Abstract
Antimicrobial peptides (AMPs) are widely expressed and rapidly induced at epithelial surfaces to repel assault from diverse infectious agents including bacteria, viruses, fungi and parasites. Much information suggests that AMPs act by mechanisms that extend beyond their capacity to serve as gene-encoded antibiotics. For example, some AMPs alter the properties of the mammalian membrane or interact with its receptors to influence diverse cellular processes including cytokine release, chemotaxis, antigen presentation, angiogenesis and wound healing. These functions complement their antimicrobial action and favor resolution of infection and repair of damaged epithelia. Opposing this, some microbes have evolved mechanisms to inactivate or avoid AMPs and subsequently become pathogens. Thus, AMPs are multifunctional molecules that have a central role in infection and Inflammation.
Antimicrobial peptides
Initially, the importance of antimicrobial peptides (AMPs) to mammalian immunity was underestimated compared to their role in less complex immune systems such as those found in plants and invertebrates. However, as the importance of innate immune defense systems of mammals was steadily uncovered, the essential role of AMPs in mammalian immunity became firmly established. Today, we are beginning to see that much of the importance of AMPs in mammals might lie in their multifunctional role. To understand this topic, we must first discuss the extreme diversity of molecules referred to as AMPs.
So far, more than 1200 antimicrobial peptides of different origins have been identified or predicted. For a list of some of these, see the Antimicrobial Peptide Database (APD: http://aps.unmc.edu/AP/main.php). Most of these peptides maintain certain common features, such as being small (12–50 amino acids), containing positive charge and an amphipathic structure. Based on their amino acid composition, size and conformational structures, AMPs can be divided into several categories, such as peptides with α-helix structures, peptides with β-sheet structures stabilized by disulfide bridges or peptides with extended or loop structures (Figure 1). The expression of AMPs differs depending on the cell and tissue type, but in most cases AMPs are co-expressed as groups that act together. For example, in skin, more than 20 antimicrobial peptides and proteins have been identified [1], including cathelicidins, β-defensins and others. Relatively little information is available on the immunological function for most of these peptides, and it is unlikely that the immunomodulatory actions of the mammalian AMPs are also a component of the biology of the hundreds of AMPs produced by most prokaryotes and invertebrates. Thus, the first important concept to confer in a discussion of the AMPs is that they are similar only by their capacity to directly kill or inhibit the growth of microbes. Because this function is often through their capacity to influence lipid membrane structures, AMPs have some distant structural similarities. However, the specific immunological effect of AMPs is likely to be highly specific and isolated in particular AMP families. For purposes of illustration, we briefly describe two of the most extensively studied mammalian gene families of AMPs, the cathelicidins and defensins.
Figure 1.
Structure of selected antimicrobial peptides (AMPs). AMPs are present in a wide variety of structural conformation, such as peptides with α-helix structures, peptides with β-sheet structures stabilized by disulfide bridges or peptides with extended or loop structures. (a) α-helix. NMR-structure of the LL-37 core peptide of cathelicidin bound to detergent micelles [PDB ID: 2FBS). (b) β-sheet Solution structure of the defensin hBD2 by two-dimensional proton nuclear magnetic resonance spectroscopy (PDB ID: 1FQQ). (c) extended structure, NMR structure of the bovine antimicrobial peptide indolicidin bound to dedecytphosphocholine (OPC) micelles (PDB ID: 1G89). (d) loop structure. 3D structure of a cyclic defensin from the leukocytes of rhesus macaques (PDB ID: 1HVZ). PDB ID: ID of peptide structure in Research Collaboratory for Structural Bioinformatics (RCSB) protein data bank (http://www.rcsb.org/pdb/home/home.do). The style of LL37 in (a) is shown as secondary coloring shortcuts, whereas the styles of peptides in (b-d) are shown in rainbow coloring shortcuts.
Cathelicidins
The cathelicidins have been described in both invertebrate species such as hagfish and vertebrate species including fish, birds, snakes and mammals [2-6]. In humans, a single cathelicidin gene (CAMP) is known that encodes an inactive precursor protein with an approximate mass of 18 KDa, and is thus named hCAP18. Cathelicidins are so named based on the highly conserved N-terminal region known as the cathelin domain. This protein contains two disulfide bonds between cysteine residues C85-C96 and C107-C124 [7,8] and was given its name based on an ability to inhibit the protease cathepsin-L. The cathelicidins are only considered to be a gene family because of the conservation seen in this region of the gene, encoding a protein that acts as a cysteine protease inhibitor and also possesses some antimicrobial activity [9]. The peptides generated by cathelicidin genes found between species show little similarity to each other and are referred to as a group solely because of the similarity of the precursor protein that is predominated by the large cathelin domain. In human neutrophils and other cell types hCAP18 is processed to release from the carboxy (C-) terminus of the precursor protein an antimicrobial peptide of 37 amino acids beginning with two leucines, named LL37 hCAP18 is stored in neutrophil granules at a molar concentration as high as 40 μM or 630 μg 10−9 cells [10] and is also produced in other granulocytes such as NK-cells and mast cells. It is also expressed, released or secreted by epithelial cells in the skin, lung, gut, mammary gland and epididymis [11-14]. As a mature peptide, LL37 has rapid, potent and broad-spectrum antimicrobial activity when released from the C-terminus of hCAP18 [15]. Its processing is essential for activation of its antimicrobial activity in vivo [16] and is accomplished by serine proteases from keratinocytes such as kallikreins [17] and neutrophil proteases such as proteinase 3 [18].
The structures of the cathelicidin peptides vary both within and between species because cathelicidins tend to show very little homology beyond the N-terminal cathelin domain. In species such as the pig, multiple catbelicidin genes encode peptides that include those with linear structures and rich in amino acids such as tryptophan, proline or arginine, peptides that have disulfide bonds and tend to form a β-sheet structure or peptides that can adopt an α helical confirmation (Figure 1). In humans, LL37 is rich in lysine and arginine and adopts an α-helical structure in detergent micelles (Figure 1a) [19] or in solutions with ionic compositions similar to human plasma, interstitial fluid or intracellular fluid [20]. In human neutrophils, cathelicidin is usually processed to release LL37, whereas at the human skin surface, LL37 is further processed by serine proteases or other proteases produced by skin microflora into different derivatives (e.g. RK-31, KS-30 and K20) with varied activities [12]. Overall, cathelicidin peptides should be considered to be a highly heterogeneous group of molecules both within and between species [21].
Defensin
In mammalian species, around 50 α-defensins and 90 β-defensins have been identified which are either stored in the granules of neutrophils and Paneth cells, or are generated by monocyte, macrophages [22]. keratinocytes or mucosal epithelial cells of the respiratory, digestive, urinary and reproductive systems. All defensins are cationic, microbicidal, lack glycosyl- or acyl- side-chain modifications and contain six highly conserved cysteine residues which form three pairs of intramolecular disulfide bonds (Figure 1b). Although mammalian defensin peptides exhibit a large variability in their sequences, the six cysteines are retained. Based on sequence homology and the connectivity of the six oonserved cysteine residues, mamma-lian defensins are classified into α-, β- and θ-defensins. The six cysteines in α-defensins are linked in a 1–6, 2–4 and 3–5 pattern, whereas the disulfide connectivity in β-defensins is in a 1–5, 2–4 and 3–6 pattern. The structure of θ-defensins is circular without a free N- or C-terminus (Figure 1d). The disulfide paring of θ-defensins is different from α- and β-defensins because θ-defensins are formed by two bemi-α-defensins, each of which contributes three cysteines. Similar to cathelicidins, defensins are activated by proteolytic processing from an inactive precursor [23]. However, unlike the cathelicidins, diversity in the mature peptides has not been described based on post-translational processing.
Expression and regulation of AMPs
Most AMPs are encoded in groups in the genome. For example, the human α-defensins HNP1 and 4 and HD5 and 6, and the β-defensins HNP1 and 2 are all mapped to a similar chromosomal location, 8p23, whereas human cathelicidin is located in chromosome 3p21.3 [24]. Their expression can be regulated both at the transcriptional and post-transcriptional levels, and the coordinated transcriptional regulation of AMP genes can lead to expression of multiple AMPs at a single site. As discussed earlier, because AMPs are expressed as pro-peptides followed by proteolytic processing to release the biologically active peptides, regulation of function is as dependent on the expression of appropriate proteases for processing as it is on the expression of the AMP gene product itself. These are accomplished differentially depending on the specific peptide and the tissue or cell type.
Recent observations have shown that the constitutive expression of AMPs is under strict developmental control and influenced by age and sexual maturation. For example, high constitutive expression of the cathelin-related antimicrobial peptide (CRAMP) was observed in neonatal mouse small intestinal epithelium, a site at which cathelicidin is not normally expressed in the adult. The presence of the peptide was limited to the first two weeks after birth and gradually disappeared with the onset of increased stem cell proliferation and epithelial cell migration along the crypt-villus axis. In this context, CRAMP conferred in neonates’ protection from the enteric pathogen Listeria monocytogenes indicating that CRAMP expression might regulate bacterial Colonization and the establishment of gut homeostasis [25]. Anothar example of developmental control of AMPs is Bin1b, a rat epididymis-specific β-defensin with antimicrobial activity. This is maximally expressed during sexual maturation and can be upregulated by inflammation [26,27]. Bin1b binds to the sperm head in different regions of the epididymis with varied binding patterns and then induces progressive sperm motility in immotile immature sperm.
In the adult, the expression of many AMPs is greatly increased following injury or infection, or the AMP is stored at high concentrations as an inactive precursor in intracellular granules. Induction of AMP expression often involves signaling mediated by pattern-recognition receptors such as Toll-like receptors (TLRs) or responses to the release of pro-inflammatory cytokines. In keratinocytes, hBD1 mRNA is constitutively expressed, but the expression of hBD2-hBD4 are only induced by stimulation with TLR ligands, tumor necrosis factor (TNF), interleukin-1β, interferon (IFN)-γ or phorbol myristate acetate [28]. By contrast, cathelicidin expression in humans is less abundantly induced directly by TLRs or cytokines, but rather relies on histone acetylation, the action of the Vitamin D receptor (VDRE) and the modulation of Vitamin D action in specific tissues [29-34]. Inhibitors of histone deacetylase can be an important inducer of cathelicidin [29,30,34]. For instance, increased histone acetylation by the histone deacetylase inhibitor butyrate or trichostatin A enhances the expression of cathelicidin in gastrointestinal cells and lung epithelial cells. This mode of regulation combined with the action of VDRE is complex, with several groups showing that vitamin D regulates cathelicidin expression in multiple ways. Hormonally active vitamin D(3)-1,25-dihydroxyvitamin D(3) (1,25 D3) directly induces cathelicidin expression via a consensus VDRE in the CAMP promoter [31]. This stimulation occurs in different cell types from humans, including isolated human keratinocytes, monocytes, neutrophils, in addition to normal human bone marrow (BM) -derived macrophages, but is less active in control of CAMP expression in colonic epithelial cells, which require inhibition of histone deacetylase [31-33]. Rapid upregulation of cathelicidin that is necessary for immunologically relevant responses in a local environment is possible through the enzymatic action of cytochrome P450-containing hydroxylase, CYP27B1, which converts 25OH vitamin D3 (25D3) to active 1,25D3 [35]. Notably, histone acetylation further amplifies 1,25 D3-regulated cathelicidin in skin keratinocytes [34]. Therefore, 1,25 D3 acts as a signaling molecule in immunity by enhancing the expression of cathelicidin. Furthermore, 1,25 D3 increases pattern recognition by increasing CD14 and TLR2 [35,36], thus enabling the host to recognize and respond to microbes and to protect against infection. To summarize a complex pathway, local stored pools of 25 D3 are first rapidly CDoverted to 1,25 D3 following the activation of CYP27B1. Active 1,25 D3 induces AMPs directly and indirectly through the capacity to both increase trarncription of cathelicidin and pattern recognition molecules. Increased TLR and CD14 expression can further increase defensin expression and other innate responses triggered by the TLR pathways. This process is further ccntrolled by histone acetylation that provides fine control in some epithelia, such as that in the colon.
The flipside of AMP regulation occurs when expression is turned off and is often associated with increased susceptibility to infection by pathogens otherwise sensitive to the AMPs. For example, psychological stress has been shown to have adverse effects on cathelicidin expression through the induction of endogenous glucocorticoids (GCs). Indeed, psychological stress and systemic or topical GC administration has been observed to downregulate epidermal expression of murine cathelicidin and β-defensin 3, leading to increased severity of group A Streptococcus pyogenes skin infection [37]. The capacity to decrease AMP expression is also a microbial virulence factor and is discussed later.
The selective antimicrobial activity of AMPs
In vitro, most AMPs act against many different types of microbes including gram-negative and gram-positive bacteria, protozoa, fungi and some viruses. This is particularly true for the mammalian AMPs that have maximal effectiveness against specific groups of organisms relevant to the tissue where the AMP is expressed. For instance, β-defensins exhibit activity against Staphylococcus aureus and Pseudomonas aerugirosa [38], which are relevant to skin infection, whereas α-defensins expressed in the gut and in granulocytes are able to inactivate certain enveloped viruses (such as HIV) in addition to killing important bacterial pathogens such as Salmonella [39]. Some mammalian AMPs have a role in many types of infection. For example in vivo, cathelicidin knockout mice (Camp-/-) have been found to be susceptible to Group A Streptococcus [40,41], herpes simplex virus [42]. Escherichia coli [43] and vaccinia virus [44]. Similarly, knocking-out mouse β-defensin 1 results in delayed clearance of Haemophilus influenzae from the lung [45] and increased colonization by Staphylococcus species in the bladder [46]. Usually, the expression of these AMPs is increased at the onset of infection in response to stimuli. Therefore, the antimicrobial activity of AMPs is one of most important countermeasures that the mammalian host has developed to contend with microbial invagion and/or infection.
How do antimicrobial peptides exert their antimicrobial activity? Depending on the specific AMP studied, most show the capacity to directly disrupt the microbial cell membrane and thereby result in killing. Disruption of lipid bilayers by AMPs occurs through a variety of mechanisms (Box 1) [47]. Although the models shown in Box 1 used for the illustration of AMPs mechanisms are based on specific cationic antimicrobial peptides and are not applicable to all antimicrobial peptides, the structure-function models of the Shai-Matsuzaki-Huang (SMH) model [48] provide a reasonable explanation fOr the antimicrobial activity of most of these ccmpounds [49]. In general, cationic AMPs are attracted by electrostatic forces to the negative phospholipid headgroups on the membrane surface provided by capsular polysaccharides, which include lipopolysaccarides (LPS) in Gram-negative bacteria and teichoic acids (TA), lipoteichoic acids (LTA) and lysylphosphatidylglycerol in Gram-positive bacteria. Once AMPs gain access to the cytoplasmic membrane they interact with the lipid bilayer [47], followed by displacement of lipids, alteration of membrane structure and the creation of a physical hole causing cellular contents to leak out. Collectively, all these are well established models representative of the mechanisms for AMPs, and each model provides a different view of peptide activity, but none of these models is capable of adequately explaining their effectiveness in vivo.
Box 1. Membrane action of AMPs.
There are several mechanisms by which different classes of AMPs could act on the microbial target membrane [47]. In the ‘carpet model’. peptides accumulate on and orient parallel to the membrane surface. At a certain high concentration of peptides, AMPs disrupt the bilayer membrane in a detergent-like manner, resulting in the formation of micelles and leakage of cellular contents. In the ‘toroidal-hole model’, the polar heads of the peptides face the polar head groups of the lipids, inducing the lipids to form a continuous bend from the top to the bottom in the fashion of a toroid which is lined by both the peptides and the lipid head groups. Some cationic AMPs adopt an α-helical configuration, attach to, aggregate and insert into oriented bilayers that are hydrated with water vapor, leading to the formation of ‘barrel-stave’ holes in the membrane. For this model, the hydrophobic peptide regions align with the lipid core region of the bilayer and the hydrophilic peptide regions form the interior region of the pore. Notably, the differences between the toroidal model from the barrel-stave model is that peptides in the former are always associated with the lipid head groups even when they are perpendicularly inserted in the lipid bilayer. Finally, although most AMPs have been shown to disrupt cell membranes and induce microbial killing, a few AMPs kill bacteria without any detectable lysis. These AMPs can penetrate the cell membrane and bind to different targets, such as DNA, to inhibit bacterial growth.
Because the mechanism of action for most of the cationic AMPs depends on the interaction between the peptide and the microbial membrane to promote membrane destabilization, a question arises as to how these cationic AMPs can function in vivo but are not toxic to host cells. To address this point, several hypotheses have been proposed. One commonly cited explanation is that AMPs produced by mammals will not act on eukaryotic cells because of the cholesterol content of eukaryotic membranes. Unlike eukaryotic cell membranes, bacterial cell membranes are free of cholesterol. For example, the AMP sarcotoxin IA Was shown to be less disruptive to liposomes containing cholesterol than cholesterol-free liposomes [50]. Because cholesterol is known to cause condensation of phospholipid bilayers, it might prevent AMPs from penetrating into the cytoplasmic membrane of eukaryotic cells. Besides cholesterol, the asymmetric distribution of phospholipids in the cytoplasmic membrane of eukaryotic cells might also contribute to the insensitivity of eukaryotic cells to AMPs. The outer monolayer of the host membrane consists of neutral phospholipids without net charge, whereas most of the lipids with negatively charged headgrouPs, which should have affinity to cationic AMPs, are segregated into the inner leaf1et facing the cytoplasm [49,51]. Moreover, the cytotoxic effects ofAMPs to eukaryotic host cells in vivo are also substantially attenuated by serum components such as apolipoprotein A-I and B, or other lipoproteins, which have been shown to bind to LL37 [52]. This binding mechanism indicates an important role for serum lipoproteins in protecting host cells from damage caused by LL37. However, the direct antimicrobial activity ofmost AMPs in vitro and in vivo is often blunted by the physiological concentration of salt (e.g. 150 mM NaCl), increasing concentrations of divalent cations and serum proteins [53]. In spite of this, under physiological conditions bacteria also change their membrane structure and gene expression in vivo and remain susceptible to AMPs even in high salt concentrations [54]. Thus, the membrane activity of AMPs might be enough to kill microbes, but it is not cytotoxic to host cells with distinct lipid composition and in sequestered environments in which ionic composition of the extracellular fluid, and serum or extracellular matrix proteins, act to protect the host.
Despite the selective activity of many AMPs for microbial membranes, several studies have also shown that these AMPs at high concentrations will also destroy eukaryotic cells in vitro, such as T lymphocytes [55], leading to the hypothesis that they might also have a role in tumor surveillance [56-58]. In spite of this, the cytotoxic concentrations of AMPs are, in general, higher than the concentrations required for elimination of microbes, revealing a cell-selective killing mechanism [59].
AMP modulation of host inflammatory responses
As discussed previously, AMPs exhibit multiple functions related to their capacity to disrupt membranes. They have the ability to confer protection against a variety of pathogens and the potential to act as cytotoxic agents against certain type of cancers. However, the direct antimicrobial activity implicit to the term ‘AMP’ has strongly biased interpretation of the function of these peptides as ‘natural antibiotics’, This bias perhaps has delayed discovery of their other roles in immunity.
The minimal inhibitory concentrations (MIC) of AMPs against microbes in vitro are typically much higher than the physiological concentrations of peptides found in vivo under resting conditions. For instance, the concentration of LL37 or β-defensins is less than 2 μg ml−1 at mucosal sites, whereas the MIC of LL37 in vitro for E.coli is more than 32 μg ml−1. Thus, the question arises; how do AMPs exert their antimicrobial function in vivo if the effective concentration is too low? One explanation for this is that they act synergistically with other classes of AMPs to exert their desired effect. For example, LL37 can act with BD2, lysozyme and lactoferrin that are co-expressed to exert optimal killing. Another explanation is that in situations of inflammation, AMPs often accumulate at a high local concentration sufficiently above the MIC, thus acting alone as a classic AMP. However, increasing evidence indicates that some AMPs can confer protection by an indirect mechanism and not simply because they can kill microbes. They can function as potent immune regulators, altering host gene expression, acting as chemokines and/or inducing chemokine production, inhibiting LPS- or hyaluronan-induced pro-inflammatory cytokine production, promoting wound healing and modulating the responses of dendritic cells or T cells of the adaptive immune response (Figure 2). In this way, AMPs can be seen as acting as a bridge between innate and adaptive immunity. All of these functions favor resolution of infection and reverse potentially harmful inflammation, and complement the direct antimicrobial action. Here, we review some of the important functions of AMPs that are not related to their direct antimicrobial action.
Figure 2.
Multiple functions of antimicrobial peptides in host defense. AMPs induce a variety of responses in host Innate immune cells such as monocytes, macrophages, neutrophils and epithelial cells. They alter gene expression of host cells, induce production of chemokines and cytokins, promote leukocyte recruitment to the site of infection, influence cell differentiation and activation and block or activate TLR signaling. The outcome of the selective Immunomodulation by AMPs results in innate Immune responses, leading to protection against infection, selective control of inflammation, promotion of wound healing and initiation of adaptive immune responses. Abbrevations: AMP, anti-microbial peptide; DC, dendritic cell; LPS, lipopolysaccharide; pDC, plasmacytold dendritic cell; PMN, polymorphonucleocyte; TLR, Toll-like receptor.
Chemotactic activity
Upon stimulation by microbial pathogens, local cells release AMPs at the site of the infection or injury [60]. In addition to inhibiting microbial growth, an additional function of some of these AMPs is that they act to directly recruit leukocytes or induce the expression of chemokines or cytokines including CXCL8 (IL-8), CCL2 (monocyte chemoattractant protein, MCP-1) and IFN-α, thereby indirectly promoting recruitment of effector cells such as neutrophils, monocytes, macrophages, immature dendritic cells and T cells. For example, human α-defensins (HNP-1 and HNP-2, but not HNP-3) have chemotactic activity mediating the recruitment of monocytes to inflammatory sites [61]. Subsequently, the defensins hBD3 and 4 have been reported to be chemotactic for monocytes and macrophages [62], and hBD2 has chemoattractant activity for mast cells [63]. Notably, both human α and β-defensins are chemotactic for memory T cells and immature dendritic cells. Human α-defensins selectively induce the migration of human nalve CD4+CD45RA+ and CD8+ cells, but not CD4+CD45RA+ memory T cells [64]. whereas β-defensins are chemotactic for immature dendritic cells and CD4+CD45RO+ memory T cells. This chemotactic effect of human defensins on both T cells and dendritic cells is pertussis toxin-sensitive and inhibited by antibodies to CCR6 [65]. Therefore, the chemotactic activity of human defensins has been suggested to promote cellular immune responses by recruiting dendritic and T cells to the site of microbial invasion through interactions with the G protein-coupled receptor, CCR6 (Figure 3). However, structure-function studies have shown conflicting data in this process. On one hand, intramolecular disulfide bonding in hBD3 was reported to be required for binding and the activation of receptors for chemotaxis, but dispensable for its antimicrobial function [66]. On the other hand, recent data have also shown that chemoattractant activity of hBD3 is not dependent on disulfide bonds but is cysteine V-dependent [67] and CCR6 might not be a functional receptor for β-defensins in memory lymphocytes and dendritic cells [68]. These contrasting results might be because of mismatched intramolecular disulfide bonding of hBD3 or different cell lines and experimental conditions used, but at any rate serve to reinforce the function of defensins in chemotaxis and emphasize the complexity of these systems.
Figure 3.
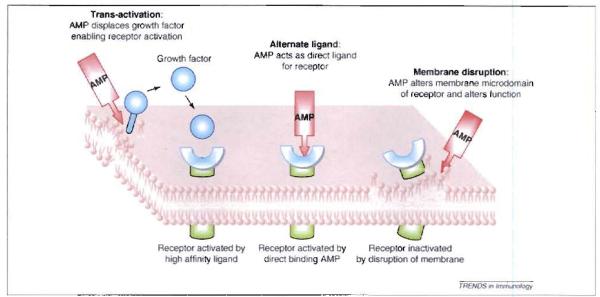
Alternative models for host activation by antimicrobial peptides. Three mechanisms have been proposed to explain how AMPs activate mammalian cells. Alternate ligand model; some AMPs, such as defensins and cathelicidin, might directly bind to a specific receptor, resulting in initiation of receptor signaling. This has been proposed for CCR6 and FPRL-1. Membrane disruption model in this model AMPs associate with and modify the membrane containing the receptor. This membrane activity indirectly results in a change in receptor function such that it can signal without a ligand or becomes insensitive to binding by its specific ligand. This has been proposed for inactivation of TLR4. Trans-activation model: AMPs stimulate the release of a membrane-bound growth factor, which then binds to its high affinity receptor and activates it. This has been proposed for HB-EGF (heparin binding epidermal growth factor) and the EGF receptor.
Like the βdefensins, cathelicidins have also been shown to be chemotactic. LL37, is chemotactic for neutrophils, monocytes and T cells, but not for dendritic cells. In some experimental sygtems, the chemotactic activity of this peptide is mediated by the G protein-coupled formyl peptide receptor-like 1 (FPRL-l) [69]. The mouse cathelicidin., CRAMP, like LL37, was found to be chemotactic for human monocytes, neutrophils, macrophages and mouse peripheral blood leukocytes [70]. CRAMP also induced the chemotaxis of human embryonic kidney 293 cells transfected with either FPRLI or mouse formyl peptide recaptor-2, the mouse homologue of FPRL1, but not by untransfected parental human embryonic kidney 293 cells, confirming the use of FPRL1 or mouse formyl peptide receptor-2 by CRAMP [70] (Figure 3).
In addition to direct chemotactic activity, AMPs can exert indirect chemotactic activity by stimulating chemokine and cytokine secretion from a variety of cell types through receptor-dependent mechanism [64,71]. LL37 synergistically enhances the IL-1β-induced production of cytokines (IL-6, IL-10) and chemokines such as MCP-1 and MCP-3 [72] in human peripheral blood monocytes (PBMC). hBD3 can induce expression of the costimulatory molecules CD80, CD86 and CD40 by interaction with TLRs 1 and 2 on monocytes and dendritic cells resulting in MyD88 signaling, leading to IL-1 receptor-associated kinase-1 phosphorylation [73]. In human kerationcytes, hBD-2, -3, -4 and cathelicidin, but not hBD-1, act through G-protein coupled receptors and increase gene expression and protein production of IL-6, IL-10, IP-10, MCP-1, MIP-3α and RANTES [74]. Interestingly, although structurally very different, β-defensins and cathelicidin show somewhat similar effects On the activation of the ERK mitogen activated protein kinase (MAPK) pathway to induce inflammatory cytokine production in epithelial cells [74,75]. Also in normal human keratinocytes, hBD-2, -3, -4 and LL37, but not hBD-1, induced. phosphorylation of the MAPKs ERK1/2 and p38, but not JNK. The activation of ERK1/2 led to the activation of the downstream transcription factor, Elk-1, resulting in the secretion of Elk-1-controlled IL-18, which activated T, B and NK cells to produce high levels ofIFN-γ, and increased IgE production in CD4+ T cells [76,77). Notably, the combination of peptides resulted in a synergistic effect on IL-18 secretion. Thus, the ability of hBDs and cathelicidin to induce cytokine and chemokine secretion demonstrates that these AMPs influence the outcome of infection in at least three ways; they act as direct antimicrobials, they are chemotactic and they induce the release of multiple cytokines and chemokines that further refine and amplify the innate immune response.
Modulation of TLR-dependent inflammatory responses
AMPs have diverse and complementary effects on cellular inflammatory responses that are beyond the stimulation of chemotaxis. In particular, mammalian AMPs have a crucial role in regulation of TLR-dependent inflammatory responses. Our studies in a mouse model of allergic contact dermatitis showed cathelicidins inhibited TLR4- and CD44-mediated induction of cytokine release in dendritic cells and macrophages [78,79]. In dendritic cells, cathelicidins inhibited TLR4- but not TLR2-mediated induction of dendritic cell maturation and cytokine release, and this inhibition was associated with an alteration of cell membrane function and structure by cathelicidin (Figure 3) [78]. A similar effect of cathelicidin was also observed in macrophages for another TLR4 ligand, small hyaluronan (HA), which is relesed following injury and is in endogenous ligand for TLR4 and CD44. Cathelicidin inhibited HA induced MTP-2 release from mouse bone-marrow-derived macrophages by interfering with membrane binding events mediated by HA in a CD44-dependent manner, but independent of G-protein coupled receptor or epidermal growth factor (EGF) receptor signaling [79]. Both observations provide evidence that cathelicidin can act to inhibit TLR4. In contrast to models in which AMPs have been proposed to act directly on the receptor, these results indicate that the membrane active peptide influences the local membrane environment of the receptor and thus alters its activation state (Figure 3). Similar phenomena have been observed in several other systems in which LL37 has been found to selectively suppress TNF-α release from human monocytes and macrophages stimulated by LPS or LTA, suggesting AMPs might have a role in preventing sepsis [80,81]. Genomic approaches showed that LL37 significantly inhibited the expression of specific proinflammatory genes upregu1ated by NF-κB in the presence of LPS, including NF-κBI (p105/p50) and TNF-α-induced protein 2 (TNFA1P2). In contrast, LL37 did not inhibit LPS-induced genes that antagonize inflammation, such as TNF-α-induced protein 3 (TNFAIP3) and the NF-κB inhibitor, NF-κBIA or certain chemokine genes that are classically considered proinflammatory [80]. However, an alternative mechanism for how LL37 influences LPS responses also lies in the capacity of this, and other cationic peptides, to bind and neutralize LPS [82-85]. In this scenario, the AMP acts as a scavenger for the activating ligand, removing it before it can trigger inflammation.
Collectively, all these observations indicate that AMPs have an important role in regulating and balancing inflammatory responses to microbes. Although central questions regarding the mechanisms of the immunomodulatory effects of AMPs are yet to be clarified, the overall capacity of AMPs to influence multiple steps in host cell activation leads to the conclusion that some AMPs are not only involved in suppressing uncontrolled microbial growth but also that they modify inflammation. Recent clinical observations in human disease states support this conclusion, and are discussed later.
Promotion of wound healing
Another important role suggested for AMPs is to promote wound healing. This can occur by many mechanisms. As mentioned earlier, cathelicidins in addition to the defensins hBD2 and hBD3 rapidly and dramatically increase at the wound bed and edge [86,87]. hBD2 stimulates migration, proliferation and tube formation of endothelial cells in wounds, leading to accelerated wound closure [87]. Cathelicidin peptides have also been shown in several studies to affect the wound repair process. Our initial observation that AMPs were present in mammalian skin was made when studying wound repair in a porcine model. Here, cathelicidin peptide in pigs (PR-39) induced the heparan sulfate proteoglycans syndecan-1 and -4 in wounds, which in turn regulate cell proliferation and migration induced by heparin-binding growth factors [88]. The human cathelicidin LL37 has been shown to initiate metalloproteinase-dependent cleavage of membrane bound heparin-binding-EGF (HB-EGF). This soluble form of HB-EGF then binds to and initiates phosphorylation of EGFR and subsequently activates STAT1 and STAT3 (Figure 3). This trans-activation of the EGFR has been suggested to lead to enhanced keratinocyte migration required for re-epithelialization of the wound [89]. LL37 has also been proposed to increase wound closure by inducing the transcription factors Snail and Slug and activating the MAPK, phosphoinositide 3-kinase (P13K) and Akt signaling pathways [90]. Furthermore, LL37 directly activates FPRL-1 expresaed on endothelial cells to increase proliferation and formation of vessel-like structures, and induces functional angiogenesis important for cutaneous wound neovascularization [91]. LL37 acts directly on dermal fibroblasts, inhibiting transforming growth factor (TGF)-induced collagen expression, resulting in anti-fibrotic effects that might benefit normal wound repair [92]. Together these observations indicate that antimicrobial peptides can serve both protective and regenerative roles after injury that are unrelated to direct defense against infection.
AMPs and their participation In disease
As discussed previously, AMPs have been shown to participate in alerting, mobilizing and amplifying innate and adaptive immune respooses of the host, and will confer protection against microbial infections. Decreased expression of AMPs can increase susceptibility to infectious diseases. For example, the expression of AMPs such as cathelicidin, hBD2 and hBD3 is not increased in individuals with atopic dermatitis, despite the presence of skin inflammation. By contrast, patients with psoriasis have high expression of these AMPs in their inflammatory lesions (Figure 4). The differences in AMP expression between psoriasis and atopic dermatitis gain immunOlogical relevance in light of the proven antimicrobial activity of these AMPs against known superinfecting pathogens of atopic dermatitis, including Group A Streptococcus (GAS) [60]. S.aureus [93] and vaccinia virus [44]. In addition, the variability in defensin gene copy numbers can contribute to differences in individual resistance to infections [94,95]. For instance, individuals with lower gene copy number of hBD2 (≤3) have a significantly higher risk of developing Crohn’s disease (CD) than individuals with ≥4 copies [94].
Figure 4.
Model of the initiation and maintenance of autoimmune skin inflammation by LL37 in psoriasis. Skin injury and infections induce a rapid expression of the cathelicidin hCAPl8 in keratinocytes or infiltrated neutrophils. The mature peptide LL37 is cleaved from the precursor hCAP18 by Kalllikreins or proteinese 3. Subsequently LL37 combines with self-DNA released by damagad cells to form a complex. which triggers TLR9 in pDC to produce type 1 Interferons (IFNα and β). Type 1 IFNs trigger local maturation of myeloid dendritic cell to activate autoreactive Th1 or Th17 cells, resulting in the production of INF-γ,.IL-22 and IL-17. The sustained production of IL-22 and IL-17 leads to the expression of LL37 that forms a feedback loop to maintain inflammation in psoriasis.
The presence of AMPs can be a double-edged sword because they might also exaggerate inflammatory responses and lead to disease. In this context, two models for the action of AMPs in promoting undesirable inflammation have been reported. In one, associated with the human facial skin disease rosacea, an excess of cathelicidin in the form of LL37 drives inflammation and abnormal blood vessel growth by mechanisms of cell activation [96]. In a second and novel mechanism, the abundance LL37 in psoriasis has been proposed as a mechanism that breaks tolerance to self-DNA and drives autoimmunity [97]. Here, LL37 binds extracellular self-DNA fragments and enables them to enter plasmacytoid dendritic cells (pDCs). This in turn triggers a robust TLR9-mediated IFN-α response. As a consequence, local maturation of myeloid dendritic cells are triggered by the type I IFNs to activate autoreactive Th1 or Th17 cells, resulting in the production of IFN-γ, IL-22 and IL-17 [98-99) (Figure 4). It is not clear how patients without psoriasis are able to control this self-perpetuating cycle. Thus, although recent clinical studies have shown the expression of AMPs is associated with the pathogenesis of several diseases (Table 1, much remains to be learned about how they function. Clearly, regulation of AMP expression and processing has an important role.
Table 1.
Diseases associated with AMPs.
| Diseases | Peptides | Expression levels | Refs |
|---|---|---|---|
| Inflammatory skin diseases | |||
| Atopic dermatitis | LL37, defensins | Downregulatad | [93] |
| Psoriasis | LL37, defensins | Overexpressed | [93] |
| Rosacea | cathelicidins | Increased | [96] |
| Acne vulgaris | Defensins, granulysin | Induced | [78,110] |
| Lupus and contact dermatitis | LL37 | Increased | [111] |
| Inflammatory bowel diseases | |||
| Ulcarative colitis | HD5.6; hBD2–4; lysozyme | upregulated | [112] |
| Crohn’s disease | α-defensins hBD2 |
Reduced in Paneth cells; low gene copy number of hBD2(≤3) |
[94,113] |
| Gastrointestinal infections | LL37, hBD1 | Downregulated | [113] |
| Respiratory diseases | |||
| Cystic fibrosis | LL37, β-defensins | Reduced antimicrobial activity because of salt accumulation |
[114] |
Microbial resistance to AMPs
Thus far, we have discussed AMPs as multifunctional peptides, acting directly to kill microbes and also through multiple indirect strategies that influence cellular function in the host. What is the benefit of a multidimensional strategy to respond to microbial invasion? The answer might lie in the lessons learned through use of pharmaceutically produced antibiotics, and how ineffective they can become with the evolution of microbial resistance. By acting through a single approach, antibiotic function can be completely evaded by a single resistance system. It is apparent today that many pathogenic microbes have evolved mechanisms to avoid the antimicrobial action of AMPs, yet the expression of AMPs still protects the host (Box 2).
Box 2. AMPs conquer microbial resistance.
Microbes have evolved several mechanisms to degrade AMPs, yet the expression of AMPs continues to provide protection against infection. Why? Several theories can be advanced to explain their effectiveness in mammalian immunity. First, even though most pathogens have developed some countermeasures against AMPs, strict regulation of the expression of AMPs at the infection site leads to extremely high concentrations of AMPs in vivo that cannot be completely overcome by the microbe [115,116]. This combined with simultaneous production of a variety of different AMPs, impedes microbial colonization and growth [25]. In addition, the high costs for microbes to develop and express resistance genes preclude modifications that allow total resistance. In particular, it is difficult to evade the membrane-disturbing activity of AMPs while preserving the functional and structural integrity of the microbial cell wall and membrane. Furthermore, a strategy of destruction of the AMPs also poses several problems for the microbe. Most peptides are created from sequences of amino acids lacking unique epitopes that could serve as the recognition site of a specifically evolved protease that would be required for selective destruction of the AMP. Thus, it is difficult to design a degradation mechanism that would not also degrade necessary host proteins for attachment or microbial proteins necessary for survival. Finally, we have seen that all multicellular organisms attack microbes with multiple peptides of different structural classes, hence destruction of one peptide might not suffice to avoid the lethal assault of a diverse group of AMPs [49].
Several mechanisms have been described for microbial resistance to AMPs. Some microbes use advanced strategies to downregulate the expression of AMPs or produce proteases to degrade AMPs. For instance, Shigella spp. (S.dysenteriae, S.flexmeri, S.boydii. and S.sonnei), highly contagious bacteria that cause bacillary dysentery of varying severity, release plasmid DNA to turn off the expression of antimicrobial peptides in epithelial cells or monocytes [100], and this helps the bacteria evade AMP function, Another strategy for resistance is for the microbe to enzymatically inactivate the AMP before it can act. S. aureus secretes proteases V8 and aureolysin to inactivate cationic antimicrobial peptide LL37 [101]. S. epidermidis produces metalloprotease sepA to degrade the anionic antimicrobial peptide dermcidin [102]. Another effective strategy used by microbes to evade the effects of AMPs is to change the composition of their cell membrane. Both S. aureus and S. epidermidis control the three-component antimicrobial peptide-sensing system (aps) to regulate L-lysine and D-alanine synthesis. This modifies membrane lipid phophatidylglycerol with lysine and adds D-alanine to teichoic acid, resulting in the reduction of the net negative charge of the bacterial membrane so as to repel cationic AMPs [97,103-105]. Similarly, under certain circumstances, Gram-negative bacteria also control their two-component signal transduction systems, PhoP-PhoQ and PmrA-PmrB, to modify the structure of their LPS [106]. Salmonella enterica Typhimurium can form hepta-acylated lipid A (via the addition of palmitate by the bacterial protein PagP), add phosphate and phosphoethanolamine to the core polysaccharide and modify lipid A phosphate groups with ethanolamine and aminoarabinose. These alterations, and severa1 others not discussed here [107-109], decrease the susceptibility of microbes to AMPs and contribute to their pathogenicity.
Conclusions and perspectives
Great progress has been achieved in the last decade with respect to the mechanisms of AMP action and their complex role in our immune system. Along with the capacity of AMPs to directly kill microbes, AMPs also boost specific innate immune responses and exert selective immunomodulatory effects on the host. Upon exposure to danger, AMPs create an overall balance by inhibiting microbial growth, attenuating exacerbated inflammatory responses and stimulating certain beneficial aspects of inflammation. This combined functional profile highlights the unique tempora1 niche that AMPs occupy in microbial defense (i.e. they are ideally suited for rapid but transient responses). These characteristics also make AMPs promising agents as beneficial therapeutics. Therefore, understanding the function of antimicrobial peptides as ‘natural antibiotics’ and their mechanism of action as ‘immune regulators’ has great promise for yielding new strategies in the control of human disease.
Acknowledgments
Work in the laboratory of R.L.G. is supported by NIH grants R01AR052728, RO1AI052453, contract HHSN266200400029 and a VA Merit Award. We apologize to those authors whose work could not be cited because of space constraints.
References
- 1.Braff MH, Gallo RL. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr. Tap. Microbiol. Immunol. 2006;306:91–110. doi: 10.1007/3-540-29916-5_4. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, et al. Snake cathelicidin from BungaruS fascatus is a potent peptide antibiotics. PLoS One. 2008;3:e3217. doi: 10.1371/journal.pone.0003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao H, et al. Identification and characterization of novel reptile cathelicidins from elapid snakes. Peptides. 2008;29:1685–1691. doi: 10.1016/j.peptides.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 4.van Dijk A, et al. CMAP27, a novel chicken cathelicidin-like antimicrobial protein. Vet. Immunol. Immunopathol. 2005;106:321–327. doi: 10.1016/j.vetimm.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Uzzell T, et al. Hagfish intestinal antimicrobial peptides are ancient cathelicidins. Peptides. 2003;24:1655–1667. doi: 10.1016/j.peptides.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Zanetti M, et al. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374:1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez JF, et al. Overexpression and structural study of the cathelicidin motif of the protegrin-3 precursor. Biochemistry. 2002;41:21–30. doi: 10.1021/bi010930a. [DOI] [PubMed] [Google Scholar]
- 8.Storici P, et al. Purification and structural characterization of bovine cathelicidins, precursors of antimicrobial peptides. Eur. J. Biochem. 1996;238:769–776. doi: 10.1111/j.1432-1033.1996.0769w.x. [DOI] [PubMed] [Google Scholar]
- 9.Zaiou M, et al. Antimicrobial and protease inhibitory functions of the human cathelicidin (hCAP18/LL-37) prosequence. J. Invest. Dermatol. 2003;120:810–816. doi: 10.1046/j.1523-1747.2003.12132.x. [DOI] [PubMed] [Google Scholar]
- 10.Sorensen O, et al. Ao ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J. Immunol. Methods. 1997;206:53–59. doi: 10.1016/s0022-1759(97)00084-7. [DOI] [PubMed] [Google Scholar]
- 11.Murakami M, et al. Expression and secretion of cathelicidin antimicrobial peptides in murine mammary glands and human milk. Pediatr. Res. 2005;57:10–15. doi: 10.1203/01.PDR.0000148068.32201.50. [DOI] [PubMed] [Google Scholar]
- 12.Murakami M, et al. Postsecretory processing generates multiple cathelicidins for enhanced topical antimicrobial defense. J. Immunol. 2004;172:3070–3077. doi: 10.4049/jimmunol.172.5.3070. [DOI] [PubMed] [Google Scholar]
- 13.Bala R, et al. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. U. S. A. 1998;95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malm J, et al. The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect. Immun. 2000;68:4297–4302. doi: 10.1128/iai.68.7.4297-4302.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- 16.Cole AM, et al. Inhibition of neutrophil elastase prevents cathelicidin activation and impairs clearance of bacteria from wounds. Blood. 2001;97:297–304. doi: 10.1182/blood.v97.1.297. [DOI] [PubMed] [Google Scholar]
- 17.Yamasaki K, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidians in skin. FASEB J. 2006;20:2068–2080. doi: 10.1096/fj.06-6075com. [DOI] [PubMed] [Google Scholar]
- 18.Sorensen O, et al. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–2803. [PubMed] [Google Scholar]
- 19.Li X, et al. Solution structures of human LL-37 fragments and NMR-based identification of a minimal membrane-targeting antimicrobial and anticancer region. J. Am. Chem. Soc. 2006;128:5776–5785. doi: 10.1021/ja0584875. [DOI] [PubMed] [Google Scholar]
- 20.Braff MH, et al. Cutaneous defense mechanisms by antimicrobial peptides. J. Invest. Dermatol. 2005;125:9–13. doi: 10.1111/j.0022-202X.2004.23587.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhu S. Did cathelicidins, a family of multifunctional host-defense peptides, arise from a cysteine protease inhibitor? Trends Microbiol. 2008;16:353–360. doi: 10.1016/j.tim.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Duits LA, et al. Expression of beta-defensin 1 and 2 mRNA by human monocytes, macrophages and dendritic cells. Immunology. 2002;106:517–525. doi: 10.1046/j.1365-2567.2002.01430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson CL, et al. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 24.Yang D, et al. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu. Rev. Immunol. 2004;22:181–215. doi: 10.1146/annurev.immunol.22.012703.104603. [DOI] [PubMed] [Google Scholar]
- 25.Menard S, et al. Developmental switch of intestinal antimicrobial peptide expression. J. Exp. Med. 2008;205:183–193. doi: 10.1084/jem.20071022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li P, et al. An antimicrobial peptide gene found in the male reproductive system of rats. Science. 2001;291:1783–1785. doi: 10.1126/science.1056545. [DOI] [PubMed] [Google Scholar]
- 27.Zhou CX, et al. An epididymis-specific beta-defensin is important for the initiation of sperm maturation. Nat. Cell Biol. 2004;6:468–464. doi: 10.1038/ncb1127. [DOI] [PubMed] [Google Scholar]
- 28.Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 29.Kida Y, et al. Sodium butyrate up-regulates cathelicidin gene expression via activator protein-1 and histone acetylation at the promoter region in a human long epithelial cell line, EBC-1. Mol. Immunol. 2006;43:1972–1981. doi: 10.1016/j.molimm.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Schauber J, et al. Histone-deacetylase inhibitors induce the the cathelicidin LL-37 in gastrointestinal cells. Mol. Immunol. 2004;41:847–854. doi: 10.1016/j.molimm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Wang TT, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004;173:2009–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 32.Gombart AF, et al. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 33.Weber G, et al. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J. Invest. Dermatol. 2005;124:1080–1082. doi: 10.1111/j.0022-202X.2005.23687.x. [DOI] [PubMed] [Google Scholar]
- 34.Schauber J, et al. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J. Invest. Dermatol. 2008;128:816–824. doi: 10.1038/sj.jid.5701102. [DOI] [PubMed] [Google Scholar]
- 35.Schauber J, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J. Clin. Invest. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu PT, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 37.Aberg KM, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J. Clin. Invest. 2007;117:3339–3349. doi: 10.1172/JCI31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang L.c., et al. In vitro activity of human beta-defensin 2 against Pseudomonas aeruginosa in the presence of tear fluid. Antimicrob. Agents Chemother. 2007;51:3853–3880. doi: 10.1128/AAC.01317-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehrer RI, Ganz T. Antimicrobial peptides in mammalian and insect host defence. Curr. Opin. Immunol. 1999;11:23–27. doi: 10.1016/s0952-7915(99)80005-3. [DOI] [PubMed] [Google Scholar]
- 40.Nizet V, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 41.Lee PH, et al. Expression of an additional cathelicidin antimicrobial peptide protects against bacterial skin infection. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3750–3755. doi: 10.1073/pnas.0500268102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Howell MD, et al. Cathelicidin deficiency predisposes to eczema herpeticum. J. Allergy Clin. Immunol. 2006;117:836–841. doi: 10.1016/j.jaci.2005.12.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chromek M, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 2006;12:636–641. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 44.Howell MD, et al. Selective killing of vaccinia virus by LL-37: implications for eczema vaccinatum. J. Immunol. 2004;172:1763–1767. doi: 10.4049/jimmunol.172.3.1763. [DOI] [PubMed] [Google Scholar]
- 45.Moser C, et al. beta-Defensin 1 contributes to pulmonary innate immunity in mice. Infect. Immun. 2002;70:3068–3072. doi: 10.1128/IAI.70.6.3068-3072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrrison G, et al. Characterization of the mouse beta defensin 1, Defbl mutant mouse model. Infect. Immun. 2002;70:3053–3060. doi: 10.1128/IAI.70.6.3053-3060.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 48.Shai Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helica1 antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta. 1999;1462:55–70. doi: 10.1016/s0005-2736(99)00200-x. [DOI] [PubMed] [Google Scholar]
- 49.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 50.Nakajima Y, et al. Interaction between 1iposomes and sarcotoxin IA, a potent antibacterial protein of Sarcophaga peregrina (flesh fly) J. Biol. Chem. 1987;262:1665–1669. [PubMed] [Google Scholar]
- 51.Matsuzaki K. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim. Biophys. Acto. 1999;1462:1–10. doi: 10.1016/s0005-2736(99)00197-2. [DOI] [PubMed] [Google Scholar]
- 52.Sorensen O, et al. The human antibacterial cathelicidin, hCAP-18, is bound to lipoproteins in plasma. J. Biol. Chem. 1999;274:22445–22451. doi: 10.1074/jbc.274.32.22445. [DOI] [PubMed] [Google Scholar]
- 53.Maisetta G, et al. Evaluation of the inhibitory effects of human serum components on bactericidal activity of human beta defensin 3. Peptides. 2008;29:1–6. doi: 10.1016/j.peptides.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 54.Darschner RA, et al. mammalian ionic environment dictates microbial susceptibility to antimicrobial defense peptides. FASEB J. 2006;20:35–42. doi: 10.1096/fj.05-4406com. [DOI] [PubMed] [Google Scholar]
- 55.Johansson J, et al. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J. Biol. Chem. 1998;273:3718–3724. doi: 10.1074/jbc.273.6.3718. [DOI] [PubMed] [Google Scholar]
- 56.Bullard RS, et al. Functional analysis of the host defense peptide Human Beta Defensin-1: new insight into its potential role in cancer. Mol. Immunol. 2008;45:839–848. doi: 10.1016/j.molimm.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coffelt SB, Scandurro AB. Tumors sound the alarmin(s) Cancer Res. 2008;68:6482–6485. doi: 10.1158/0008-5472.CAN-08-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun CQ, et al. Human beta-defensin-1, a potential chromosome 8p tumor Suppressor: control of transcription and induction of apoptosis in renal cell carcinoma. Cancer Res. 2006;66:8542–8549. doi: 10.1158/0008-5472.CAN-06-0294. [DOI] [PubMed] [Google Scholar]
- 59.Kai-Larsen Y, Agerberth B. The role of the multifunctional peptide LL-37 in host defense. Front. Biosci. 2008;13:3760–3767. doi: 10.2741/2964. [DOI] [PubMed] [Google Scholar]
- 60.Dorschner RA, et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J. Invest. Dermatol. 2001;117:91–97. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- 61.Territo MC, et al. Monocyte-chemotactic activity of defensins from human neutrophils. J. Clin. Invest. 1989;84:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang D, et al. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 2002;23:291–296. doi: 10.1016/s1471-4906(02)02246-9. [DOI] [PubMed] [Google Scholar]
- 63.Niyonsaba F, et al. Epithelial cell-derived human beta-defensin-2 acts as a chemotaxin for mast cells through a pertussis toxin-sensitive and phospholipase C-dependent pathway. Int. Immunol. 2002;14:421–426. doi: 10.1093/intimm/14.4.421. [DOI] [PubMed] [Google Scholar]
- 64.Yang D, et al. Participation of mammalian defensins and cathelicidins in anti-microbial immunity: receptors and activities of human defensins and cathelicidin (LL-37) J. Leukoc. Biol. 2001;69:691–697. [PubMed] [Google Scholar]
- 65.Yang D, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCRS. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 66.Wu Z, et al. Engineering disulfide bridges to dissect antimicrobial and chemotactic activities of human beta-defensin 3. Proc. Nall. Acad. Sci. U. S. A. 2003;100:8880–8885. doi: 10.1073/pnas.1533186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taylor K, et al. Analysis and separation of residues important for the chemoattractant and antimicrobial activities of beta-defensin 3. J. Biol. Chem. 2008;283:6631–6639. doi: 10.1074/jbc.M709238200. [DOI] [PubMed] [Google Scholar]
- 68.Soruri A, et al. beta-Defensins chemoattract macrophages and mast cells but not lymphocytes and dendritic cells: CCR6 is not involved. Eur. J. Immunol. 2007;37:2474–2486. doi: 10.1002/eji.200737292. [DOI] [PubMed] [Google Scholar]
- 69.Yang D, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chamoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kurosaka K, et al. Mouse cathelin-related antimicrobia1 peptide chemoattracts leukocytes using formyl peptide receptor like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J. Immunol. 2005;174:6257–6265. doi: 10.4049/jimmunol.174.10.6257. [DOI] [PubMed] [Google Scholar]
- 71.Elssner A, et al. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J. Immunol. 2004;172:4987–4994. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 72.Yu J, et al. Host defense peptide LL-37, in synergy with inflammatory mediator IL-1beta, augments immune responses by multiple pathways. J. Immunol. 2007;179:7684–7691. doi: 10.4049/jimmunol.179.11.7684. [DOI] [PubMed] [Google Scholar]
- 73.Funderburg N, et al. Human -defensin-3 activates professional antigen-presenting cells via Toll-like receptors 1 and 2. Proc. Natl. Acad. Sci. U. S. A. 2007;104:18631–18635. doi: 10.1073/PNAS.0702130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Niyonsaba F, et al. The human beta-defensins(-1, -2, -3, -4) and cathelicidin LL-37 induce IL-18 secretion through p38 and ERK MAPK activation in primary human keratinocytes. J. Immunol. 2005;175:1776–1784. doi: 10.4049/jimmunol.175.3.1776. [DOI] [PubMed] [Google Scholar]
- 75.Niyonsaba F, et al. Antimicrobial peptides human beta-defensins stimulate epidermal keratinocyte migration, proliferation, and production of proinflamatory cytokines and chemokines. J. Invest. Dermatol. 2007;127:594–604. doi: 10.1038/sj.jid.5700599. [DOI] [PubMed] [Google Scholar]
- 76.Yoshimota T, et al. IL-18 induction of IgE: dependence on CD4+ T cells, IL-4 and STAT6. Nat. Immunol. 2000;1:132–137. doi: 10.1038/77811. [DOI] [PubMed] [Google Scholar]
- 77.Nakanishi K, et al. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 78.Di Nardo A, et al. Cathelicidin antimicrobial peptides block dendritic cell TLR4 activation and allergic contact sensitization. J. Immunol. 2007;178:1829–1834. doi: 10.4049/jimmunol.178.3.1829. [DOI] [PubMed] [Google Scholar]
- 79.Morioka Y, et al. Cathelicidin antimicrobial peptides inhibit byaluronan-induced cytokine release and modulate chronic allergic dermatitis. J. Immunol. 2008;181:3915–3922. doi: 10.4049/jimmunol.181.6.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mookherjee N, et al. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J. Immunol. 2006;176:2455–2464. doi: 10.4049/jimmunol.176.4.2455. [DOI] [PubMed] [Google Scholar]
- 81.Mookherjee N, et al. Bovine and human cathelicidin cationic host defense peptides similarly suppress transcriptional responses to bacterial lipopolysaccharide. J. Leukoc. Biol. 2006;80:1563–1574. doi: 10.1189/jlb.0106048. [DOI] [PubMed] [Google Scholar]
- 82.Tydell CC, et al. Bovine peptidoglycan recognition protein-S: antimicrobial activity, localization, secretion, and binding properties. J. Immunol. 2006;176:1154–1162. doi: 10.4049/jimmunol.176.2.1154. [DOI] [PubMed] [Google Scholar]
- 83.Yibin G, et al. A synthesized cationic tetradecapeptide from hornet venom kills bacteria and neutralizes lipopolysaccharide in vivo and in vitro. Biochem. Pharmacol. 2005;70:209–219. doi: 10.1016/j.bcp.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 84.Rosenfeld Y, et al. Endotoxin (lipopolysaccharide) neutralization by innate immunity host-defense peptides. Peptide properties and plasible modes of action. J. Biol. Chem. 2006;281:1636–1643. doi: 10.1074/jbc.M504327200. [DOI] [PubMed] [Google Scholar]
- 85.Chen X, et al. Topomimetics of amphipathic beta-sheet and helix-forming bactericidal peptides neutralize lipopolysaccharide endotoxins. J. Med. Chem. 2006;49:7754–7765. doi: 10.1021/jm0610447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hailborn JD, et al. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J. Invest. Dermatol. 2003;120:379–389. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- 87.Baroni A, et al. Antimicrobial human beta-defensin-2 stimuletes migration, proliferation and tube formation of human umbilical vein endothelial cells. Peptides. 2009;32:267–272. doi: 10.1016/j.peptides.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 88.Gallo RL, et al. Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc. Natl. Sci. U. S. A. 1994;91:11035–11039. doi: 10.1073/pnas.91.23.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tokumaru S, et al. Induction of karatinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J. Immunol. 2005;176:4662–4668. doi: 10.4049/jimmunol.175.7.4662. [DOI] [PubMed] [Google Scholar]
- 90.Carretero M, et al. In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J. Invest. Dermatol. 2008;128:223–236. doi: 10.1038/sj.jid.5701043. [DOI] [PubMed] [Google Scholar]
- 91.Koczulla R, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J. Clin. lnvest. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park HJ, et al. Collagen synthesis is suppressed. in dermal fibroblasts by the human antimicrobial peptide LL-37. J. Invest. Dermatol. 2008 doi: 10.1038/jid.2008.320. DOI: 10.1038/jid.2008.320 (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ong PY, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 94.Fellermann K, et al. A chromosome 8 gene-cluster polymorphism with low human beta-defensin 2 gene copy number predisposes to Crohn disease of the colon. Am. J. Hum. Genet. 2006;79:439–448. doi: 10.1086/505915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Linzmeier RM, Ganz T. Human defensin gene copy number polymorphisms: comprehensive analysis of independent variation in alpba- and beta-defensin regions at 8p22-p23. Genomics. 2005;86:423–430. doi: 10.1016/j.ygeno.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 96.Yamasaki K, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat. Med. 2007;13:975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- 97.Lande R, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 98.Ma HL, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J. Clin. Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wilson NJ, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 100.Islam D, et al. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat. Med. 2001;7:180–185. doi: 10.1038/84627. [DOI] [PubMed] [Google Scholar]
- 101.Sieprawska-Lupa M, et al. Degradation of human antimicrobial peptide LL37 by Staphylococcus aureus-derived proteinases Antimicrob. Agents Chemother. 2004;48:4673–4679. doi: 10.1128/AAC.48.12.4673-4679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lai Y, et al. The human anionic antimicrobial peptide dermcidin induces proteolytic defence mechanisms in staphylococci. Mol. Microbiol. 2007;63:497–506. doi: 10.1111/j.1365-2958.2006.05540.x. [DOI] [PubMed] [Google Scholar]
- 103.Peschel A, et al. Staphylococcus aureus resistance to human defensins and evasion of neutrophil killing via the noval virulence factor MprF is based on modification of membrane lipids with l-lysine. J. Exp. Med. 2001;193:1067–1076. doi: 10.1084/jem.193.9.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li M, et al. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol. Microbiol. 2007;66:1136–1147. doi: 10.1111/j.1365-2958.2007.05986.x. [DOI] [PubMed] [Google Scholar]
- 105.Li M, et al. Gram-positive three-component antimicrobial peptide-sensing system. Proc. Natl. Acad. Sci. U. S. A. 2007;104:9469–9474. doi: 10.1073/pnas.0702159104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gunn JS, et al. Genetic and functional analysis of a PmrA-PmrB-regulated locus necessary for lipopolysaccharide modification, antimicrobial peptide resistance, and oral virulence of Salmonella enterica serovar typhimurium. Infect. Immun. 2000;68:6139–6146. doi: 10.1128/iai.68.11.6139-6146.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weidenmaier C, Peschel A. Teichoic acids and related cell-wall glycopolymers in Gram-positive physiology and host interactions. Nat. Rev. Microbiol. 2008;6:276–287. doi: 10.1038/nrmicro1861. [DOI] [PubMed] [Google Scholar]
- 108.Kristian SA, et al. D-alanylation of teichoic acids promotes group a streptococcus antimicrobial peptide resistance, neutrophil survival, and epithelial cell invasion. J. Bacteriol. 2005;187:6719–6725. doi: 10.1128/JB.187.19.6719-6725.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bader MW, et al. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell. 2005;122:461–472. doi: 10.1016/j.cell.2005.05.030. [DOI] [PubMed] [Google Scholar]
- 110.Guarna M. Marta, et al. Anti-inflammatory activity of cationic peptides: application to the treatment of acne vulguris. FEMS Microbiol. Lett. 2006;257:1–6. doi: 10.1111/j.1574-6968.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 111.Frohm M, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 1997;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 112.Fahlgren A, et al. beta-Defensin-3 and -4 in intestinal epithelial cells display increased mRNA expression in ulcerative colitis. Clin. Exp. Immunol. 2004;137:379–385. doi: 10.1111/j.1365-2249.2004.02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wehkamp J, et al. Inducible and constitutive beta-defensins are differentially expressed in Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 2003;9:215–223. doi: 10.1097/00054725-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 114.Boman HG. Innate immunity and the normal microflora. Immunol. Rev. 2000;173:5–16. doi: 10.1034/j.1600-065x.2000.917301.x. [DOI] [PubMed] [Google Scholar]
- 115.Ghosh D, et al. Paneth cell trypsin is the processing enryme for human defensm-5. Nat. Immunol. 2002;3:583–590. doi: 10.1038/ni797. [DOI] [PubMed] [Google Scholar]
- 116.Diamond G, et al. Transcriptional regulation of beta-defensin gene expression in tracheal epithelial cells. Infect. Immun. 2000;68:113–119. doi: 10.1128/iai.68.1.113-119.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]