 2500 cGy delivered in a hypofractionation treatment platform can inhibit β1 integrin expression in DU-145 and PC3 prostate cancer cells [14]. This was not observed at lower doses of radiation therapy. There is evidence that the presence of β3 and β1 integrins in prostate cancer cells further promotes resistance to radiation treatment as well as promote tumor-growth kinetics. This implies that several biological factors may have an influence on radiation therapy treatment response. Targeting these areas for additive therapies to facilitate cell death during radiation therapy is an important area of translational science.
As tools for evaluation continue to improve, there is expanded opportunity to evaluate the tumor microenvironment. 3D matrices are becoming more robust and evaluation with imaging of these constructs will provide an opportunity to review tumor/stroma interactions as well as evaluate conditions within the microenvironment that may influence tumor cell death and/or repair. Finally, there are areas of hypoxia within prostate cancers. Hypoxia has been associated with tumor resistance to radiation therapy [3]. Modifying the environment for this issue may convert potentially lethal damage from radiation therapy into a lethal event. The impact of integrin therapy in this area remains to be studied.
IGF-IR modulation
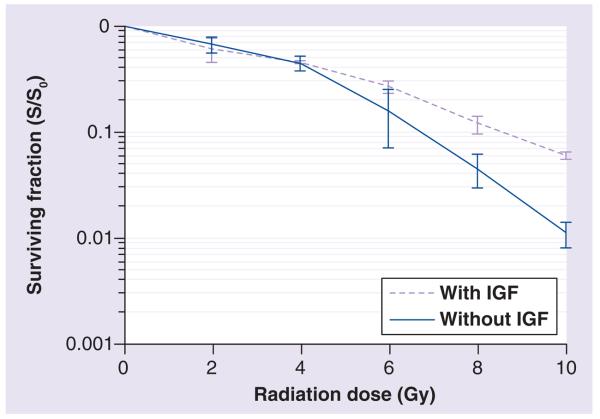
Intrinsic radiosensitivity is one of the critical factors that determine the probability of a successful tumor cure or local control following radiotherapy. The IGF-I receptor (IGF-IR) is a transmembrane receptor with intrinsic tyrosine kinase activity. IGF-IR mediates the mitogenic, transforming, differentiating and antiapoptotic activities of IGF-I and -II [19-21]. Several reports indicate that IGF-IR can induce radiation resistance in different tumor cells [22-24]. Thus, study of this receptor is likely to shed light on the downstream pathways leading to this phenomenon. Clonogenic radiation survival of small-cell lung cancer H226 and H460 cells, grown under anchorage-dependent conditions, is impaired by a fully humanized anti-IGF-IR monoclonal antibody A12 demonstrating a radiation dose-enhancing effect for IGF-IR blockade. In the H460 xenograft model, combining A12 and radiation significantly enhances antitumor efficacy compared with either modality alone [25]. The activation of IGF-IR by its ligands protects several cancer cell types from cell death caused by anticancer agents including ionizing radiation [25,26]. Moreover, β1 integrins, known to increase radio-resistance, selectively modulate IGF-IR signaling in response to IGF stimulation in prostate cancer cells [27]. Thus, inhibition of IGF-IR may become a critical factor in determining the success of radiotherapy. It is clear that prostate cancer cells expressing IGF-IR are more resistant to radiation therapy [28,29]. As evidenced in Figure 2 and [29], DU-145 cells stimulated with IGF demonstrate higher resistance to ionizing radiation with cell survival measured by standard clonogenic assays. This may become another important pathway to target in selected patients showing upregulation of this receptor.
Figure 2. IGF increases surviving fraction of DU-145 cells exposed to ionizing radiation.
DU-145 cells were starved in serum-free culture medium for 24 h followed by addition of 50 ng/ml IGF-I for 15 min immediately before radiation. For the clonogenic assay, cells were exposed to 0, 2, 4, 6, 8 or 10 Gy x-ray at room temperature in the presence or absence (control) of IGF. After irradiation, cells were cultured in the culture medium for 2 weeks and cell colony with more than 50 cells was counted to score the surviving fraction (p < 0.01 at radiation doses of 8 and 10 Gy).
c-JUN N-terminal kinase (JNK) role
JNK kinases, also called Sap kinases, are known to be activated by several stresses such as UV light or γ-radiation [30] as well as by integrins which act through focal adhesion kinase (FAK) pathways [31].
It was recently reported that Bcl-2 inhibitor treatment followed by irradiation induces apoptosis and JNK phosphorylation plays an important role in apoptosis induced by this combined treatment [32]. Su et al. demonstrated that combined treatment of γ-radiation and mda-7/IL-24 increases JNK phosphorylation, which results in apoptosis [33]. Restoration of expression of the retinoblastoma (RB) gene to DU-145 cells sensitizes them to apoptosis induced by γ-irradiation. It was shown that, after ionizing radiation, intact RB mediates transcriptional activation that leads to the activation of JNK that is required for RB-mediated DU-145 cell death after ionizing radiation [34]. These data suggest that JNK modulation in combination with radiation represents a potential treatment that could improve the therapeutic outcome for prostate cancer patients.
Runx2 amplification
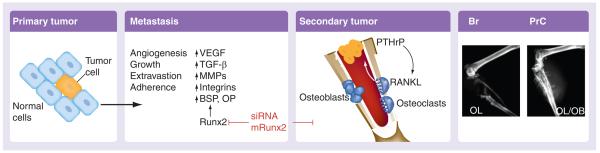
The family of Runx proteins are essential for normal development of hematopoietic tissue (Runx1), bone formation (Runx2) and gut and neuronal cell development (Runx3). Runx proteins are mutated, deleted, silenced or ectopically expressed in a variety of solid tumors and leukemias [35]. Runx1 and 3 function as tumor suppressors in leukemias and gastric cancer, respectively. By contrast, Runx2 is aberrantly expressed at high levels in breast and prostate tumors cells that aggressively metastasize to the bone environment. Tumor explants and highly metastatic cell lines placed directly into mouse femur and tibia readily form tumors in bone, a rich environment for tumor growth. These tumors are extremely destructive to the bone, resulting in osteoblastic and osteolytic lesions (Figure 3). The lytic nature causes profound changes in metabolism (hypocalcaemia) and bone structure. Runx2 expression correlates with their metastatic potential and coincides with higher expression of angiogenic and metastatic gene markers such as VEGF, matrix metalloproteinases, and osteopontin, all of which are direct targets of Runx2. Runx2 responds to growth factors, IGF-1, TGFβ and bone morpho-genetic protein signaling and act to facilitate tumor growth, as well as mediate tumor cell activities that form either osteolytic or osteoblastic lesions, which cause the breakdown of bone structure leading to high incidence of fractures. The tumor suppressor properties of Runx2 observed in normal osteo-blasts appear to fail in tumor cells [36]. Runx2 upregulation in tumor cells does not impede cell-cycle progression as it does in normal bone cells. In normal cells, the absence of Runx2 results in genomic instability and a marked delay in DNA repair after a sublethal dose of ionizing irradiation. Thus, with Runx2 being expressed in tumor cells, Runx2 may also contribute to tumor cell viability at this level as well. In a broader biological context, recent studies demonstrate that Runx factors facilitate genetic and epigenetic control of proliferation and cell growth [37,38]. Runx2-deficient osteoblasts lack components of the Mre11/Rad50/Nbs1 DNA-repair complex and exhibit a delayed DNA-repair response when cells were exposed to 5 Gy ionizing radiation [36]. Establishing a strategy to inhibit Runx2 expression may be of importance in the inhibition of the development of metastatic lesions. Studies have already shown that inhibition of functional Runx2 by mutation of its essential transactivation domain, the nuclear-matrix targeting signal, can prevent tumor growth and osteolytic lesions associated with breast cancer metastasis [39].
Figure 3. Summary of the functional roles of Runx2 in facilitating metastasis and tumor-induced lesions in the bone environment.
Br: Breast cancer; BSP: Bone sialoprotein; MMP: Matrix metalloproteinase; OB: Osteoblastic; OL: Osteolytic; OP: Osteopontin; PrC: Prostate cancer; PTHrP: Parathyroid hormone-related protein.
Expert commentary
There is extraordinary opportunity for developing strategies for clinical protocols that could potentially improve the application of radiation therapy for patients treated for ADC of the prostate. Cell-adhesion modifiers appear to be a very good target for integration treatment strategies and merit evaluation in the clinical trial setting. The actual mechanism of action for Casodex remains to be fully characterized; however, it appears to have properties that influence tumor cell adhesion, these being most pronounced in irradiated tumor. This would imply that Casodex could be given to patients as a copartner with radiation therapy during teletherapy or brachytherapy during the decay of radioactive seeds and potentially improve patient outcome. There is interest in limited hormone application in patients with both favorable and intermediate risk factors for treatment failure, therefore, a clinical trial in this area with both tumor control and normal tissue end points may be the appropriate next step for evaluation. Although these therapies may not be robust as monotherapy in a Phase I setting, their optimal use may be with copartnering with traditional therapies such as radiation therapy.
Integrins remain an interesting target for evaluation. There is increasing evidence that they play a key role in tumorigenesis and may predict for aggressive tumor behavior in experimental models of prostate cancer studied to date (Figure 1). A recent study has also demonstrated the role of α6 integrin cleavage in modulation of tumor radiosensitivity. This study was performed using PC3 cells conditionally expressing either a cleavable wild-type form of α6 integrin (PC3N-α6-WT) or a mutated noncleavable form of this integrin (PC3N-α6-RR). Their data demonstrate that treatment with fractionated doses of ionizing radiation inhibited tumor growth of cells expressing the mutant form as compared with the wild-type form. These results suggest that blocking α6 integrin cleavage in vivo may be efficacious for increasing responsiveness to irradiation of slow growing, prometastatic human prostate cancer [40].
There is evidence in other tumor systems that integrin expression influences patient outcome with radiation therapy in a negative fashion. Integrins also appear to be associated with resistance to radiation therapy as observed in our study using cell lines. A protocol could be designed to use integrin modulation with radiation therapy without hormone and determine if PSA response and freedom from progression could mimic the established excellent outcome with hormone-radiotherapy in patients with low- and intermediate-risk factors for treatment failure. This would potentially hold hormone therapy in reserve for patients who progress in spite of integrin modulation therapy coupled with radiation management or who do not demonstrate reasonable PSA response to initial management. The advantage of such an approach would be that the induction of hormone-resistant disease could potentially be delayed or decreased by not introducing hormone therapy early in the treatment process. These issues remain to be studied; however, they may prove to be interesting protocols to initiate in a clinical trials process.
Enhancing our knowledge of signaling pathways and their response to radiation management is an important scientific effort. Radiation therapy plays an important role in the treatment of patients with prostate cancer. Both brachytherapy and intensity-modulated radiotherapy teletherapy have demonstrated outstanding clinical outcome both with and without hormone intervention as part of primary management. Understanding the influence of hormones on pathways is important as it may change strategies for intervention with radiation therapy. It is clear there exists opportunity to promote tumor cell death with radiation therapy alongside integrin modulation or IGF-IR modulation, and other strategies may play an important role moving forward in improving the clinical outcome for patients affected with this disease. Likewise, if adhesion modulation appears to be a valuable copartner to radiation therapy, protocols can be designed to either limit and/or hold hormone therapy in patient care in order not to influence the development of hormone-resistant disease. As science matures, integrating our knowledge into translational protocols will hopefully serve to further improve clinical outcome.
Five-year view
Adenocarcinoma of the prostate remains an important public health problem as it is one of the leading cancers affecting men. Despite improvements in patient detection and stage migration at the time of disease presentation and in spite of optimal patient management with traditional therapies including hormone deprivation, advanced technology radiation therapy and surgery, many patients remain vulnerable to tumor recurrence and disease progression. In order to improve patient outcome for those with intermediate- and high-risk features for tumor recurrence, process improvements are needed in patient care, as we have likely best optimized the use of traditional therapies in patient care. We need to look to alternative strategies to improve patient outcome. To do so, we need to revisit basic principles of prostate cancer tumor biology and develop treatment strategies which, to date, have been less visible to the translational scientist.
Improvements in molecular medicine, including proteomic analysis, have yielded interesting findings in cell culture. More importantly, the data appear to be validated in animal models. Cell adhesion appears to be an important feature in prostate cancer tumor progression as demonstrated by the influence of integrin expression. Accelerated expression of integrins in this disease appears to be associated with a less favorable outcome, thus making adhesion modulation a potential target for treatment. Integrin expression also appears to induce resistance to radiation therapy in this disease, thus adhesion molulation coupled with radiation therapy may be an excellent translational science effort in the future. Another important issue is that the prolonged use of hormone therapy may impart the transition to hormone-resistant disease in patients. Therefore, identifying translational science strategies that mimic the positive effect of hormone manipulation without imparting/inducing therapeutic resistance is of crucial clinical importance. Interestingly, cell adhesion may be the bridge between these objectives as hormone therapy appears to have an impact on the tumor at this level. Therefore, adhesion modulation may prove to be very important as it could provide the necessary therapeutic sensitization to traditional therapies while permitting hormones to be delayed or given in a shorter course. This may decrease the risk of developing hormone-insensitive disease.
Key issues.
Targeted therapy in prostate cancer.
Alteration in signal transduction in prostate cancer.
New strategies for the treatment of prostate cancer.
Radiation therapy in prostate cancer.
Alteration of the effect of radiation therapy in prostate cancer.
Cell adhesion and the role of resistance to radiation therapy in prostate cancer.
Signal transduction modification with radiation therapy in prostate cancer.
Future management strategies for prostate cancer.
Acknowledgments
Support for this article was from NIH-RO1 CA-89720 and RO1 CA-109874.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
TJ FitzGerald, Department of Radiation Oncology, University of Massachusetts Medical School, 55 Lake Avenue North Worcester, MA 01605, USA Tel.: +1 508 856 5551 Fax: +1 508 856 5006 fitzgert@ummhc.org.
Tao Wang, Department of Radiation Oncology & Cancer Biology, University of Massachusetts Medical School, 55 Lake Avenue North Worcester, MA 01605, USA Tel.: +1 508 856 1721 Fax: +1 508 856 1310 tao.wang@umassmed.edu.
Hira Lal Goel, Department of Cancer Biology, University of Massachusetts Medical School, 55 Lake Avenue North Worcester, MA 01605, USA Tel.: +1 508 856 1721 Fax: +1 508 856 1310 hira.goel@umassmed.edu.
Jiayi Huang, Department of Radiation Oncology, University of Massachusetts Medical School, 55 Lake Avenue North Worcester, MA 01605, USA Tel.: +1 508 856 2173 Fax: +1 508 856 6781 jiayi.huang@umassmed.edu.
Gary Stein, Department of Cancer Biology, Cell Biology and the Cancer Center, University of Massachusetts Medical School, 55 Lake Avenue North Worcester, MA 01605, USA Tel.: +1 508 856 5625 Fax: +1 508 856 6800 gary.stein@umassmed.edu.
Jane Lian, Department of Cancer Biology, Cell Biology and the Cancer Center, University of Massachusetts Medical School, 55 Lake Avenue North Worcester, MA 01605, USA Tel.: +1 508 856 5941 Fax: +1 508 856 6800 jane.lian@umassmed.edu.
Roger J Davis, Program in Molecular Medicine, Program in Gene Expression & Function, University of Massachusetts Medical School, 55 Lake Avenue North Worcester, MA 01605, USA Tel.: +1 508 856 6054 Fax: +1 508 856 3210 roger.davis@umassmed.edu.
Steven Doxsey, Program in Molecular Medicine and the Cancer Center, University of Massachusetts Medical School, 55 Lake Avenue North Worcester, MA 01605, USA Tel.: +1 508 856 1613 Fax:+1 508 856 5657 stephen.doxsey@umassmed.edu.
KC Balaji, Department of Surgery, University of Massachusetts Medical School, 55 Lake Avenue North Worcester, MA 01605, USA tel.: +1 508 856 5821 Fax:+1 508 856 3137 kc.balaji@umassmed.edu.
Jesse Aronowitz, Department of Radiation Oncology, University of Massachusetts Medical School, 55 Lake Avenue North Worcester, MA 01605, USA Tel.: +1 508 334 6550 Fax: +1 508 334 5624 aronowij@ummhc.org.
Lucia R Languino, Department of Cancer Biology, Cell Biology and the Cancer Center, University of Massachusetts Medical School, 55 Lake Avenue North Worcester, MA 01605, USA Tel.: +1 508 856 1606 Fax: +1 508 856 3710 lucia.languino@umassmed.edu.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J. Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.FitzGerald TJ, Simon E, Meyer J. Prostate carcinoma: opportunities for translational research. J. Cell Biochem. 2004;91(3):433–442. doi: 10.1002/jcb.10693. [DOI] [PubMed] [Google Scholar]
- 3.Hall E, Giacca AJ, editors. Radiology for the Radiologist. Lippincott, Williams & Wilkins, Columbia University; NY, USA: 2006. [Google Scholar]
- 4.Ahmed MM. Regulation of radiation-induced apoptosis by early growth response-1 gene in solid tumors. Curr. Cancer Drug Targets. 2004;4(1):43–52. doi: 10.2174/1568009043481704. [DOI] [PubMed] [Google Scholar]
- 5.Calaf GM, Hei TK. Ionizing radiation induces alterations in cellular proliferation and c-myc, c-jun and c-fos protein expression in breast epithelial cells. Int. J. Oncol. 2004;25(6):1859–1866. doi: 10.3892/ijo.25.6.1859. [DOI] [PubMed] [Google Scholar]
- 6.Hehlgans S, Haase M, Cordes N. Signalling via integrins: implications for cell survival and anticancer strategies. Biochim. Biophys. Acta. 2007;1775(1):163–180. doi: 10.1016/j.bbcan.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Cordes N, Blaese MA, Plasswilm L, Rodemann HP, Van Beuningen D. Fibronectin and laminin increase resistance to ionizing radiation and the cytotoxic drug Ukrain in human tumour and normal cells in vitro. Int. J. Radiat. Biol. 2003;79(9):709–720. doi: 10.1080/09553000310001610240. [DOI] [PubMed] [Google Scholar]
- 8.Onoda JM, Piechocki MP, Honn KV. Radiation-induced increase in expression of the aIIb β3 integrin in melanoma cells: effects on metastatic potential. Radiat. Res. 1992;130(3):281–288. [PubMed] [Google Scholar]
- 9.Burke PA, DeNardo SJ, Miers LA, Lamborn KR, Matzku S, DeNardo GL. Cilengitide targeting of αvβ3 integrin receptor synergizes with radioimmunotherapy to increase efficacy and apoptosis in breast cancer xenografts. Cancer Res. 2002;62(15):4263–4272. [PubMed] [Google Scholar]
- 10.Cao Q, Cai W, Li T, et al. Combination of integrin siRNA and irradiation for breast cancer therapy. Biochem. Biophys. Res. Commun. 2006;351(3):726–732. doi: 10.1016/j.bbrc.2006.10.100. [DOI] [PubMed] [Google Scholar]
- 11.Cordes N, Seidler J, Durzok R, Geinitz H, Brakebusch C. β1-integrin-mediated signaling essentially contributes to cell survival after radiation-induced genotoxic injury. Oncogene. 2006;25(9):1378–1390. doi: 10.1038/sj.onc.1209164. [DOI] [PubMed] [Google Scholar]
- 12.Choi SH, Cho KJ, Nam SY, Lee SW, Kang J, Kim SY. Clinical significance of β1 integrin expression as a prediction marker for radiotherapy in early glottic carcinoma. Laryngoscope. 2006;116(7):1228–1231. doi: 10.1097/01.mlg.0000224499.93774.77. [DOI] [PubMed] [Google Scholar]
- 13.Hazelbag S, Kenter GG, Gorter A, et al. Overexpression of the αvβ6 integrin in cervical squamous cell carcinoma is a prognostic factor for decreased survival. J. Pathol. 2007;212(3):316–324. doi: 10.1002/path.2168. [DOI] [PubMed] [Google Scholar]
- 14.Simon EL, Goel HL, Teider N, Wang T, Languino LR, Fitzgerald TJ. High dose fractionated ionizing radiation inhibits prostate cancer cell adhesion and β1 integrin expression. Prostate. 2005;64(1):83–91. doi: 10.1002/pros.20227. [DOI] [PubMed] [Google Scholar]
- 15.Wang T, Huang J, Alavian MR, Goel HL, Languino LR, Fitzgerald TJ. αvβ3 integrin promotes resistance of prostate cancer cells to ionizing radiation. Int. J. Rad. Onc. Biol. Phys. 2007;69(3 Suppl):S611. [Google Scholar]
- 16.Altieri DC. The molecular basis and potential role of survivin in cancer diagnosis and therapy. Trends Mol. Med. 2001;7(12):542–547. doi: 10.1016/s1471-4914(01)02243-2. [DOI] [PubMed] [Google Scholar]
- 17.Masiello D, Cheng S, Bubley GJ, Lu ML, Balk SP. Bicalutamide functions as an androgen receptor antagonist by assembly of a transcriptionally inactive receptor. J. Biol. Chem. 2002;277(29):26321–26326. doi: 10.1074/jbc.M203310200. [DOI] [PubMed] [Google Scholar]
- 18.Alavian MR, Wang T, Goel HL, Languino LR, Fitzgerald TJ. Bicalutamide inhibits androgen promotion of prostate cancer cell adhesion when exposed to ionizing radiation; Presented at: 53rd Annual Meeting of the Radiation Research Society; PA, USA. 5–8 November 2006. [Google Scholar]
- 19.Valentinis B, Baserga R. IGF-I receptor signalling in transformation and differentiation. Mol. Pathol. 2001;54(3):133–137. doi: 10.1136/mp.54.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan PJ, Mohan S, Cohen P, Foster BA, Greenberg NM. The insulin-like growth factor axis and prostate cancer: lessons from the transgenic adenocarcinoma of mouse prostate (TRAMP) model. Cancer Res. 1999;59(9):2203–2209. [PubMed] [Google Scholar]
- 21.Werner H, LeRoith D. The role of the insulin-like growth factor system in human cancer. Adv. Cancer Res. 1996;68:183–223. doi: 10.1016/s0065-230x(08)60354-1. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe H, Miura M, Sasaki T. Differential effects of the insulin-like growth factor I receptor on radiosensitivity and spontaneous necrosis formation of human glioblastoma cells grown in multicellular spheroids. Exp. Cell Res. 1999;250(1):99–111. doi: 10.1006/excr.1999.4498. [DOI] [PubMed] [Google Scholar]
- 23.Yu D, Shibuya H, Miura M. Roles of the insulin-like growth factor I receptor C-terminus in cellular radioresistance. Biochem. Biophys. Res. Commun. 2003;311(1):174–178. doi: 10.1016/j.bbrc.2003.09.195. [DOI] [PubMed] [Google Scholar]
- 24.Turner BC, Haffty BG, Narayanan L, et al. Insulin-like growth factor-I receptor overexpression mediates cellular radioresistance and local breast cancer recurrence after lumpectomy and radiation. Cancer Res. 1997;57(15):3079–3083. [PubMed] [Google Scholar]
- 25.Allen GW, Saba C, Armstrong EA, et al. Insulin-like growth factor-I receptor signaling blockade combined with radiation. Cancer Res. 2007;67(3):1155–1162. doi: 10.1158/0008-5472.CAN-06-2000. [DOI] [PubMed] [Google Scholar]
- 26.Peretz S, Jensen R, Baserga R, Glazer PM. ATM-dependent expression of the insulin-like growth factor-I receptor in a pathway regulating radiation response. Proc. Natl Acad. Sci. USA. 2001;98(4):1676–1681. doi: 10.1073/pnas.041416598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goel HL, Fornaro M, Moro L, et al. Selective modulation of type 1 insulin-like growth factor receptor signaling and functions by β1 integrins. J. Cell Biol. 2004;166(3):407–418. doi: 10.1083/jcb.200403003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu D, Watanabe H, Shibuya H, Miura M. Redundancy of radioresistant signaling pathways originating from insulin-like growth factor I receptor. J. Biol. Chem. 2003;278(9):6702–6709. doi: 10.1074/jbc.M209809200. [DOI] [PubMed] [Google Scholar]
- 29.Wang T, Languino LR, Fitzgerald TJ. Insulin-like growth factor type I receptor (IGF-IR) mediates radioresistance of DU-145 and PC3 human prostate cancer cells during ionizing radiation; Presented at: 52nd Annual Meeting of the Radiation Research Society; 2005. [Google Scholar]
- 30.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr. Opin. Cell Biol. 2007;19(2):142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Almeida EA, Ilic D, Han Q, et al. Matrix survival signaling. From fibronectin via focal adhesion kinase to c-jun nh(2)-terminal kinase. J. Cell Biol. 2000;149(3):741–754. doi: 10.1083/jcb.149.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.An J, Chervin AS, Nie A, Ducoff HS, Huang Z. Overcoming the radioresistance of prostate cancer cells with a novel Bcl-2 inhibitor. Oncogene. 2007;26(5):652–661. doi: 10.1038/sj.onc.1209830. [DOI] [PubMed] [Google Scholar]
- 33.Su ZZ, Lebedeva IV, Sarkar D, et al. Ionizing radiation enhances therapeutic activity of mda-7/IL-24: overcoming radiation- and mda-7/IL-24-resistance in prostate cancer cells overexpressing the antiapoptotic proteins bcl-xL or bcl-2. Oncogene. 2006;25(16):2339–2348. doi: 10.1038/sj.onc.1209271. [DOI] [PubMed] [Google Scholar]
- 34.Bowen C, Birrer M, Gelmann EP. Retinoblastoma protein-mediated apoptosis after g-irradiation. J. Biol. Chem. 2002;277(47):44969–44979. doi: 10.1074/jbc.M202000200. [DOI] [PubMed] [Google Scholar]
- 35.Blyth K, Cameron ER, Neil JC. The RUNX genes: gain or loss of function in cancer. Nat. Rev. 2005;5(5):376–387. doi: 10.1038/nrc1607. [DOI] [PubMed] [Google Scholar]
- 36.Zaidi SK, Pande S, Pratap J, et al. Runx2 deficiency and defective subnuclear targeting bypass senescence to promote immortalization and tumorigenic potential. Proc. Natl Acad. Sci. USA. 2007;104(50):19861–19866. doi: 10.1073/pnas.0709650104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young DW, Hassan MQ, Yang XQ, et al. Mitotic retention of gene expression patterns by the cell fate-determining transcription factor Runx2. Proc. Natl Acad. Sci. USA. 2007;104(9):3189–3194. doi: 10.1073/pnas.0611419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young DW, Hassan MQ, Pratap J, et al. Mitotic occupancy and lineage-specific transcriptional control of rRNA genes by Runx2. Nature. 2007;445(7126):442–446. doi: 10.1038/nature05473. [DOI] [PubMed] [Google Scholar]
- 39.Javed A, Barnes GL, Pratap J, et al. Impaired intranuclear trafficking of Runx2 (AML3/CBFA1) transcription factors in breast cancer cells inhibits osteolysis in vivo. Proc. Natl Acad. Sci. USA. 2005;102(5):1454–1459. doi: 10.1073/pnas.0409121102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pawar SC, Dougherty S, Pennington ME, et al. α6 integrin cleavage: sensitizing human prostate cancer to ionizing radiation. Int. J. Rad. Biol. 2007;83(11–12):761–767. doi: 10.1080/09553000701633135. [DOI] [PMC free article] [PubMed] [Google Scholar]