Abstract
The acrosome reaction of spermatozoa is a complex, calcium-dependent, regulated exocytosis. Fusion at multiple sites between the outer acrosomal membrane and the cell membrane causes the release of the acrosomal contents and the loss of the membranes surrounding the acrosome. However, very little is known about the molecules that mediate and regulate this unique fusion process. Here, we show that N-ethylmaleimide-sensitive factor (NSF), a protein essential for most fusion events, is present in the acrosome of several mammalian spermatozoa. Moreover, we demonstrate that calcium-dependent exocytosis of permeabilized sperm requires active NSF. Previously, we have shown that the addition of the active (GTP-bound) form of the small GTPase Rab3A triggers exocytosis in permeabilized spermatozoa. In the present report we show that Rab3A is necessary for calcium-dependent exocytosis. The activation of Rab3A protects NSF from N-ethylmaleimide inhibition and precludes the exchange of the endogenous protein with recombinant dominant negative mutants of NSF. Furthermore, Rab3A activation of acrosomal exocytosis requires active NSF. Our results suggest that, upon calcium stimulation, Rab3A switches to its active GTP-bound form, triggering the formation of a protein complex in which NSF is protected. This process is suggested to be an essential part of the molecular mechanism of membrane fusion leading to the release of the acrosomal contents.
After leaving the testis, the mammalian spermatozoon must undergo a series of modifications to become capable of fertilizing an egg (1). Several morphological and biochemical changes occur during the transit through the epididymis and later in the female tract. Finally, when the spermatozoon adheres to the zona pellucida of an unfertilized egg, it must release the contents of its secretory granule, i.e., the acrosome, to penetrate the zona pellucida and fuse with the egg (2). Acrosomal exocytosis is triggered by the coordinate action of binding proteins and/or activation of receptors on the sperm surface upon binding to glycoproteins from the zona pellucida (3).
Acrosomal exocytosis is a specialized type of regulated secretion leading to a massive fusion between the outer acrosomal membrane and the cell membrane. Because fusion between the two membranes occurs at multiple points, the contents of the acrosome granule are lost and the external acrosomal membrane together with the plasma membrane and the cytoplasm surrounding the acrosome likewise is lost (1). The molecular mechanisms involved in this remarkable membrane fusion process are unknown. The study of the factors involved in the acrosome reaction is difficult because the spermatozoon is a nondividing cell that does not synthesize proteins, thus, hindering the use of several standard biochemical and molecular biology techniques. Most studies thus are limited to a pharmacological approach. We previously have described a model of streptolysin O-permeabilized spermatozoa capable of undergoing acrosomal content release upon calcium stimulation that is suitable for unveiling some of the factors involved in this special, regulated exocytotic event (4, 5).
Regulated exocytosis is a complex process that involves a large set of proteins (6). Some of them are common to different membrane fusion events that occur during intracellular transport, and others are specific to exocytosis. Two main sets of proteins play crucial roles in intracellular trafficking: SNAP receptor (SNARE) and SNARE-interacting proteins and Rab and Rab-interacting proteins. SNAREs are a group of membrane-associated proteins with coiled-coil domains capable of forming extremely stable complexes (7). The disassembly of the SNARE complexes is mediated by N-ethylmaleimide-sensitive factor (NSF), a chaperone-like ATPase, in association with α-SNAP, a protein that regulates the ATPase activity of NSF (8). The actual function of NSF in membrane fusion is still controversial. It is generally accepted that it plays a role in an early priming step, disassembling preformed cis complexes present in the membranes. Unassembled SNAREs then are free to interact in trans with active SNAREs from other membranes (8).
Rabs constitute a family of small GTPases necessary for membrane fusion. Their function probably is related to the recruitment of a complex of Rab-associated proteins capable of tethering vesicles to the site at which fusion is going to occur (9). It has been shown recently that these oligomeric complexes associate with SNAREs (10) and that Rabs may contribute to activate SNAREs (11). Rab3 isoforms (Rab3A, B, C, and D) are associated specifically with exocytosis (12). Rab3A is one of the best-characterized Rabs, whose three-dimensional crystal structure in association with a putative effector has been solved at 2.6-A resolution (13). Curiously, Rab3 isoforms seem to be negative regulators in several exocytotic events (12), although an activating role also has been proposed in other exocytotic models (14–16).
Regulated exocytosis presents an additional layer of complexity because it requires activation of the fusion process generally by a cytoplasmic increase of free calcium. Several proteins involved in transport present calcium-binding domains. In particular, synaptotagmins and calmodulin have been postulated as sensors of free calcium responsible for triggering the final membrane fusion step preceding exocytosis (17, 18). In addition, calcium may play a role in several other steps of the exocytotic process (19).
In a previous report, we have shown that permeabilized spermatozoa undergo the acrosome reaction when GTPases are activated and, more specifically, when activated Rab3A was added to the assay. In the present work, we address the characterization of the molecular mechanism of calcium-dependent acrosomal exocytosis in permeabilized human sperm cells. We report the presence of NSF in the male gamete and demonstrate that active NSF is required for acrosomal exocytosis. Our data also indicate that Rab3A is activated upon calcium stimulation and stabilizes NSF on the membranes as part of the molecular mechanism mediating acrosomal exocytosis.
Materials and Methods
Reagents.
Streptolysin O (SLO) was obtained from Wellcome. Gamete Preparation Media (Serono, Geneva) was used as culture medium. Commercial anti-NSF antibody (rabbit polyclonal, whole antiserum) was from Synaptic Systems (Göttingen, Germany). Recombinant (His6-tagged) NSF wild type and the negative mutants D1EQ and D1KA (20) as well as an anti-NSF antibody (mouse monoclonal, clone 6E6) (21) were generously provided by M. I. Colombo (University of Cuyo, Argentina). The expression plasmid pGEX-2t containing the cDNA of human rab3A (GenBank accession no. NP002857) and bovine GDIα (GenBank accession no. P21856) were generously provided by M. I. Colombo and P. D. Stahl (Washington University, St. Louis). All other reagents were from Sigma.
SLO Permeabilization of Human Spermatozoa.
Human semen samples from healthy donors of proven fertility (motility ≥ 50%, motile spermatozoa ≥ 60 × 106/ml) were handled as described previously (5). After swim-up separation for 1 h, highly motile sperm cells were recovered. The concentration was adjusted to 5–10 × 106/ml, and incubation proceeded for an additional 2 h under capacitating conditions. Permeabilization was performed as described previously (4). In brief, spermatozoa were resuspended in cold PBS containing 0.4 unit/ml SLO for 15 min at 4°C. After incubation, the cells were washed twice with PBS and the pellets were resuspended in ice-cold sucrose buffer (SB; 250 mM sucrose/20 mM Hepes-K/0.5 mM EGTA, pH 7) containing 2 mM DTT. In those experiments in which the sperm cells were treated with N-ethylmaleimide (NEM), the pellets were resuspended in ice-cold SB without DTT and containing the required NEM concentration and then incubated for 15 min at 4°C. Finally, 2 mM DTT was added to the tubes. Permeabilization at this step of the protocol was 100%, as assessed by staining with the supravital dye eosyn-Y (0.5%).
Acrosome Reaction Assay.
The acrosome reaction was evaluated for each condition by the fluorescein isothiocyanate Pisum sativum lectin method according to Mendoza et al. (22). At least 200 cells were scored by using a Zeiss microscope equipped with epifluorescence optics (×630). The acrosome reaction also was evaluated in some instances by transmission electron microscopy. For these experiments, the samples were fixed in 2% glutaraldehyde (vol/vol in SB) at the end of the assay, embedded in plastic, sectioned, stained by standard procedures, and analyzed in a Siemens Elmiskop I electron microscope (Siemens, Iselin, NJ). Negative (no stimulation) and positive (calcium stimulation) controls were included in all experiments. For each experiment, the data were normalized by subtracting the number of reacted spermatozoa in the negative control from all values and expressing the resulting values as a percentage of the acrosome reaction observed in the positive control.
Recombinant GDIα and Rab3A.
The proteins were expressed in Escherichia coli and purified by following standard procedures (5). Rab3A was prenylated in vitro as described (5). Just before use, aliquots of the prenylated protein were loaded with the required nucleotide [GDP or guanosine 5′-[γ-thio]triphosphate (GTP[γS])] as described (5).
SDS/PAGE, Western Blots, and Immunofluorescence.
Proteins were extracted in sample buffer from human (23), mouse (24), and rat (25) spermatozoa (whole cells). Human sperm membranes were obtained according to Bohring and Krause (26). SDS/PAGE, Western blot analysis, and immunofluorescence were performed as described (5).
Statistical Analysis.
Differences between experimental and control conditions were tested by a two-way ANOVA and Fisher's protected least significant difference tests. Percentages (not normalized) were transformed to the arc-sine before analysis. Only significant differences (P < 0.05) among experimental groups are discussed in the text.
Results
NSF Is Present in the Acrosomal Region in Human Sperm.
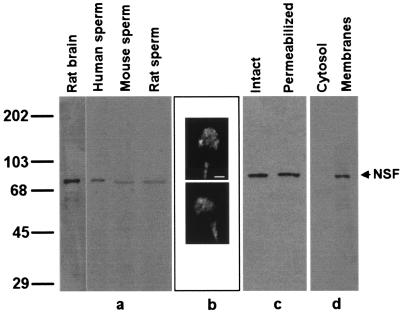
Very little is known about the presence of proteins required for regulated exocytosis in sperm cells. Therefore, the presence of NSF, a key protein for most exocytotic events, was assessed in the spermatozoa of several mammalian species. A mAb raised against recombinant NSF was used to identify NSF in spermatozoa (Fig. 1a). Immunoblot analysis of whole-cell extracts from human, mouse, rat (Fig. 1a), and ram (not shown) sperm demonstrate the presence of a single protein band comigrating with rat brain NSF, suggesting that related, if not identical, forms are present in these cells. Identical results were obtained when human sperm samples were probed with a polyclonal antibody raised against a synthetic peptide corresponding to residues 733–744 in human NSF (data not shown).
Figure 1.
NSF is present in membranes of human spermatozoa and localizes to the acrosomal region. (a) Proteins from rat brain and human, mouse, and rat sperm were extracted in Laemmli sample buffer and analyzed by Western blot using an anti-NSF mAb as probe. Lanes: rat brain, 1 μg of a postnuclear membrane pellet from rat brain; human sperm, proteins from 5 × 106 cells; mouse sperm, proteins from 10 × 106 cells; rat sperm, proteins from 4 × 106 cells. Molecular mass standards (kDa × 10−3) are indicated to the left. (b) Human sperm were fixed and permeabilized and immunostained with a rabbit polyclonal anti-NSF antibody followed with a TRITC-labeled goat anti-rabbit antibody. Shown are epifluorescence micrographs of two typically stained cells. (Bar = 2 μm.) (c) Proteins extracted in 1% Triton X-100 from intact (intact lane) or SLO-permeabilized (permeabilized lane) were denatured in Laemmli sample buffer and analyzed by Western blot using an anti-NSF mAb as probe (7 × 106 cells per lane). (d) Percoll-washed human sperm were separated into soluble (cytosol lane) and particulate (membranes lane) fractions and probed on blots with an anti-NSF mAb. Ten micrograms of proteins derived from 1.7 × 108 cells was loaded per lane.
Indirect immunostaining was used to localize NSF on fixed, permeabilized sperm. The antibody reacted specifically with the sperm head in the acrosomal region (Fig. 1b). Seventy to 80% of the cells displayed acrosomal staining. Most cells also displayed fluorescence in the midpiece. However, the label in the midpiece also was observed when the NSF antibody was substituted by a preimmune serum.
Similar amounts of NSF were present in Triton X-100 extracts made from SLO-permeabilized sperm when compared with untreated cells, suggesting that NSF did not leak from the cells. This finding might be because of two reasons: cytosolic NSF is so large (>430 kDa in its hexameric form) that it does not leak through the SLO pores or NSF is not cytosolic but membrane-bound. To determine the cellular localization of NSF in human sperm, membrane and cytosolic fractions were prepared and probed with an anti-NSF antibody on Western blots (Fig. 1d). Our data indicate that NSF is present predominantly in a membrane-bound and, therefore, SLO-resistant form in human sperm.
NSF Is Necessary for Calcium-Triggered Acrosomal Exocytosis.
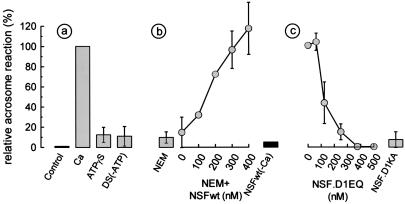
The presence of NSF in spermatozoa encouraged us to assess the function of this ATPase in the acrosome reaction of permeabilized sperm cells. Because ATP is an essential cofactor for NSF function, the ATP requirement of acrosomal exocytosis triggered by calcium was assessed in permeabilized spermatozoa. Exocytosis was observed without the addition of exogenous ATP. However, ATP depletion and the presence of adenosine 5′-[γ-thio]triphosphate (ATP[γS]) completely abrogated exocytosis, indicating that the process requires ATP hydrolysis (Fig. 2a). It is well known that NSF is sensitive to NEM, an alkylating reagent that irreversibly inhibits its ATPase activity. Pretreatment of the spermatozoa with NEM abolished the calcium-induced acrosome reaction. Because other NEM-sensitive factors have been implicated in membrane fusion, it was important to assess whether recombinant NSF was sufficient to restore acrosomal exocytosis in NEM-treated spermatozoa. For these experiments, the NEM concentration was reduced to the minimum that caused full inhibition (0.6 mM). Fig. 2b shows that addition of nanomolar concentrations of the recombinant protein restored exocytosis to the untreated control levels. NSF alone—in the absence of calcium—was not effective, ruling out an unspecific activating effect of the recombinant protein preparation by itself (Fig. 2b). In addition, two NSF mutants, one defective in ATP hydrolysis (NSF.D1EQ) and the other in ATP binding (NSF.D1KA), inhibited calcium-dependent acrosomal exocytosis at concentrations similar to those described in other systems (27–29) (Fig. 2c). NSF.D1EQ associates irreversibly to αSNAP–SNARE complexes (20), displacing endogenous NSF and, therefore, blocking membrane fusion (27–29). Mutants defective in ATP binding—such as NSF.D1KA—interact weakly with αSNAP–SNARE complexes (20), but they are inhibitory for some transport assays (28, 29). They may prevent NSF interaction with effector proteins other than the αSNAP–SNARE complex (29).
Figure 2.
Acrosomal exocytosis in permeabilized human spermatozoa requires ATP hydrolysis and active NSF. Acrosome reactions were evaluated in permeabilized spermatozoa after incubation under the following conditions. (a) Control, no additions; Ca, 0.5 mM CaCl2 in the presence of 0.5 mM EGTA (same stimulus was applied to the rest of the conditions represented with shaded bars or circles); ATP[γS], 0.2 mM ATP[γS]; DS(-ATP), ATP-depleting system (0.1 mg/ml hexokinase/5 mM mannose/5 μM rotenone). (b) NEM, spermatozoa were treated with 1 mM NEM during permeabilization; NEM+NSFwt, pretreatment with 0.6 mM NEM and addition of increasing concentrations of wild-type NSF (0.2 mM ATP was included in this set of experiments to stabilize recombinant NSF); NSFwt(-Ca), pretreatment with 0.6 mM NEM and addition of 285 nM NSFwt in the absence of calcium stimulation. (c) NSF.D1EQ, increasing concentrations of the D1EQ mutant of NSF; NSF.D1KA, 285 nM recombinant D1KA mutant of NSF. The values were normalized as explained in Material and Methods (ranges for negative and positive controls were 17–28% and 31–53%, respectively). The data represent the mean ± SEM of at least three independent experiments.
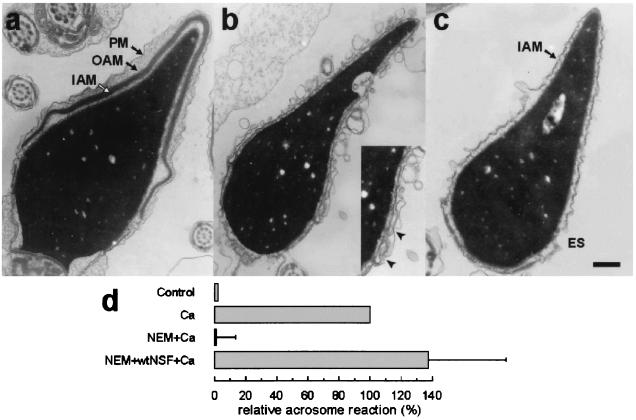
The acrosome reaction is a complex process that causes not only the release of the acrosomal contents but also the vesiculation of the outer acrosomal and plasma membranes to expose the inner acrosomal membrane. To observe the morphological characteristics of the permeabilized spermatozoa undergoing acrosomal exocytosis, the sperm cells were fixed after incubation under different conditions and observed by transmission electron microscopy. Permeabilization and NEM treatment of spermatozoa did not cause any noticeable morphological alterations (Fig. 3a). Addition of wild-type NSF to these cells increased the percentage of cells lacking the acrosome and exposing the inner acrosomal membrane (Fig. 3c). A few spermatozoa were observed in the process of vesiculation, where fusion events between the outer acrosomal and the plasma membrane were observed (Fig. 3b and Inset). The percentage of sperm cells lacking the acrosome under each condition was evaluated by electron microscopy. The percentages obtained were consistent with the data obtained using the lectin-binding method (compare Fig. 3d with Fig. 2b).
Figure 3.
Morphological observation of acrosomal exocytosis in permeabilized spermatozoa. The acrosome reaction was evaluated by transmission electron microscopy in permeabilized spermatozoa after incubation under the following conditions: Control, no additions; Ca, 0.5 mM CaCl2 in the presence of 0.5 mM EGTA; NEM+Ca, spermatozoa were treated with 0.6 mM NEM during permeabilization and stimulated with calcium; NEM+wtNSF+Ca, pretreatment with 0.6 mM NEM and addition of 300 nM wild-type NSF and calcium. At the end of the incubation, the samples were fixed in 2% glutaraldehyde and analyzed by transmission electron microscopy. Examples of unreacted and reacted spermatozoa are shown in a–c. The unreacted sperm (NEM+Ca) in a present intact acrosomal granule and plasma membrane (PM, plasma membrane; OAM, outer acrosomal membrane; IAM, inner acrosomal membrane). In b, a vesiculated spermatozoon (NEM+NSF+Ca), presumptively undergoing acrosomal exocytosis, is shown. Notice the multiple interactions between the outer acrosomal and plasma membrane, particularly evident in the equatorial segment enlarged in the Inset (arrowheads). (c) A completely reacted spermatozoon (NEM+NSF+Ca). Only the IAM is observed above the equatorial segment (ES). (Bar = 0.5 μm.) (d) The percentage of spermatozoa lacking acrosomes was evaluated for each condition and normalized as explained in Material and Methods (ranges for negative and positive controls were 10–13% and 20–29%, respectively). The data represent the means and ranges of two independent experiments.
Therefore, functional assays performed by using two independent methods indicate that the NSF present in human spermatozoa is necessary for calcium-triggered acrosomal exocytosis.
A Role for Rab3A in Calcium-Dependent Acrosomal Exocytosis.
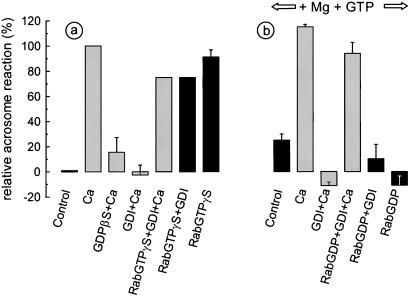
In a previous paper we have shown that recombinant Rab3A loaded with GTP[γS] triggers the acrosome reaction in permeabilized spermatozoa (5). We also have reported that guanosine 5′-[β-thio]diphosphate (GDP[βS]), a general inhibitor of monomeric and heterotrimeric GTPases, abrogated calcium-dependent exocytosis. To assess whether calcium-dependent exocytosis requires small GTPases, sperm cells were pretreated with GDIα. This protein complexes several Rab proteins, releasing them from membranes and locking them in the GDP-bound (inactive) conformation (30). As shown in Fig. 4a, calcium-dependent acrosomal exocytosis was inhibited by GDP[βS] and by GDIα. The effect of GDIα was not due to a deleterious effect of the protein on the sperm cells, because normal exocytosis took place in the presence of GDIα when GTP[γS]-bound (active) Rab3A was added to the system. As reported previously, active Rab3A by itself triggered acrosomal exocytosis (Fig. 4a).
Figure 4.
Calcium-triggered acrosomal exocytosis in permeabilized human spermatozoa requires Rab3A. Acrosomal exocytosis was evaluated in permeabilized spermatozoa after incubation under the following conditions. (a) Control, no additions; Ca, 0.5 mM CaCl2 in the presence of 0.5 mM EGTA (same stimulus was applied to the rest of the conditions represented with shaded bars); GDP[βS]+Ca, 0.2 mM GDP[βS] plus calcium; GDI+Ca, 4 μM GDIα plus calcium; RabGTPγS+GDI+Ca, 300 nM GST-Rab3A loaded with GTP[γS] plus 4 μM GDIα plus calcium; RabGTPγS+GDI, 4 μM GDIα plus 300 nM GST-Rab3A loaded with GTP[γS]; RabGTPγS, 300 nM GST-Rab3A loaded with GTP[γS]. (b) For the following conditions, 0.7 mM MgCl2 and 0.2 mM GTP were included in the assay. Control, no further additions; Ca, 0.5 mM CaCl2 in the presence of 0.5 mM EGTA; GDI+Ca, 4 μM GDIα plus calcium; RabGDP+GDI+Ca, 300 nM GST-Rab3A loaded with GDP plus 4 μM GDIα plus calcium; RabGDP+GDI, 300 nM GST-Rab3A loaded with GDP plus 4 μM GDIα; RabGDP, 300 nM GST-Rab3A loaded with GDP. The values were normalized as explained in Material and Methods (ranges for negative and positive controls were 14–23% and 33–48%, respectively). The data represent the mean ± SEM of at least three independent experiments.
The effect of GDIα on the acrosome reaction indicates that a small GTPase of the Rab family is necessary for the calcium-triggered acrosomal exocytosis. Because GDIα can bind several Rabs, it was not possible to conclude which specific Rab protein was required. Rab3A is a likely candidate because it overcomes GDIα inhibition (Fig. 4a). However, active Rab3A is able to trigger acrosomal exocytosis in the absence of calcium. Therefore, it could trigger exocytosis by a different mechanism. To directly prove that Rab3A was required for calcium-dependent exocytosis, we searched for conditions under which Rab3A would reverse GDIα inhibition but only upon calcium stimulation. We found that these requirements could be fulfilled by loading Rab3A with GDP, increasing the concentration of magnesium to avoid spontaneous exchange with GTP, and adding free GTP to the assay. Under these conditions, Rab3A did not trigger the acrosome reaction in the presence or absence of GDIα, suggesting that Rab3A remained in its inactive, GDP-bound form at low calcium concentrations (Fig. 4b). However, Rab3A reversed GDIα inhibition when calcium was added to the system (Fig. 4b). Taken together, these data support a stimulatory role for Rab3A in the calcium-dependent acrosome reaction. They also suggest that calcium promotes Rab3A activation, presumably by boosting the exchange of GTP for GDP and the dissociation from GDI.
Rab3A Requires a Membrane-Associated Pool of NSF to Trigger Exocytosis.
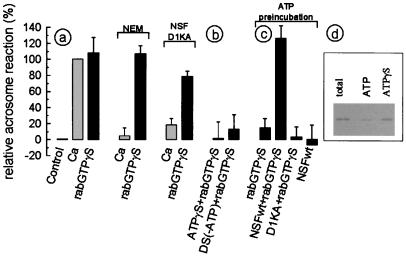
Based on the results of the above experiments, calcium-dependent acrosomal exocytosis requires active NSF and Rab3A, two key proteins involved in most fusion processes. However, the functional relationship between these two proteins is not yet clear. It then was of interest to assess whether exocytosis triggered by active Rab3A required NSF. As shown in Fig. 5a, fusion triggered by Rab3A was resistant to NEM and the negative mutant of NSF whereas these treatments inhibit calcium-dependent exocytosis in the same samples. These observations suggested that NSF is not required downstream of Rab3A activation. However, ATP[γS] and depletion of ATP inhibited Rab3A-promoted exocytosis, suggesting that the activity of an ATPase still was required after Rab3A activation (Fig. 5b).
Figure 5.
Rab3A-triggered acrosomal exocytosis in permeabilized human spermatozoa requires NSF. Acrosomal exocytosis was evaluated in permeabilized spermatozoa after incubation under the following conditions. (a) Control, no additions; Ca, 0.5 mM CaCl2 in the presence of 0.5 mM EGTA; RabGTPγS, 300 nM GST-Rab3A loaded with GTP[γS]. The same stimuli were applied to spermatozoa pretreated with 1 mM NEM or in the presence of 285 nM of the NSF mutant D1KA. (b) ATPγS+RabGTPγS, 0.2 mM ATP[γS] plus 300 nM GST-Rab3A loaded with GTP[γS]; DS(-ATP)+RabGTPγS, ATP-depleting system (0.1 mg/ml hexoquinase/5 mM mannose/5 μM rotenone) plus 300 nM GST-Rab3A loaded with GTP[γS]. (c) For the following conditions, spermatozoa were preincubated for 15 min at 37°C with 0.2 mM ATP to promote the dissociation of NSF from the membranes. Afterward, the spermatozoa were incubated with: RabGTPγS, 300 nM GST-Rab3A loaded with GTP[γS]; NSFwt+RabGTPγS, 285 nM wild-type NSF plus 300 nM GST-Rab3A loaded with GTP[γS]; D1KA+RabGTPγS, 285 nM NSF.D1KA plus 300 nM GST-Rab3A loaded with GTP[γS]; NSFwt, 285 nM wild-type NSF. The values were normalized as explained in Material and Methods (ranges for negative and positive controls were 14–28% and 35–53%, respectively). The data represent the mean ± SEM of at least three independent experiments. (d) Western blot probed with anti-NSF of membranes before (total) and after 15 min of incubation at 37°C with 0.2 mM ATP (ATP) or 0.2 mM ATP[γS] (ATPγS).
As shown in Fig. 1d, NSF is associated mostly with membranes in the spermatozoon. Hence, it is conceivable that in this environment NSF could be protected from NEM and unavailable to exchange with the negative mutant of NSF during the exocytosis promoted by Rab3A. To assess whether Rab3A-triggered acrosomal exocytosis requires membrane-associated NSF, the exchange of the membrane-associated pool was stimulated by incubation with ATP. This treatment released a substantial amount of NSF from the membranes (Fig. 5d) and inhibited Rab3A-promoted exocytosis (Fig. 5c). This effect was a result of NSF release because it could be rescued by the addition of recombinant wild-type NSF but not by the addition of an inactive NSF mutant. Wild-type NSF alone, in the absence of Rab3A, did not trigger acrosomal exocytosis, ruling out an unspecific effect of the protein. These observations indicate that NSF is protected from NEM inhibition and prevented from exchanging with the cytosolic protein in the presence of active Rab3A. The results also indicate that active NSF is required for the effect of Rab3A on acrosomal exocytosis.
Discussion
The acrosome reaction is considered a form of regulated exocytosis, although it has several special features that make the process unique. The sperm cells possess a single acrosome granule that forms a cap surrounding the nucleus. This special shape differentiates an outer acrosomal membrane in close proximity to the plasma membrane and an inner acrosomal membrane in close proximity to the nucleus. During the acrosome reaction, the outer acrosomal membrane fuses at multiple points with the plasma membrane. The formation of a large number of fusion pores destabilizes the structure of the acrosome, causing the release of the contents from the acrosomal matrix and the loss of the plasma and outer acrosomal membranes together with the cytoplasm surrounding the acrosome (1). Therefore, the process is irreversible and occurs just once during the lifetime of the spermatozoon.
The molecular mechanisms involved in acrosomal exocytosis are still mostly unknown. However, it is reasonable to assume that it will share key factors involved in other exocytotic events. We and several other laboratories have reported that the acrosome reaction can be triggered by an increase of cytoplasmic calcium and by activation of GTPases (3). Calcium and GTP[γS], separately or in combination, also trigger regulated exocytosis in several other cell types (e.g., chromaffin and mast cells) (31).
Exocytosis is, in essence, a membrane-fusion event. A large set of experimental data coming from genetic and reconstitution studies in the secretory and endocytic pathways has established the basis for a consensus mechanism for membrane fusion. The central molecules of this model are SNAREs and SNARE-interacting proteins (8) as well as Rab and Rab-interacting proteins (9). Although it is assumed that these molecules are involved in all intracellular membrane fusion events from yeast to mammalian cells, information about their role is scarce in many specific transport events. In sperm, the only components of the fusion machinery identified so far are Rab3A in rat (32), mouse (33), and human (5) and homologues of the SNARE proteins VAMP, syntaxins, and SNAP-25 (34) in sea urchin.
In this report we present evidence that NSF is present in several mammalian spermatozoa and that the calcium-triggered acrosome reaction requires NSF. The protein is mostly membrane-associated even after permeabilization of sperm cells. Although NSF is a soluble protein, it associates with membranes through interactions with SNAP and SNARE proteins and with other poorly characterized binding sites (29). The negative mutants of NSF compete with endogenous NSF to inhibit calcium-dependent exocytosis, indicating that some exchange between membrane-bound and soluble NSF occurs upon calcium stimulation. Acrosomal exocytosis is also sensitive to NEM, and NEM inhibition is completely reversed by wild-type NSF, suggesting that no other proteins essential for exocytosis are inhibited by NEM. The electron microscopic analysis of the samples indicates that calcium triggers a complex exocytotic process in permeabilized spermatozoa very similar to the acrosome reaction in intact sperm cells. The quantification of the electron microscopic observations corroborates that active NSF is necessary for acrosomal exocytosis.
Although it is well established that activation of GTPases promotes exocytosis, the actual proteins responsible for this effect is still not known. The more conspicuous GTPases found in secretory granules with a function in exocytosis are Rab3 isoforms, but most studies report an inhibitory role for these proteins (12). However, a stimulatory role has been suggested in some secretory events (14–16). In this report we show that Rab3A is essential for the calcium-dependent acrosome reaction. A stimulatory role for the Rab involved in exocytosis is more consistent with the experimental data from most other intracellular transport events in the secretory and endocytic pathways. Activation of Rabs recruits a series of proteins that are necessary for the tethering of the membranes involved in fusion (9). Our results strongly indicate that upon Rab3A activation, membrane-associated NSF is protected from NEM inhibition and prevented from exchanging with the soluble protein. The results also indicate that active NSF is required for the effect of Rab3A on acrosomal exocytosis. These findings are consistent with the report showing that NSF and a SNARE (syntaxin 13) are part of a transient complex formed with the Rab5-interacting protein EEA1 (10). In the same system, membrane fusion promoted by EEA1 requires NSF and αSNAP-dependent priming of SNAREs (35). Interestingly, high concentrations of NEM (3 mM) cause only a 40% inhibition in this model, suggesting that NSF is protected upon addition of excess EEA1. NEM-resistant fusion events have been described in other in vitro reconstitution assays (36–38). Our data suggest that activation of Rab promotes not only association of tethering proteins but also of NSF and probably other factors involved in SNARE activation. NSF would be protected in this macromolecular complex and would be ready to activate SNARE to promote the formation of trans complexes between the outer acrosomal and plasma membranes.
At present, it is difficult to reconcile the opposite effects of Rab3A in different secretion events (6). In neuronal and chromaffin cells, two pools of secretory vesicles can be distinguished: (i) a readily releasable pool already attached to the plasma membrane and (ii) a pool of vesicles that need to be recruited to the plasma membrane before exocytosis (6, 39). Rabs probably are required for the recruitment of new vesicles to the plasma membrane. However, in mice lacking Rab3A, the rate at which the readily releasable pool is replenished with new vesicles after exocytosis is not altered (40). Other still unidentified Rabs might be involved in this process. Alternatively, a Rab-independent mechanism of tethering may exist for these vesicles. Vesicles that are already docked to the plasma membrane probably have passed the tethering step; therefore, activation of Rabs would not be necessary for their exocytosis. For these vesicles, Rab3A may have a regulatory role as part of the final membrane-fusion control of exocytosis. Calmodulin, a late-acting factor in membrane fusion (18), interacts with Rab3A, and this interaction is important for Rab3A regulation of exocytosis (41). The activating role of Rab3A in acrosomal exocytosis indicates that, despite the close proximity between the external acrosomal membrane and the plasma membrane, the acrosome cannot be considered a readily releasable secretory granule.
More studies will be necessary to assess the function of other factors required for the acrosome reaction. The unveiling of the molecular mechanism involved in the acrosomal exocytosis will be of great importance for understanding the timely regulation of the process required for a successful sperm–egg interaction. These studies also may enlighten the process of regulated exocytosis in other cell types, particularly in nonneuronal cells.
Acknowledgments
We thank A. Challa for excellent technical assistance, G. S. Kopf, M. I. Colombo, and S. Patterson for critical reading of the manuscript, P. D. Stahl and M. I. Colombo for reagents, and S. Belmonte for rat sperm extracts. This work was supported partly by an International Research Scholar Award from the Howard Hughes Medical Institute and by grants from Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina and Universidad Nacional de Cuyo.
Abbreviations
- NSF
N-ethylmaleimide-sensitive factor
- SNARE
SNAP receptor
- ATP[γS]
adenosine 5′-[γ-thio]triphosphate
- GTP[γS]
guanosine 5′-[γ-thio]triphosphate
- NEM
N-ethylmaleimide
- SLO
streptolysin O
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.180206197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.180206197
References
- 1.Yanagimachi R. In: The Physiology of Reproduction. Knobil E, Neill J D, editors. New York: Raven; 1994. pp. 189–281. [Google Scholar]
- 2.Fraser L R. Hum Reprod. 1998;13, Suppl 1:9–19. doi: 10.1093/humrep/13.suppl_1.9. [DOI] [PubMed] [Google Scholar]
- 3.Snell W J, White J M. Cell. 1996;85:629–637. doi: 10.1016/s0092-8674(00)81230-1. [DOI] [PubMed] [Google Scholar]
- 4.Diaz A, Dominguez I, Fornes M W, Burgos M H, Mayorga L S. Andrologia. 1996;28:21–26. doi: 10.1111/j.1439-0272.1996.tb02753.x. [DOI] [PubMed] [Google Scholar]
- 5.Yunes R, Michaut M, Tomes C, Mayorga L S. Biol Reprod. 2000;62:1084–1089. doi: 10.1095/biolreprod62.4.1084. [DOI] [PubMed] [Google Scholar]
- 6.Jahn R, Sudhof T C. Annu Rev Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- 7.Sutton R B, Fasshauer D, Jahn R, Brunger A T. Nature (London) 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 8.Gerst J E. Cell Mol Life Sci. 1999;55:707–734. doi: 10.1007/s000180050328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfeffer S R. Nat Cell Biol. 1999;1:E17–E22. doi: 10.1038/8967. [DOI] [PubMed] [Google Scholar]
- 10.McBride H M, Rybin V, Murphy C, Giner A, Teasdale R, Zerial M. Cell. 1999;98:377–386. doi: 10.1016/s0092-8674(00)81966-2. [DOI] [PubMed] [Google Scholar]
- 11.Lupashin V V, Waters M G. Science. 1997;276:1255–1258. doi: 10.1126/science.276.5316.1255. [DOI] [PubMed] [Google Scholar]
- 12.Geppert M, Sudhof T C. Annu Rev Neurosci. 1998;21:75–95. doi: 10.1146/annurev.neuro.21.1.75. [DOI] [PubMed] [Google Scholar]
- 13.Ostermeier C, Brunger A T. Cell. 1999;96:363–374. doi: 10.1016/s0092-8674(00)80549-8. [DOI] [PubMed] [Google Scholar]
- 14.Conner S, Wessel G M. Dev Biol. 1998;203:334–344. doi: 10.1006/dbio.1998.9057. [DOI] [PubMed] [Google Scholar]
- 15.Tasaka K, Masumoto N, Mizuki J, Ikebuchi Y, Ohmichi M, Kurachi H, Miyake A, Murata Y. J Endocrinol. 1998;157:267–274. doi: 10.1677/joe.0.1570267. [DOI] [PubMed] [Google Scholar]
- 16.Ohnishi H, Samuelson L C, Yule D I, Ernst S A, Williams J A. J Clin Invest. 1997;100:3044–3052. doi: 10.1172/JCI119859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis A F, Bai J, Fasshauer D, Wolowick M J, Lewis J L, Chapman E R. Neuron. 1999;24:363–376. doi: 10.1016/s0896-6273(00)80850-8. [DOI] [PubMed] [Google Scholar]
- 18.Peters C, Mayer A. Nature (London) 1998;396:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- 19.Burgoyne R D, Morgan A. Cell Calcium. 1998;24:367–376. doi: 10.1016/s0143-4160(98)90060-4. [DOI] [PubMed] [Google Scholar]
- 20.Nagiec E E, Bernstein A, Whiteheart S W. J Biol Chem. 1995;270:29182–29188. doi: 10.1074/jbc.270.49.29182. [DOI] [PubMed] [Google Scholar]
- 21.Tagaya M, Wilson D W, Brunner M, Arango N, Rothman J E. J Biol Chem. 1993;268:2662–2666. [PubMed] [Google Scholar]
- 22.Mendoza C, Carreras A, Moos J, Tesarik J. J Reprod Fertil. 1992;95:755–763. doi: 10.1530/jrf.0.0950755. [DOI] [PubMed] [Google Scholar]
- 23.Tomes C N, Carballada R, Moses D F, Katz D F, Saling P M. Mol Hum Reprod. 1998;4:17–25. doi: 10.1093/molehr/4.1.17. [DOI] [PubMed] [Google Scholar]
- 24.Tomes C N, McMaster C R, Saling P M. Mol Reprod Dev. 1996;43:196–204. doi: 10.1002/(SICI)1098-2795(199602)43:2<196::AID-MRD9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 25.Belmonte S A, Challa A, Gutierrez L S, Bertini F, Sosa M A. Int J Androl. 1998;21:277–282. doi: 10.1046/j.1365-2605.1998.00124.x. [DOI] [PubMed] [Google Scholar]
- 26.Bohring C, Krause W. Electrophoresis. 1999;20:971–976. doi: 10.1002/(SICI)1522-2683(19990101)20:4/5<971::AID-ELPS971>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 27.Whiteheart S W, Rossnagel K, Buhrow S A, Brunner M, Jaenicke R, Rothman J E. J Cell Biol. 1994;126:945–954. doi: 10.1083/jcb.126.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sumida M, Hong R M, Tagaya M. J Biol Chem. 1994;269:20636–20641. [PubMed] [Google Scholar]
- 29.Colombo M I, Taddese M, Whiteheart S W, Stahl P D. J Biol Chem. 1996;271:18810–18816. doi: 10.1074/jbc.271.31.18810. [DOI] [PubMed] [Google Scholar]
- 30.Ullrich O, Stenmark H, Alexandrov K, Huber L A, Kaibuchi K, Sasaki T, Takai Y, Zerial M. J Biol Chem. 1993;268:18143–18150. [PubMed] [Google Scholar]
- 31.Avery J, Jahn R, Edwardson J M. Annu Rev Physiol. 1999;61:777–807. doi: 10.1146/annurev.physiol.61.1.777. [DOI] [PubMed] [Google Scholar]
- 32.Iida H, Yoshinaga Y, Tanaka S, Toshimori K, Mori T. Dev Biol. 1999;211:144–155. doi: 10.1006/dbio.1999.9302. [DOI] [PubMed] [Google Scholar]
- 33.Ward C R, Faundes D, Foster J A. Mol Reprod Dev. 1999;53:413–421. doi: 10.1002/(SICI)1098-2795(199908)53:4<413::AID-MRD7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 34.Schulz J R, Sasaki J D, Vacquier V D. J Biol Chem. 1998;273:24355–24359. doi: 10.1074/jbc.273.38.24355. [DOI] [PubMed] [Google Scholar]
- 35.Christoforidis S, McBride H M, Burgoyne R D, Zerial M. Nature (London) 1999;397:621–625. doi: 10.1038/17618. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez L, Stirling C J, Woodman P G. Mol Biol Cell. 1994;5:773–783. doi: 10.1091/mbc.5.7.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alvarez-Dominguez C, Barbieri A M, Beron W, Wandinger-Ness A, Stahl P D. J Biol Chem. 1996;271:13834–13843. doi: 10.1074/jbc.271.23.13834. [DOI] [PubMed] [Google Scholar]
- 38.Mukherjee K, Siddiqi S A, Hashim S, Raje M, Basu S K, Mukhopadhyay A. J Cell Biol. 2000;148:741–754. doi: 10.1083/jcb.148.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu T, Ashery U, Burgoyne R D, Neher E. EMBO J. 1999;18:3293–3304. doi: 10.1093/emboj/18.12.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geppert M, Goda Y, Stevens C F, Sudhof T C. Nature (London) 1997;387:810–814. doi: 10.1038/42954. [DOI] [PubMed] [Google Scholar]
- 41.Coppola T, Perret-Menoud V, Luthi S, Farnsworth C C, Glomset J A, Regazzi R. EMBO J. 1999;18:5885–5891. doi: 10.1093/emboj/18.21.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]