Abstract
INTRODUCTION
Cervical cancer is the second commonest cancer to affect women with over half a million cases world-wide yearly. Screening programmes have reduced the incidence and death rate dramatically in Western societies. At the same time, professional and social pressures may delay child bearing such that a significant number of women will present with early stage disease, but be anxious to retain their fertility potential. Standard treatment by radical hysterectomy or radiotherapy has good results, but inevitably renders the women infertile. The rationale for extensive surgery resecting parametrium or destructive radiotherapy treating the whole pelvis in all cases of cervical cancer has been questioned.
PATIENTS AND METHODS
Lessons learnt from the less radical surgical approach to breast cancer can be applied to cervical cancer whilst still observing Halstead's principles of surgical oncology. Wide, local excision of early stage small tumours by radical vaginal trachelectomy combined with a laparoscopic pelvic lymphadenectomy utilises modern technology with traditional surgery. Radical vaginal trachelectomy comprises the distal half of a radical abdominal (Wertheim's) or vaginal (Schauta's) hysterectomy. An isthmic–vaginal anastomosis restores continuity of the lower genital tract after insertion of a cerclage that is necessary to maintain competence during future pregnancies.
RESULTS
A total of 142 cases were performed between 1994 and 2006, most (98%) in women with Stage 1B carcinoma of the cervix with a mean follow-up of 57 months. Twelve (9%) had completion treatment, 11 with chemo/radiotherapy and one radical hysterectomy. There were four recurrences (3%) among the women who did not have completion treatment, and two (18%) in those that did. There were 72 pregnancies in 43 women and 33 live births in 24 women. The 5-year accumulative pregnancy rate among women trying to conceive was 53%. Delivery was by classical caesarean section in a high-risk feto-maternal units with 8 babies (25%) born before 32 weeks.
CONCLUSIONS
Radical vaginal trachelectomy appears safe when performed in centres with appropriate experience of radical vaginal surgery and laparoscopic techniques. The impact of this new approach questions traditional teaching whilst preserving potential fertility in hitherto impossible circumstances.
Keywords: Cervical cancer, Fertility sparing surgery, Radical vaginal trachelectomy
The forefather of modern-day surgery who pioneered an understanding of anatomy and, therefore, pathology by careful descriptive dissection was John Hunter, Founder of The Royal College of Surgeons of England. He was a 19-year-old lad, who travelled from Glasgow to London to study and subsequently work with his brother William Hunter, a graduate of Edinburgh University. John had no formal medical training, but became the leading anatomist and subsequently one of the country's finest surgeons through self-education and training, initially by working as an anatomy dissector, teacher and demonstrator in London and subsequently as a surgeon in the battlefields of Europe. For a while, he studied under Sir Percival Pott at St Bartholomew's Hospital, but later was appointed to the staff of St George's Hospital.
Amongst his many prosected specimens, most of which are in the Hunterian Museum at The Royal College of Surgeons of England, are specimens of advanced cervical cancer demonstrating the natural history and route of spread locally within the pelvis. Other models of his dissected specimens include women at various stages of pregnancy exhibited in the Anatomy Museum at the University of Glasgow. All these indicate that he had an amazing grasp of pelvic anatomy and pathology.
Cervical cancer: a third world problem, but a first world responsibility
There are over 500,000 cancers of the cervix world-wide every year. The great majority of these occur in the third world (over 80%) and most of these will be at an advanced stage beyond surgical cure.
Hippocrates in ancient Greece recognised cancer of the uterus and subsequently Actius of Amida used vaginal irrigation with herbal compounds to relieve pain. Ambroise Paré recommended cervical amputation and, in 1652, Tulpius described cervical amputation as treatment. Dr Marion Sims1 used galvanocaustic loops to amputate and cauterise the cervix (1879).
Early stage disease is eminently treatable by surgery carried out following the oncological principles as laid down by William Halstead for the treatment of solid tumours. In 1895, at the John Hopkins Hospital, he described and demonstrated his surgical technique for the treatment of breast cancer. At that hospital another surgeon, John Clark,2 followed these principles and performed the first abdominal radical hysterectomy for cervical cancer the same year. Whilst Pawlick and Czerny in Europe, some years before, had carried out a less radical procedure, it was Ernst Wertheim in Vienna in 18983 who described what has become the gold standard for surgical treatment of this disease. His teacher, Fredrick Schauta,4 at the same time advocated a radical vaginal approach. Whilst Wertheim removed bulky and enlarged pelvic nodes, Schauta was unable to carry this out with his vaginal surgery. Subsequently, Mitra,5 in 1954, described an extra peritoneal approach for removing pelvic lymph nodes and then performing a radical vaginal hysterectomy.
At the same time, following the discovery of radium by Marie and Paul Currie in 1889 and of X-rays by William Conrad Roentgen in 1895, techniques were developed to treat cervical cancer with radiotherapy. History records that over the subsequent 100 years battles raged between surgeons and radiotherapists as to which method of treatment was better, less morbid, and more successful. Swings and roundabouts have continued until this day and age.
Victor Bonney,6 Vice President of The Royal College of Surgeons of England, championed his technique for radical hysterectomy combined with a pelvic node dissection in order to avoid radiotherapy (1949). He was vehemently opposed to the development of the separate specialty and College of Obstetrics and Gynaecology. He was one of London's finest surgeons on the staff of the Middlesex Hospital and the Chelsea Hospital for Women. Subsequently, John Howkins, Consultant Gynaecological Surgeon at St Bartholomew's Hospital added the radical vaginal component to Bonney's technique, but also operated on women following radical radiotherapy, which regrettably resulted in a large and significant morbidity.
Hans Hinselmann invented the colposcope in Hamburg in 1925.7 This allowed magnification of the cervix up to 8–40 times so that pre-cancerous lesions (carcinoma in situ or dysplasia, cervical intra-epithelial neoplasia) to be seen as well as invasive cancer with its distinctive changes in the epithelium. In 1928, George Papanicolaou,8 a Greek emigré from the Island of Simni, working as a laboratory technician and, subsequently, a pathologist in New York, described his cytological method for examining fluid from the posterior fornix of the vagina in order to assess exfoliated uterine or endometrial cells. Following this, his ‘Papanicolaou smear’ technique of scraping the surface of the uterine cervix in order to obtain further exfoliated cells allowed pre-cancers of the cervix to be diagnosed; thus potential screening was devised and invented (1941).9
As a result of this, successful screening programmes have been developed in different parts of the developed world. This allows pre-cancerous, intra-epithelial conditions to be diagnosed and treated before an invasive cancer develops.10 Following Zur Hausen's work (1986), it is now known that the human papilloma virus is responsible for the development of all cervical cancers, especially with certain co-factors, such as smoking, association with multiple partners, and early onset of coitus. These, when active physiological metaplasia is occurring on the cervix, will lead to a higher risk of developing pre-cancerous, cervical, intra-epithelial neoplasia, and hence cancer.
The screening programme in England was started in 1988; since then, the incidence of invasive cancer has halved as has the death rate. However, an increasing number of young women with early stage disease are also being discovered. Hitherto, standard treatment has been a radical hysterectomy as described by Wertheim or Schauta or alternatively radiotherapy. Both of these modalities compromise fertility, which is consequently, therefore, impossible.
Novak in Ljubljana (1948) described a radical vaginal approach to removing the cervix for cervical pathology, but the technique fell into disrepute. Aburel11 in Bucharest (1956) performed the procedure abdominally, again with little success. Erik Burghardt in Graz in 1977,12 having carried out major and radical surgery for cervical cancer, ultimately realised that it was not necessary to remove the corpus uteri in all cases of early cervical cancer. The rationale for extensive surgical resection of the parametrium in all cases has been questioned.13
With this knowledge in mind, Daniel Dargent in Lyon described in 199414 a small group of patients in whom he had performed a radical vaginal excision of the cervix, but conserved the uterus and, at the same time, performed a pelvic node dissection. He visited St Bartholomew's Hospital, and at a surgical workshop, discussed this technique. Subsequently, after careful peer review and ethical committee approval, a modification of the Dargent technique was introduced to both St Bartholomew's Hospital and The Royal Marsden Hospital.15
Patients and Methods
Between July 1994 and December 2006, 215 patients have been referred with an established diagnosis of invasive carcinoma of the cervix for consideration of radical vaginal trachelectomy. Of these, 142 subsequently underwent the procedure after careful review and assessment. All patients were advised that standard treatment was either radical hysterectomy or radiotherapy but that, in certain selected small tumours, fertility sparing surgery was possible. Careful pathology review was carried out on all patients. All patients had undergone a prior cone biopsy, either by cold knife conisation or loop diathermy excision of the transformation zone. Of these, 85 patients have had two conisation procedures and a further 19, three conisations. Magnetic resonance imaging (MRI) was carried out according to a protocol developed at the beginning of this study.16 Staging by MRI with assessment of the position of the tumour, its size and distance from the internal os or isthmus was measured, as well as the position of the tumour ensuring that it did not breach the cervical margin.17 Lymph node enlargement was assessed as many patients were part of a further study assessing molecular imaging by ultra-small iron oxide particles (sinerem).18
Surgical Techniques
A total of 142 patients elected for radical vaginal trachelectomy and pelvic node dissection. The laparoscopic node dissection was initially performed via a 4-port approach removing the external, internal and lower common iliac nodes as well as the obturator nodes. Any suspicious nodes were sent for frozen section assessment; if positive nodes were discovered, the procedure was abandoned.
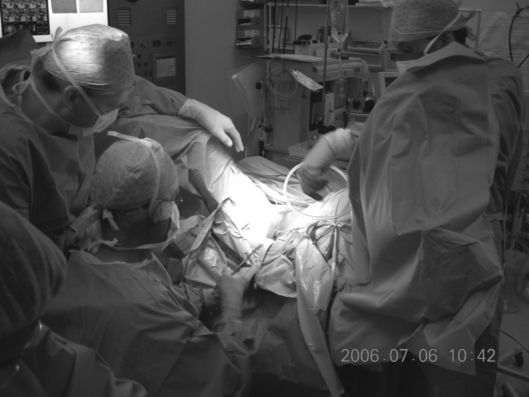
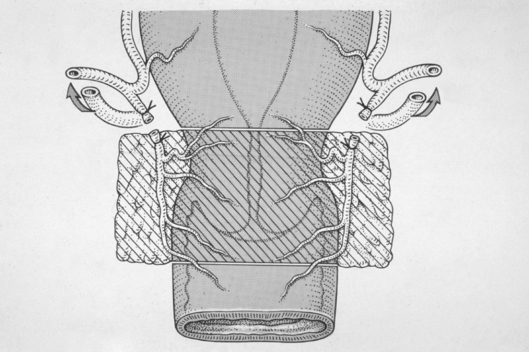
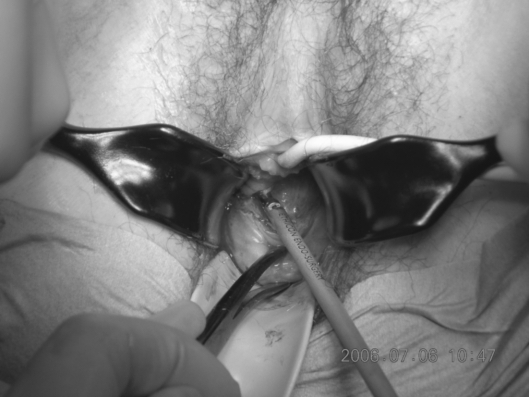
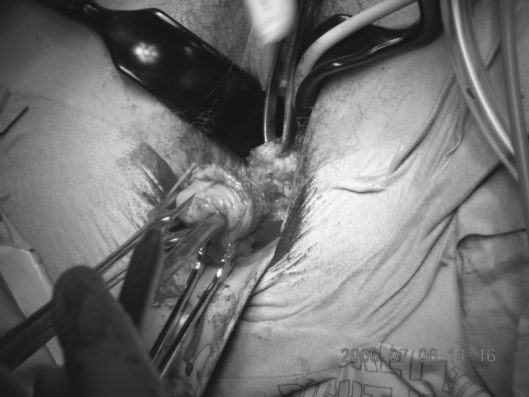
Following the bilateral pelvic node dissection, the patient was placed in an extended lithotomy position and a vaginal approach taken to performing a radical vaginal trachelectomy (Fig. 1). This is as for the distal part or first part of a Schauta's radical vaginal hysterectomy (Fig. 2). After infiltrating the cervix and paravaginal tissues with 0.25% marcaine and 1 in 200,000 adrenalin, a circumferential incision is made using cutting diathermy around the cervix to include a 2-cm cuff of vagina (Fig. 3). The bladder may then be separated and pushed proximally identifying the bladder pillars (Fig. 4). The descending branch of the uterine artery (the cervical branch) is identified and divided. The Harmonic scalpel is used for this procedure; alternatively, vessels and pedicles may be clamped, and then under-run using no. 1 vicryl sutures for transfixion. The ureter is palpated proximal and lateral to the bladder pillar. The bladder pillar is then mobilised by sharp dissection thus elevating and moving laterally the ureter by sharp and blunt dissection. The ureteric tunnel is not formally opened, but it is important to identify not only the ureter, but where it is crossed by the uterine arteries. It may thus be pushed and elevated proximately out of the operative field and carefully conserved and preserved throughout the procedure (Fig. 2). The lateral ligaments are identified with paracervical tissue, and a good 1 cm is taken, again being divided using the Harmonic scalpel. Similarly, the uterosacral ligament is divided posterolaterally and the incision deepened. It is not necessary to open the posterior peritoneum in the Pouch of Douglas as this may be reflected and pushed proximally up to and above the isthmus. By not opening the Pouch of Douglas, the operative field is not contaminated by any collected fluid and blood from the previous laparoscopic lymphadenectomy that has pooled in the Pouch of Douglas. In addition, ascending infection and contamination may be avoided.
Figure 1.
Patient in an extended lithotomy position to allow a vaginal approach for a radical vaginal trachelectomy.
Figure 2.
The distal or first part of a Schauta's radical vaginal hysterectomy.
Figure 3.
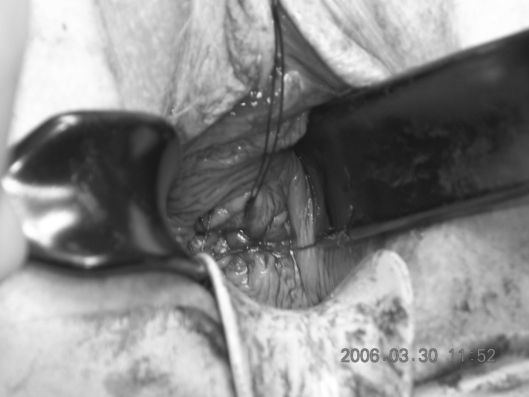
A circumferential incision is made using cutting diathermy around the cervix to include a 2-cm cuff of vagina.
Figure 4.
The bladder is separated and pushed proximally to identify the bladder pillars.
Having mobilised the paracervical and paravaginal tissue in such a way as to include a 1-cm margin all around the cervix and tumour, the body of the cervix may then be divided at the isthmus using cutting diathermy. A Hegar 6 dilator is inserted into the cervical and isthmic canal in order to identify this. The incision is taken down and around the stroma and thus the specimen may be removed. (Figs 5 and 6) The dilator is kept within the isthmus and a cerclage suture of non-absorbable no. 1 proline is inserted with 4 or 5 good bites approximately 0.5 cm proximal to the distal end of the isthmus. This knot is then tied with 7 or 8 half-turns with a knot remaining anteriorly at 12 o'-clock for identification. By tying the knot with the Hegar dilator in position, isthmic stenosis is avoided and menstruation may continue or spontaneous abortion should it occur in a subsequent pregnancy.
Figure 5.
A Hegar 6 dilator is inserted into the cervical and isthmic canal and the excision completed with cutting diathermy.
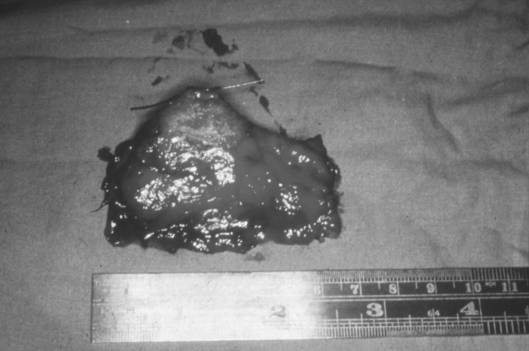
Figure 6.
Radical vaginal trachelectomy specimen.
The vaginal–isthmic anastomosis is then performed using four interrupted mattress sutures of no. 1 vicryl. Two lateral angle sutures are placed into the left and right vaginal fornices to close off the rest of the vaginal vault. The appearance, therefore, is similar to that of the end of a vaginal hysterectomy with two lateral angles (Figs 7 and 8).
Figure 7.
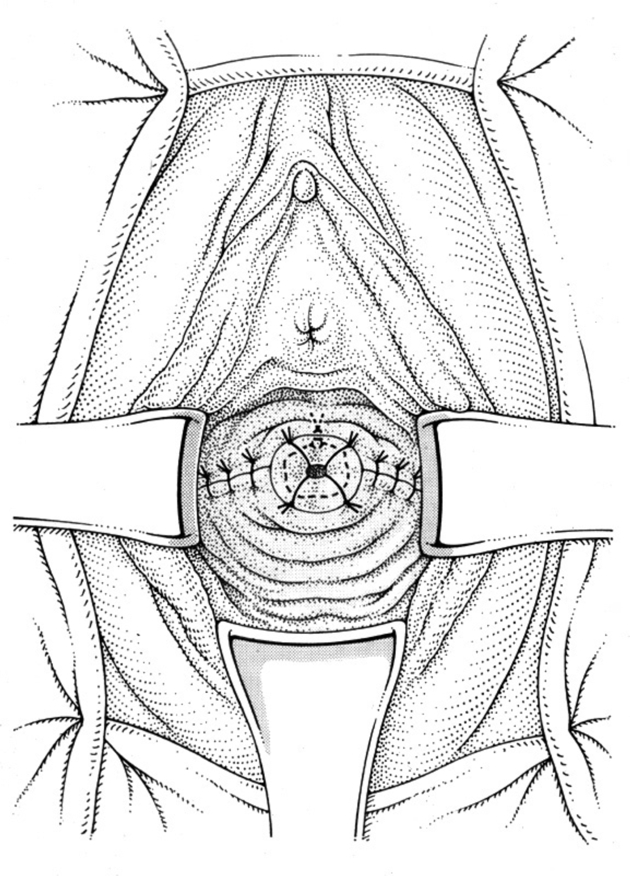
Vagino-isthmic anastomosis.
Figure 8.
Appearance following surgery.
A no. 12 Foley's catheter is inserted into the isthmus and the balloon inflated for retention with 3 ml of water. This catheter is left in situ for 3 days in order to prevent synechiae forming, and also isthmic stenosis. Similarly, a bladder catheter is left within the bladder for continuous drainage for 5 days. Pelvic floor exercises are commenced on the 4th day, and then may be continued in order to ensure adequate bladder voiding.
A vaginal pack is inserted for tamponade postoperatively, and this is removed after 24 h.
Having completed the vaginal portion of the procedure, the abdomen is further inspected laparoscopically. Haemostasis is confirmed; the ureters are inspected for satisfactory peristalsis and the uterus also as well as the Pouch of Douglas to ensure that there has been no untoward trauma. The pelvic side walls are inspected and any swabs (tonsil swabs) that had been inserted to encourage haemostasis are removed. Surgical gauze is placed into both pelvic side walls. The abdomen is then deflated and the four portal incisions are closed after removing the laparoscopy instruments under direct vision. The rectus-sheath of the two larger portals is closed with interrupted no. 1 vicryl, and 3/0 monocryl is used to close the skin.
The procedure is covered with prophylactic antibiotics and postoperative prophylactic anticoagulation is commenced with low molecular weight heparin, after checking the clotting studies the following morning.
Results
At the time of this Hunterian presentation, 142 women had undergone the procedure with a mean age of 30.6 years. The great majority (98%) had Stage 1B1 lesions with a smaller number of Stage 1A2 patients (Table 1). Most (63%) were squamous carcinomas with 33% glandular tumours (Table 2).
Table 1.
Demographic details of 142 women who elected for radical trachelectomy at St Bartholomew's and The Royal Marsden Hospitals, 1994–2006
Table 2.
Histopathological details of 142 women who elected for radical trachelectomy at St Bartholomew's and The Royal Marsden Hospitals, 1994–2006
| Histological type | n (%) |
| Squamous | 90 (63.4%) |
| Adenocarcinoma | 44 (31%) |
| Adenosquamous | 4 (2.8%) |
| Other | 4 (2.8%) |
Nineteen patients were advised to have completion treatment in view of adverse prognostic factors, which would include either close margins or positive nodes.
A number of complications have occurred mostly in the first half of this study as the technique has been developed (Table 3). There can be, however, little doubt that the surgical morbidity associated with trachelectomy is considerably less than that from radical hysterectomy as shown in another study.19 Isthmic stenosis may require careful dilatation should a haematometra occur.20
Table 3.
Complications amongst 142 women who elected for radical trachelectomy at St Bartholomew's and The Royal Marsden Hospitals, 1994–2006
| Peri-operative | |
| Ureteric damage | 3 |
| Uterine perforation | 2 |
| Laparotomy pelvic side wall bleeding | 2 |
| Failure of pneumoperitoneum | 2 |
| External iliac artery rupture | 1 |
| Bladder perforation | 1 |
| Vaginal fornix laceration | 1 |
| Other complications | |
| Isthmic stenosis | 7 |
| Temporary thigh numbness | 5 |
| Retention of urine | 5 |
| Stitch expulsion | 4 |
| Amenorrhoea | 3 |
| Unexplained limp | 2 |
| Dyspareunia | 2 |
| Mild leg oedema | 1 |
| Winged scapula | 1 |
| Psoas abscess | 1 |
| Secondary haemorrhage | 1 |
| Infected epidural site | 1 |
| Lymphocyst | 1 |
Of the 142 women undergoing trachelectomy, 24 have had 33 live births, and there has been one stillbirth (Table 4). The cumulative pregnancy rate following this procedure has been shown to be good.21 In this series there have been four recurrences, all occurring in women with squamous cell carcinomas, and presenting at 7, 26, 51 and 90 months following diagnosis and treatment. All women have died. The two later recurrences manifested with hydroureters due to large para-aortic node recurrence or pelvic node disease. The other two earlier recurrences manifested with paracervical recurrence. All four were treated with chemo/radiotherapy. All four patients had negative nodes and clear margins with two having no residual disease in the operative specimen.
Table 4.
Pregnancies amongst 142 women who elected for radical trachelectomy at St Bartholomew's and The Royal Marsden Hospitals, 1994–2006
| Live births | 33 in 24 women |
| Stillbirths | 1 in 1 woman |
| Neonatal deaths | 0 in 0 women |
| Miscarriages < 14/40 weeks | 16 in 11 women |
| Miscarriages ≥ 14/40 weeks | 11 in 8 women |
| Terminations | 2 in 2 women |
| Ectopic pregnancy | 1 woman |
| On-going pregnancy | 4 women |
| Surrogate live birth | 2 women |
| Total pregnancies | 70 |
Follow-up assessment is continued 3-monthly for the first year and 4-monthly for the second. Thereafter, patients are seen 6-monthly until 5 years and then yearly until 10 years. At each visit, a clinical examination is performed and vaginal and isthmic cytology. At 6, 12 and 24 months, colposcopy is performed with cytology and an MRI scans.
Patients are advised to continue with contraception for 6 months. Providing clinical examination as well as cytology and colposcopy are normal, and the MRI scan has shown no evidence of recurrent disease, those that wish to conceive may proceed to do so.
In the study to-date, of the 33 live births, eight (25%) have been born with severe prematurity before 32 weeks. Sixteen have been delivered electively between 36–38 weeks. Prematurity, therefore, is a risk due to ascending chorio-amnionitis. All deliveries have been by a classical or low vertical caesarean section at the onset of labour or electively at 36–38 weeks. The solitary stillbirth occurred in a patient who ruptured her membranes at 33 weeks, but was not delivered as fetal maturity was awaited. Regrettably, infection occurred resulting in fetal demise.
Consideration, therefore, should be given to either prophylactic antibiotics in the second trimester or at least taking regular vaginal swabs in a sterile fashion in order to detect infection early.
Conclusions
Trachelectomy appears to be a safe treatment for carefully selected women. Conception rates are high, although premature delivery due to ascending chorio-amnionitis can lead to spontaneous rupture of the membranes. Delivery is by elective caesarean section with a low vertical or classical incision. Antibiotics should be considered in the second trimester.
The isthmic cerclage appears to be efficient, and recurrence rates are acceptable with no significant difference between histological types or grades.
It is anticipated that, in the future when more accurate and discriminating forms of molecular imaging are available, it may be possible to avoid lymphadenectomy in patients who have truly negative nodes and select patients for non-surgical treatment in those who do have microscopic and metastatic disease. Molecular quantification may allow mapping of micrometastases.22 With a better understanding of co-expression patterns of genes involved in angiogenesis,23 true Stage 1 tumours may be identified with confidence in the future. Accurate localisation of the tumour may enable individual patients requesting fertility sparing surgery to undergo a limited, but adequate, wide local excision with conservation of some proximal cervical tissue in those women with squamous cell lesions confined to the distal or ectocervix. Glandular adenocarcinomas will always require excision of the complete length of the cervix to include the entire endocervical canal up to the isthmus.
Acknowledgments
Acknowledgement
This article is based on a Hunterian Lecture given at The Royal College of Surgeons of England on 27 November 2006.
References
- 1.Sims JM. The treatment of epithelioma of the cervix uteri. Am J Obstet. 1879;12:451–89. [Google Scholar]
- 2.Clark JG. More radical method of performing hysterectomy for cancer of the uterus. John Hopkins Hosp Bull. 1895;6:120. [Google Scholar]
- 3.Wertheim E. Zur Frage der Radi Raloperation beam Uterus Krebs. Arch Gynakol. 1900;61:627. [Google Scholar]
- 4.Schauta F. Die Operation des Gebär mutter Krebes Mittels des Schuchardt Schen Paravaginatschmittes. Monatsschrift Geburtsch Gynakol. 1902;15:133. [Google Scholar]
- 5.Mitra S. In: Surgical Treatment of Carcinoma of the Cervix. Meigs JV, editor. New York: Grune and Stratton; 1954. [Google Scholar]
- 6.Bonney V. Wertheim's operation in retrospect. Lancet. 1949;1:637. doi: 10.1016/s0140-6736(49)91740-7. [DOI] [PubMed] [Google Scholar]
- 7.Hinselmann H. Verbesserung der Inspektions-moglichkeiteur von Vulva Vagina und Portio. Munch Med Wchnschr. 1925:1733. [Google Scholar]
- 8.Papanicolaou GN. Proceedings of the 3rd Race Betterment Conference. Battle Creek: Race Betterment Foundation; 1928. New cancer diagnosis; p. 528. [Google Scholar]
- 9.Papanicolaou GN, Traut HF. The diagnostic value of vaginal smears in carcinoma of the uterus. Am J Obstet Gynecol. 1941;42:193. [PubMed] [Google Scholar]
- 10.Zur Hausen H. Intracellular surveillance of persisting viral infections. Lancet. 1986;ii:489. doi: 10.1016/s0140-6736(86)90360-0. [DOI] [PubMed] [Google Scholar]
- 11.Aburel E. Chirurgia Ginecologica technica, si tactica operatiorie (1956), vol II sub redaetia Panait Sirbu. 2nd edn. Editura Medicola Bucuresti; 1976. [Google Scholar]
- 12.Burghardt E, Holzer E. Diagnosis and treatment of micro-invasive carcinoma of the cervix uteri. Obstet Gynecol. 1977;49:641–53. [PubMed] [Google Scholar]
- 13.Hagan H, Jacobs IJ, Shepherd JH. Are we sure of the rationale for parametrial resection in the surgical management of cervical cancer? Int J Gynaecol Cancer. 2001;10:1–6. [Google Scholar]
- 14.Dargent D, Brun JL, Roy M, Mathevet P. La trachelectomie élargie: uné alternative á l'hystérectomie. Radicale dansu traitment des cancers in filtrants. Jobgyn. 1994;2:285–92. [Google Scholar]
- 15.Shepherd JH, Crawford RAF, Oram DH. Radical trachelectomy: a way to preserve fertility in the treatment of early cervical cancer. Br J Obstet Gynaecol. 1998;105:912–6. doi: 10.1111/j.1471-0528.1998.tb10238.x. [DOI] [PubMed] [Google Scholar]
- 16.Peppercorn DP, Jeyarajah AR, Woolas R, Shepherd JH, Oram DH, et al. The role of MRI in the selection of patients with early cervical carcinoma for fertility-sparing surgery: initial experience. Radiology. 1999;212:395–9. doi: 10.1148/radiology.212.2.r99au01395. [DOI] [PubMed] [Google Scholar]
- 17.Sahdev A, Wenaden AET, Sohaib SA, Shepherd JH, Reznek RH. Magnetic resonance imaging in gynaecological malignancies. Cancer Imaging. 2005;5:539. [Google Scholar]
- 18.Rockall AG, Sohaib SA, Harisinghani NG, Babar SA, Singh N, et al. Diagnostic performance nano particle-enhanced MRI in the diagnosis of lymph node metastasis in patients with endometrial and cervical cancer. J Clin Oncol. 2005;23:2813–21. doi: 10.1200/JCO.2005.07.166. [DOI] [PubMed] [Google Scholar]
- 19.Alexander-Sefre F, Chee N, Spencer C, Menon U, Shepherd JH. Surgical morbidity associated with radical trachelectomy and radical hysterectomy. Gynecol Oncol. 2006;101:450–4. doi: 10.1016/j.ygyno.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Selo-Ojeme DO, Ind TEJ, Shepherd JH. Isthmic stenosis following radical trachelectomy. J Obstet Gynaecol. 2002;22:327–8. doi: 10.1080/01443610252971302. [DOI] [PubMed] [Google Scholar]
- 21.Shepherd JH, Spencer C, Herod J, Ind TEJ. Radical vaginal trachelectomy as a fertility sparing procedure in women with early stage cervical cancer: Cumulative pregnancy rate in a series of 123 women. Br J Obstet Gynaecol. 2006;113:719–24. doi: 10.1111/j.1471-0528.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- 22.Van Trappen PO, Gyselman VG, Lowe DG, Ryan A, Oram DH, et al. Molecular quantification and mapping of lymph node micro-metastases in cervical cancer. Lancet. 2001;357:15–20. doi: 10.1016/S0140-6736(00)03566-2. [DOI] [PubMed] [Google Scholar]
- 23.Van Trappen PO, Ryan A, Carroll M, Lecoeur C, Goff L, et al. A model for co-expression pattern analysis of genes implicated in angiogenesis and tumour cell invasion in cervical cancer. Br J Cancer. 2002;87:537–44. doi: 10.1038/sj.bjc.6600471. [DOI] [PMC free article] [PubMed] [Google Scholar]