Abstract
The syncytiotrophoblast (SCT) is the outer layer of placenta which is in direct contact with maternal blood. As such it is uniquely positioned to alter maternal hemostasis and endothelial function. The syncytium is known to release anti-angiogenic factors including fms-like tyrosine kinase-1 (sFlt-1) and soluble endoglin (sEng), as well as the anti-fibinolytic factor plasminogen activator inhibitor-1 (PAI-1). Its release of microparticles has also been suggested to play a role in regulating maternal endothelial and immune cell function. It is of note that syncytial release of the abovementioned factors increases in preeclampsia, a major cause of maternal mortality and morbidity. In preeclampsia, hypoxia and reperfusion injury in the placenta is associated with activation of the maternal endothelium. In this review, I describe the interaction of syncytial factors with hypoxia, reactive oxygen species, and apoptosis in the pathophysiology of preeclampsia and intrauterine growth restriction. In addition, I detail the potential protective actions of placental ceruloplasmin in preeclampsia, recently described by our group to be a sensitive marker of syncytial hypoxia.
Keywords and phrases: placenta, syncytiotrophoblast, preeclampsia, PAI-1, reactive oxygen species, ceruloplasmin
Introduction
Release of syncytial factors in preeclampsia
Although the precise etiology of the maternal syndrome of preeclampsia remains unelucidated, there is agreement that it is preceded by failed conversion of maternal endometrial spiral arteries in the placental bed [1]. This is suggested to preclude the development of a low resistance/high capacitance utero-placental circulation requisite for normal pregnancy [2, 3]. Whether this is due to inadequate trophoblast invasion and/or maternal factors is the subject of debate [1]. The syncytium is the outer cell layer of the placenta that is in direct contact with maternal blood [4]. It is postulated in preeclampsia that hypoxia/reperfusion injury disrupts syncytial architecture and promotes increased release of soluble syncytial factors including cytokines [5, 6], eicosanoids [7], peroxides [8, 9], the anti-angiogenic factors soluble fms-like tyrosine kinase (sFlt)-1[10] and endoglin [11], as well as syncytiotrophoblast microparticles (STBM) [12, 13]. These factors are suggested to pathologically activate the maternal endothelium leading to maternal proteinuria and hypertension, clinical hallmarks of preeclampsia.
Syncytial hypoxia, reperfusion injury, and apoptosis in preeclampsia
Hypoxia and/or reperfusion injury associated with preeclampsia occurs in concert with the release of the abovementioned deleterious compounds by syncytiotrophoblasts [1, 8]. It is well known that the superoxide anion (SO), the most common reactive oxygen species (ROS), is generated during the conversion of xanthine (X) to uric acid by the enzyme xanthine oxidase (XO)[8, 14]. SO may combine with nitric oxide to produce peroxynitrite anions which damages cellular proteins [8]. Increased placental nitrosylation of proteins and oxidative stress are biochemical markers of preeclampsia [15, 16]. ROS-associated placental damage was noted in synctiotrophoblasts in pregnancies with preeclampsia and intrauterine growth restriction (IUGR) [8, 14, 16], and is suggested to promote higher levels of apoptosis noted in this cell type in these pregnancy complications [17, 18]. In vitro studies are consistent with this notion as hypoxia and reperfusion, not hypoxia alone, induced apoptosis in syncytiotrophoblasts [19]. Our group has studied apoptosis at the maternal-fetal interface in association with the expression of Fas ligand (FasL) [20-22]. FasL is a member of the tumor necrosis factor family that induces cell death following binding to Fas, its receptor on target cells [23]. We observed that placental trophoblasts express FasL across gestation, and Fas was localized to chorionic trophoblasts, amnion epithelial cells, and decidua of fetal membranes [20-22]. It has been suggested that dysregulation of the Fas/FasL signaling system in villous trophoblasts occurs in association with preeclampsia [23]; the Fas/FasL ratio increased in preeclamptic villi [24], and sera from preeclamptic women decreased trophoblast viability while increasing trophoblast sensitivity to Fas-mediated apoptosis [25]. It is of note, that in a recent study we presented a novel methodology in which laser capture microdissection (LCMD) followed by Western blotting was used to assess levels of syncytial Fas ligand (FasL) [26], suggesting that this technique may be used to elucidate changes in syncytial protein expression that occurs in preeclampsia and IUGR.
Syncytiotrophoblast microparticles (STBM)
STBM are surface membranes shed from the outer layer of the placenta directly to maternal blood [12, 13, 27, 28]. Higher levels of STBM were found in maternal blood in association with preeclampsia, and STBM were demonstrated to promote endothelial and immune cell dysfunction in vitro [12, 13, 27-29]. STBM can be isolated in vitro by mechanical means, during perfusion, and from explant culture media following centrifugation, and are quantitated using FACS and ELISA [27, 28, 30]. The presence of STBMs were specifically demonstrated to promote cell death and/or reduce proliferation of endothelial cells [28, 30]. In addition, they were shown to activate superoxide production in neutrophils isolated from women with preeclampsia [27]. It is of note, that STBM levels in maternal blood correlate with the severity of preeclampsia, whereas deportation of trophoblasts (cells) does not [31]. Enhanced shedding of STBM appears to occur in association with preeclampsia and not IUGR, suggestive of unique patterns of placental pathophysiology in these complications of pregnancy [12]. STBM are 0.2 to 2 μm in size, and they are formed by plasma membrane blebbing associated with apoptosis/necrosis [32]. STBM are distinct from exosomes which are 40 to 80 nm and are formed from fusion of intracellular vesicles with the plasma membrane [32]. An exciting recent finding suggests an apoptotic etiology for STBM generation as apoptotic DNA fragments (ladders) were found in STBMs isolated from maternal blood as well as in conditioned media from JEG-3 choriocarcinoma cells [33].
Placental damage and PAI-1 in PE and IUGR
Although fibrin deposition at the syncytial surface is a critical component of physiological repair and differentiation of the placental villous [34], aberrantly high levels of intervillous fibrin is a histological hallmark of pregnancies with preeclampsia and IUGR, and has been suggested to reduce nutrient flow between mother and fetus resulting in poor neonatal outcomes [35, 36]. Placental damage (infarct) in pregnancies with preeclampsia and IUGR was correlated with adverse fetal outcomes as well as increased placental expression of the antifibrinolytic factor plasminogen activator inhibitor -1 (PAI-1) [37, 38]. Immuohistochemistry and in situ hybridization revealed elevated syncytial expression of PAI-1 in these pregnancies, suggesting that this specific cell type was responsible for reduced perivillous fibrinolysis in preeclampsia and IUGR [38, 39]. The technique of dual (maternal+fetal) placental perfusion was used by our group to study the syncytial release of PAI-1, cytokines, microparticles, and eicosenoids [40, 41]. The maternal component of dual perfusion is perfused via cannulae inserted through the decidual surface directly into the intervillous space, thereby effectively mimicking the in vivo process in which syncytiotrophoblasts release proteins and other compounds directly to maternal blood. Thus, dual perfusion affords the investigator a physiology relevant model to study the causes of aberrant release of syncytial factors in preeclampsia.
Adaptive syncytial responses in preeclampsia; potential role of placental ceruloplasmin
As described above, there is consensus that ROS generated during ischemia/reperfusion injury in preeclampsia promotes both placental damage and the release of factors leading to maternal endothelial dysfunction. There are several antioxidant systems shown to be present in placenta that are altered in activity in association with preeclampsia [8, 9]. Glutathione peroxidase, catalase, and superoxide dismutase are a key group of enzymes that metabolize reactive oxygen species thereby limiting cellular damage. It has been suggested that increased placental expression of glutathione peroxidase, a selenium-containing enzyme that metabolizes and inactivates hydrogen peroxide, serves as an adaptive/protective mechanism to limit oxidative damage in preeclampsia [42, 43]. However, in another report, glutathione peroxidase activity was found to be lower in placentas from pregnancies with preeclampsia [42, 43]. The enzyme catalase, which also catalyzes the decomposition of hydrogen peroxide, was demonstrated to be increased in preeclamptic placentas [42, 43]. Decreased levels of mRNA and activity of the copper-zinc isoform of superoxide dismutase (i.e. an enzyme which metabolizes and inactivates SO) were reported in preeclamptic placentas [43, 44]. Additionally, it was found that there was no alteration in levels or localization of isoforms of superoxide dismutase in normal and preeclamptic placentas [45].
In order to further characterize placental factors playing a role in the pathophysiology of preeclampsia, we recently used DNA microarray gene profiling to identify mRNA differentially regulated in placentas from women with severe preeclampsia compared to both preterm and term control groups [46]. Microarray studies detected an up-regulation of mRNA for ceruloplasmin, a copper-containing iron transport protein with anti-oxidant ferroxidase properties, in preeclamptic placentas compared to preterm and term controls. Quantitative real-time PCR confirmed these results [46]. Ceruloplasmin was first described as a protein that was synthesized and released by the liver [47]. Decreased serum levels of ceruloplasmin were noted in patients with Wilson's disease, establishing the basis for a clinical test that is still in use [48]. Early animal studies showed that iron accumulated in liver as well as tissues of pigs fed a copper free diet, and administration of ceruloplasmin rapidly promoted the release of iron to the circulation [49, 50]. Of note, ceruloplasmin was also demonstrated to be a ferroxidase [51]. The ferroxidatic activity of ceruloplasmin is a critical function of this enzyme in light of the finding that even trace amounts of iron can generate hydroxyl radicals through the Fenton reaction which can destroy cellular architecture [47, 52]. Ferroxidatic activity of ceruloplasmin converts toxic ferrous iron to less toxic ferric iron, thereby reducing oxidative damage to lipids, proteins, and DNA [47, 52].
To determine which cell types in placenta synthesize ceruloplasmin, RNA was isolated from whole placental tissue and from primary cultures of syncytiotrophoblasts and placental fibroblasts, and ceruloplasmin expression was examined by PCR [46]. Results from this experiment indicated that syncytiotrophoblasts expressed ceruloplasmin mRNA and are the likely source of placental ceruloplasmin. It is of note, that preeclampsia was associated with a 60% increase in the concentration of ceruloplasmin in maternal blood in one study [53], and a 16% increase in another [54]. To date, it is not known whether placental ceruloplasmin is secreted, thus its potential contribution to the preeclampsia-associated increase of this protein in maternal serum is unknown.
The question then arises as to which factors may lead to increased placental ceruloplasmin expression in preeclampsia. One possibility is that placental hypoxia which accompanies preeclampsia [1, 2] increases placental ceruloplasmin expression as has been noted in studies using macrophages and monocytes [55]. Using primary cultures of syncytiotrophoblasts we reported that hypoxic treatment induced ceruloplasmin expression approximately 25-fold, significantly greater than the effect on expression of PAI-1 and sFlt-1 [46]. This suggests that ceruloplasmin expression is a sensitive marker of syncytial hypoxia in pregnancies with preeclampsia.
Concerning the potential role of elevated placental ceruloplasmin in preeclampsia, it is known that this syndrome is characterized by increased placental expression of ROS, lipid peroxidation, and damage to villous architecture [7, 8, 16, 56]. Thus, our findings suggest that increased levels of placental ceruloplasmin in preeclampsia would result in enhanced ferroxidatic activity, thereby converting excess ferrous iron to the less toxic ferric form. This suggests that syncytial ceruloplasmin, induced by hypoxic conditions in severe preeclampsia, plays a key role in a cellular program which mitigates the damaging effects of subsequent reperfusion injury in placenta.
Role of syncytial products in preeclampsia and IUGR
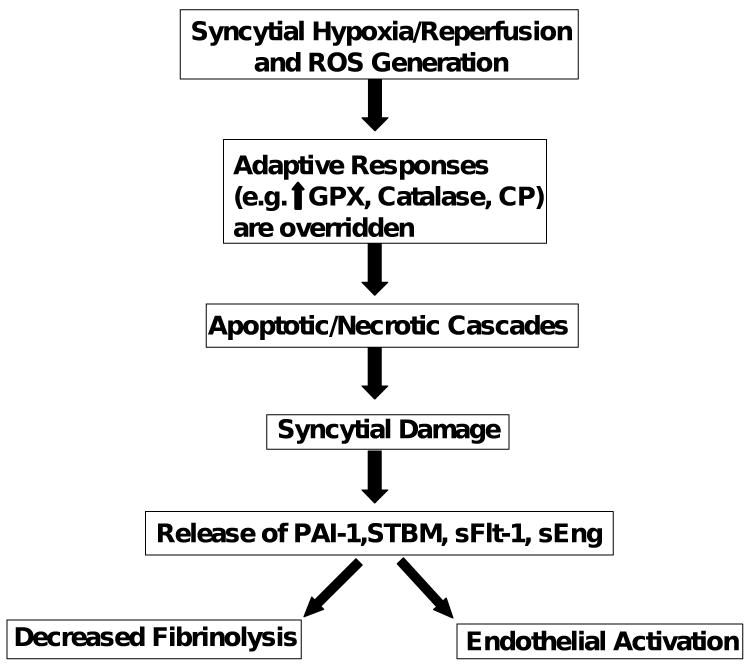
In Figure 1 we present a model which incorporates the roles of syncytial hypoxia/reperfusion and ROS in activation of the maternal endothelium and placental damage in pregnancies associated with preeclampsia and IUGR. It is indicated that hypoxia and reperfusion leads to the generation of excessive levels of ROS in placenta. In uncomplicated pregnancies ROS generation is low, and antioxidative pathways including glutathione peroxidase, catalase and ceruloplasmin are able to inactivate endogenous ROS thereby limiting placental damage. However, in pregnancies with preeclampsia and IUGR, these adaptive mechanisms are overwhelmed by enhanced production of ROS leading to an apoptotic/necrotic cascade in syncytiotrophoblasts. This is postulated to promote the release of syncytial products including sFlt-1, endoglin, eicosanoids, peroxides, and STBM. These factors aberrantly activate maternal endothelium and promote the maternal syndrome of preeclampsia. In addition, we suggest that when preeclampsia occurs in combination with enhanced levels of syncytial PAI-1, intervillous fibrin deposition, and infarction, this may critically reduce the flow of nutrients from mother to fetus leading to IUGR.
Figure 1.
Model of the putative role of the placental syncytium in the pathophysiology of preeclampsia and IUGR.
ROS, reactive oxygen species; GPX, glutathione peroxidase; CP, ceruloplasmin; PAI-1, plasminogen activator inhibitor-1; STBM, syncytiotrophoblast microparticles; sFlt-1, soluble Fms-like tyrosine kinase; sEng, soluble endoglin
Footnotes
Supported in part by NIH Grant R56HD033909
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- 3.Stepan H, Faber R, Dornhofer N, Huppertz B, Robitzki A, Walther T. New insights into the biology of preeclampsia. Biol Reprod. 2006;74:772–776. doi: 10.1095/biolreprod.105.045997. [DOI] [PubMed] [Google Scholar]
- 4.Benirschke K. Remarkable placenta. Clin Anat. 1998;11:194–205. doi: 10.1002/(SICI)1098-2353(1998)11:3<194::AID-CA8>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 5.Benyo DF, Smarason A, Redman CW, Sims C, Conrad KP. Expression of inflammatory cytokines in placentas from women with preeclampsia. J Clin Endocrinol Metab. 2001;86:2505–2512. doi: 10.1210/jcem.86.6.7585. [DOI] [PubMed] [Google Scholar]
- 6.Conrad KP, Miles TM, Benyo DF. Circulating levels of immunoreactive cytokines in women with preeclampsia. Am J Reprod Immunol. 1998;40:102–111. doi: 10.1111/j.1600-0897.1998.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 7.Walsh SW, Vaughan JE, Wang Y, Roberts LJ., 2nd Placental isoprostane is significantly increased in preeclampsia. Faseb J. 2000;14:1289–1296. doi: 10.1096/fj.14.10.1289. [DOI] [PubMed] [Google Scholar]
- 8.Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol. 2004;122:369–382. doi: 10.1007/s00418-004-0677-x. [DOI] [PubMed] [Google Scholar]
- 9.Sikkema JM, van Rijn BB, Franx A, Bruinse HW, de Roos R, Stroes ES, van Faassen EE. Placental superoxide is increased in pre-eclampsia. Placenta. 2001;22:304–308. doi: 10.1053/plac.2001.0629. [DOI] [PubMed] [Google Scholar]
- 10.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 12.Goswami D, Tannetta DS, Magee LA, Fuchisawa A, Redman CW, Sargent IL, von Dadelszen P. Excess syncytiotrophoblast microparticle shedding is a feature of early-onset pre-eclampsia, but not normotensive intrauterine growth restriction. Placenta. 2006;27:56–61. doi: 10.1016/j.placenta.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Knight M, Redman CW, Linton EA, Sargent IL. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1998;105:632–640. doi: 10.1111/j.1471-0528.1998.tb10178.x. [DOI] [PubMed] [Google Scholar]
- 14.Poston L, Raijmakers MT. Trophoblast oxidative stress, antioxidants and pregnancy outcome--a review. Placenta. 2004;25 A:S72–78. doi: 10.1016/j.placenta.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Many A, Hubel CA, Fisher SJ, Roberts JM, Zhou Y. Invasive cytotrophoblasts manifest evidence of oxidative stress in preeclampsia. Am J Pathol. 2000;156:321–331. doi: 10.1016/S0002-9440(10)64733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myatt L, Rosenfield RB, Eis AL, Brockman DE, Greer I, Lyall F. Nitrotyrosine residues in placenta. Evidence of peroxynitrite formation and action. Hypertension. 1996;28:488–493. doi: 10.1161/01.hyp.28.3.488. [DOI] [PubMed] [Google Scholar]
- 17.Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. Am J Obstet Gynecol. 2002;186:158–166. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- 18.Levy R, Smith SD, Yusuf K, Huettner PC, Kraus FT, Sadovsky Y, Nelson DM. Trophoblast apoptosis from pregnancies complicated by fetal growth restriction is associated with enhanced p53 expression. Am J Obstet Gynecol. 2002;186:1056–1061. doi: 10.1067/mob.2002.122250. [DOI] [PubMed] [Google Scholar]
- 19.Hung TH, Skepper JN, Charnock-Jones DS, Burton GJ. Hypoxia-reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res. 2002;90:1274–1281. doi: 10.1161/01.res.0000024411.22110.aa. [DOI] [PubMed] [Google Scholar]
- 20.Abrahams VM, Straszewski-Chavez SL, Guller S, Mor G. First trimester trophoblast cells secrete Fas ligand which induces immune cell apoptosis. Mol Hum Reprod. 2004;10:55–63. doi: 10.1093/molehr/gah006. [DOI] [PubMed] [Google Scholar]
- 21.Runic R, Lockwood CJ, LaChapelle L, Dipasquale B, Demopoulos RI, Kumar A, Guller S. Apoptosis and Fas expression in human fetal membranes. J Clin Endocrinol Metab. 1998;83:660–666. doi: 10.1210/jcem.83.2.4600. [DOI] [PubMed] [Google Scholar]
- 22.Runic R, Lockwood CJ, Ma Y, Dipasquale B, Guller S. Expression of Fas ligand by human cytotrophoblasts: implications in placentation and fetal survival. J Clin Endocrinol Metab. 1996;81:3119–3122. doi: 10.1210/jcem.81.8.8768884. [DOI] [PubMed] [Google Scholar]
- 23.Neale DM, Mor G. The role of Fas mediated apoptosis in preeclampsia. J Perinat Med. 2005;33:471–477. doi: 10.1515/JPM.2005.085. [DOI] [PubMed] [Google Scholar]
- 24.Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA. Placental apoptosis in preeclampsia. Obstet Gynecol. 2000;96:271–276. doi: 10.1016/s0029-7844(00)00895-4. [DOI] [PubMed] [Google Scholar]
- 25.Neale D, Demasio K, Illuzi J, Chaiworapongsa T, Romero R, Mor G. Maternal serum of women with pre-eclampsia reduces trophoblast cell viability: evidence for an increased sensitivity to Fas-mediated apoptosis. J Matern Fetal Neonatal Med. 2003;13:39–44. doi: 10.1080/jmf.13.1.39.44. [DOI] [PubMed] [Google Scholar]
- 26.Guller S, Ma YY, Fu HH, Krikun G, Abrahams VM, Mor G. The placental syncytium and the pathophysiology of preeclampsia and intrauterine growth restriction: a novel assay to assess syncytial protein expression. Ann N Y Acad Sci. 2008;1127:129–133. doi: 10.1196/annals.1434.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aly AS, Khandelwal M, Zhao J, Mehmet AH, Sammel MD, Parry S. Neutrophils are stimulated by syncytiotrophoblast microvillous membranes to generate superoxide radicals in women with preeclampsia. Am J Obstet Gynecol. 2004;190:252–258. doi: 10.1016/j.ajog.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Gupta AK, Rusterholz C, Huppertz B, Malek A, Schneider H, Holzgreve W, Hahn S. A comparative study of the effect of three different syncytiotrophoblast micro-particles preparations on endothelial cells. Placenta. 2005;26:59–66. doi: 10.1016/j.placenta.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Lok CA, Van Der Post JA, Sargent IL, Hau CM, Sturk A, Boer K, Nieuwland R. Changes in microparticle numbers and cellular origin during pregnancy and preeclampsia. Hypertens Pregnancy. 2008;27:344–360. doi: 10.1080/10641950801955733. [DOI] [PubMed] [Google Scholar]
- 30.Cockell AP, Learmont JG, Smarason AK, Redman CW, Sargent IL, Poston L. Human placental syncytiotrophoblast microvillous membranes impair maternal vascular endothelial function. Br J Obstet Gynaecol. 1997;104:235–240. doi: 10.1111/j.1471-0528.1997.tb11052.x. [DOI] [PubMed] [Google Scholar]
- 31.Johansen M, Redman CW, Wilkins T, Sargent IL. Trophoblast deportation in human pregnancy--its relevance for pre-eclampsia. Placenta. 1999;20:531–539. doi: 10.1053/plac.1999.0422. [DOI] [PubMed] [Google Scholar]
- 32.Redman CW, Sargent IL. Circulating microparticles in normal pregnancy and pre-eclampsia. Placenta. 2008;29 A:S73–77. doi: 10.1016/j.placenta.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 33.Orozco AF, Jorgez CJ, Horne C, Marquez-Do DA, Chapman MR, Rodgers JR, Bischoff FZ, Lewis DE. Membrane protected apoptotic trophoblast microparticles contain nucleic acids: relevance to preeclampsia. Am J Pathol. 2008;173:1595–1608. doi: 10.2353/ajpath.2008.080414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humphrey RG, Smith SD, Pang L, Sadovsky Y, Nelson DM. Fibrin enhances differentiation, but not apoptosis, and limits hypoxic injury of cultured term human trophoblasts. Placenta. 2005;26:491–497. doi: 10.1016/j.placenta.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Salafia CM, Minior VK, Pezzullo JC, Popek EJ, Rosenkrantz TS, Vintzileos AM. Intrauterine growth restriction in infants of less than thirty-two weeks' gestation: associated placental pathologic features. Am J Obstet Gynecol. 1995;173:1049–1057. doi: 10.1016/0002-9378(95)91325-4. [DOI] [PubMed] [Google Scholar]
- 36.Salafia CM, Pezzullo JC, Minior VK, Divon MY. Placental pathology of absent and reversed end-diastolic flow in growth-restricted fetuses. Obstet Gynecol. 1997;90:830–836. doi: 10.1016/S0029-7844(97)00473-0. [DOI] [PubMed] [Google Scholar]
- 37.Estelles A, Gilabert J, Aznar J, Loskutoff DJ, Schleef RR. Changes in the plasma levels of type 1 and type 2 plasminogen activator inhibitors in normal pregnancy and in patients with severe preeclampsia. Blood. 1989;74:1332–1338. [PubMed] [Google Scholar]
- 38.Estelles A, Gilabert J, Keeton M, Eguchi Y, Aznar J, Grancha S, Espna F, Loskutoff DJ, Schleef RR. Altered expression of plasminogen activator inhibitor type 1 in placentas from pregnant women with preeclampsia and/or intrauterine fetal growth retardation. Blood. 1994;84:143–150. [PubMed] [Google Scholar]
- 39.Estelles A, Grancha S, Gilabert J, Thinnes T, Chirivella M, Espana F, Aznar J, Loskutoff DJ. Abnormal expression of plasminogen activator inhibitors in patients with gestational trophoblastic disease. Am J Pathol. 1996;149:1229–1239. [PMC free article] [PubMed] [Google Scholar]
- 40.Di Santo S, Sager R, Andres AC, Guller S, Schneider H. Dual in vitro perfusion of an isolated cotyledon as a model to study the implication of changes in the third trimester placenta on preeclampsia. Placenta. 2007;28 A:S23–32. doi: 10.1016/j.placenta.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Bahtiyar MO, Buhimschi C, Ravishankar V, Copel J, Norwitz E, Julien S, Guller S, Buhimschi IA. Contrasting effects of chronic hypoxia and nitric oxide synthase inhibition on circulating angiogenic factors in a rat model of growth restriction. Am J Obstet Gynecol. 2007;196:72 e71–76. doi: 10.1016/j.ajog.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 42.Knapen MF, Peters WH, Mulder TP, Merkus HM, Jansen JB, Steegers EA. Glutathione and glutathione-related enzymes in decidua and placenta of controls and women with pre-eclampsia. Placenta. 1999;20:541–546. doi: 10.1053/plac.1999.0408. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Walsh SW. Antioxidant activities and mRNA expression of superoxide dismutase, catalase, and glutathione peroxidase in normal and preeclamptic placentas. J Soc Gynecol Investig. 1996;3:179–184. [PubMed] [Google Scholar]
- 44.Wang Y, Walsh SW. Increased superoxide generation is associated with decreased superoxide dismutase activity and mRNA expression in placental trophoblast cells in pre-eclampsia. Placenta. 2001;22:206–212. doi: 10.1053/plac.2000.0608. [DOI] [PubMed] [Google Scholar]
- 45.Myatt L, Eis AL, Brockman DE, Kossenjans W, Greer IA, Lyall F. Differential localization of superoxide dismutase isoforms in placental villous tissue of normotensive, pre-eclamptic, and intrauterine growth-restricted pregnancies. J Histochem Cytochem. 1997;45:1433–1438. doi: 10.1177/002215549704501012. [DOI] [PubMed] [Google Scholar]
- 46.Guller S, Buhimschi CS, Ma YY, Huang ST, Yang L, Kuczynski E, Zambrano E, Lockwood CJ, Buhimschi IA. Placental expression of ceruloplasmin in pregnancies complicated by severe preeclampsia. Lab Invest. 2008;88:1057–1067. doi: 10.1038/labinvest.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hellman NE, Gitlin JD. Ceruloplasmin metabolism and function. Annu Rev Nutr. 2002;22:439–458. doi: 10.1146/annurev.nutr.22.012502.114457. [DOI] [PubMed] [Google Scholar]
- 48.Scheinberg IH, Gitlin D. Deficiency of ceruloplasmin in patients with hepatolenticular degeneration (Wilson's disease) Science. 1952;116:484–485. doi: 10.1126/science.116.3018.484. [DOI] [PubMed] [Google Scholar]
- 49.Lee GR, Nacht S, Lukens JN, Cartwright GE. Iron metabolism in copper-deficient swine. J Clin Invest. 1968;47:2058–2069. doi: 10.1172/JCI105891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roeser HP, Lee GR, Nacht S, Cartwright GE. The role of ceruloplasmin in iron metabolism. J Clin Invest. 1970;49:2408–2417. doi: 10.1172/JCI106460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osaki S, Johnson DA, Frieden E. The possible significance of the ferrous oxidase activity of ceruloplasmin in normal human serum. J Biol Chem. 1966;241:2746–2751. [PubMed] [Google Scholar]
- 52.Patel BN, Dunn RJ, Jeong SY, Zhu Q, Julien JP, David S. Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J Neurosci. 2002;22:6578–6586. doi: 10.1523/JNEUROSCI.22-15-06578.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Engin-Ustun Y, Ustun Y, Kamaci M, Sekeroglu R. Maternal serum ceruloplasmin in preeclampsia. Int J Gynaecol Obstet. 2005;89:51–52. doi: 10.1016/j.ijgo.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 54.Serdar Z, Gur E, Develioglu O. Serum iron and copper status and oxidative stress in severe and mild preeclampsia. Cell Biochem Funct. 2006;24:209–215. doi: 10.1002/cbf.1235. [DOI] [PubMed] [Google Scholar]
- 55.Sarkar J, Seshadri V, Tripoulas NA, Ketterer ME, Fox PL. Role of ceruloplasmin in macrophage iron efflux during hypoxia. J Biol Chem. 2003;278:44018–44024. doi: 10.1074/jbc.M304926200. [DOI] [PubMed] [Google Scholar]
- 56.Cester N, Staffolani R, Rabini RA, Magnanelli R, Salvolini E, Galassi R, Mazzanti L, Romanini C. Pregnancy induced hypertension: a role for peroxidation in microvillus plasma membranes. Mol Cell Biochem. 1994;131:151–155. doi: 10.1007/BF00925951. [DOI] [PubMed] [Google Scholar]