Abstract
Purpose
To evaluate whether (i) Wnt5a expression in pancreatic cancer and malignant melanoma cells might be associated with constitutive levels of Toll-Like Receptor (TLR)-3 and/or TLR3 signaling (ii) phenylmethimazole (C10), a novel TLR signaling inhibitor, could decrease constitutive Wnt5a and TLR3 levels together with cell growth and migration, and (iii) the efficacy of C10 as a potential inhibitor of pancreatic cancer and malignant melanoma cell growth in vivo.
Experimental Design
We used a variety of molecular biology techniques including but not limited to PCR, Western, and ELISA to evaluate the presence of constitutively activated TLR3/Wnt5a expression and signaling. MTT-based technology and scratch assays were used to evaluate inhibition of cell growth and migration, respectively. TLR3 regulation of cell growth was confirmed using siRNA technology. Nude and SCID mice were implanted with human pancreatic cancer and/or melanoma cells and the effects of C10 on tumor growth were evaluated.
Results
We show that constitutive TLR3 expression is associated with constitutive Wnt5a in human pancreatic cancer and malignant melanoma cell lines, that C10 can decrease constitutive TLR3/Wnt5a expression and signaling, suggesting that they are interrelated signal systems, and that C10 inhibits growth and migration in both of these cancer cell lines. We also report that C10 is effective at inhibiting human pancreatic cancer and malignant melanoma tumor growth in vivo in nude or SCID mice, and associate this with inhibition of STAT3 activation.
Conclusions
C10 may have potential therapeutic applicability in pancreatic cancer and malignant melanoma.
Introduction
Pancreatic cancer and malignant melanoma are difficult to treat and have poor prognoses. The American Cancer Society estimates that 37,680 people will have been diagnosed with pancreatic cancer in 2008, with an expected death rate of 92%. It is the fourth leading cause of cancer deaths in the United States and has an overall survival rate of less than 4%; most die within 6 months to 1 year from time of diagnosis. Malignant melanoma exceeds many other types of cancers in lost “years of life” because it is most prevalent in younger individuals. The poor prognosis is attributable to a highly invasive nature, metastases before discovery, and a poor response to chemotherapeutic and/or surgical intervention. Uncovering a potentially effective treatment for both carcinoma of the pancreas and malignant melanoma is, therefore, of importance, particularly if the therapy has a novel molecular basis and is applicable to both.
The Wingless (Wnt) family of secreted glycoproteins control early developmental processes including cellular migration, differentiation, and proliferation [reviewed in (1)]. “Cannonical” Wnts modulate cell growth by increasing β-catenin levels, β-catenin nuclear localization, and binding to the LEF/TCF family of transcription factors, which can trigger the expression of genes controlling cell growth (2–4). Non-canonical (β-catenin-independent) Wnt signaling is thought to modulate cell proliferation by inducing the release of intracellular Ca2+ and activating both protein kinase C (PKC) (5) and calcium-calmodulin kinase II (CaMKII). Non-canonical Wnt5a is up-regulated in many types of human cancers, including malignant melanoma and pancreatic cancer (6–9). The role of Wnt5a in oncogenesis is, however, not fully understood. On the one hand, Wnt5a is categorized as a nontransforming Wnt in studies in the C57MG mouse mammary epithelial cell (10–12) ; on the other, a recent study suggests that Wnt5a activation of PKC contributes to enhanced motility and invasiveness of malignant melanoma cells (7).
Recently, we demonstrated that high constitutive Wnt5a might be linked to high constitutive TLR3 signaling and that the TLR3/Wnt5a association might be important in cancer cell growth and migration (13). Toll-like receptors (TLR) on immune cells are the basis of our multigenic, innate immune, inflammation response to signature molecules of environmental pathogens that cause tissue damage; they signal an important host defense mechanism (14). Inappropriate TLR expression in non-immune cells has, however, now been associated with disease expression (13, 15–23), for example, TLR3 was identified in thyrocytes and its overexpression associated with Hashimoto’s thyroiditis (16). TLR3 recognize dsRNA and activate genes that increase inflammatory cytokines and co-stimulatory molecules important for cell growth, as well as immune cell interactions (13, 16, 24). The dsRNA interaction with TLR3 on thyrocytes activated two distinct but critical signal pathways: the NF-κB/MAP kinase signal transduction pathway and the interferon (IFN) regulatory factor (IRF)-3 path producing IFN-β. Increased TLR3 signaling activates Signal Transducers and Activators of Transcription (STATs).
We have hypothesized that (i) TLR3-mediated activation of STAT3 might contribute to the increases in Wnt5a and to the growth and migration of PTC; (ii) TLR3 might be important in other Wnt5a-expressing cancers; and (iii) Phenylmethimazole (C10), a small molecule inhibitor that we have shown can decrease TLR3/Wnt5a expression and signaling, together with the growth and migration of PTC cells, might be useful to inhibit the growth and migration of other tumor cells with high TLR3 and Wnt5a (13). In support of these hypotheses, chronic inflammation is now recognized as an important risk factor for the development of certain cancers (25–27). Inflammation is associated with the presence of many TLR-associated pro-inflammatory cytokines, e.g. TNF-α and IL-6. IL-6 is important for the activation of STAT3, a key regulator of cancer cell growth, survival, metastasis, immune evasion and angiogenesis [reviewed in (28)]; activated STAT3 is associated with multiple types of cancers, including malignant melanoma and pancreatic cancer. Phenylmethimazole, structurally related to a drug used to treat Graves’ Disease, methimazole (MMI), was selected for its ability to suppress abnormal major histocompatibility Class I gene expression in autoimmune disease but not thyroid function (16); it appears to do this by inhibiting TLR3 signaling, including STAT activation (13, 16), in the absence of constitutively expressed Wnt5a (16, 29).
In this report, we questioned whether (i) Wnt5a expression in pancreatic cancer and malignant melanoma cells (6–9) might be associated with constitutive levels of TLR3 and/or TLR3 signaling, (ii) C10 could decrease constitutive Wnt5a and TLR3 levels in association with cell growth and migration, and (iii) C10 could inhibit pancreatic and malignant melanoma cell growth in vivo. We show that (i) constitutive TLR3 expression is associated with constitutive Wnt5a in at least some human pancreatic cancer and malignant melanoma cell lines, (ii) C10 can decrease constitutive TLR3/Wnt5a expression and signaling, again suggesting that they are interrelated signal systems, and (iii) C10 inhibits growth and migration in these pancreatic cancer and melanoma cells. Further, we show that C10 is effective at inhibiting human pancreatic tumor and malignant melanoma tumor growth in vivo in nude or SCID mice and associate this with inhibition of STAT3 activation. We suggest that C10 may have potential therapeutic applicability.
Materials & Methods
Materials
Anti-phospho-STAT3Ser727, recombinant IL-6 and the IL-6 ELISA were from BioSource International. Anti-phospho-STAT3Tyr705 and anti-β-Actin were from Cell Signaling, Poly (I:C) [a synthetic dsRNA], psiRNA-hTLR3, psiRNA-hTLR4, and psiRNA-Scrambled from InvivoGen. C10 was a gift of the Interthyr Corporation (Athens, OH) and was prepared as 200 mM stock solution in DMSO (Sigma-Aldrich). The source of all other materials was the same as previously reported or is noted below (13, 16).
Cells
The melanoma cell lines UACC647, UACC1273EV, and M93-047 have been described (10). All melanoma and BXPC-3 cells were grown in RPMI 1640 medium supplemented with 2 g/L sodium bicarbonate, 1.4 mM sodium pyruvate, 0.14 mM non-essential amino acids, and 10% fetal bovine serum, pH 7.2. PANC-1 & BXPC-3 cells were generously provided by Dr. Duxin Sun (The Ohio State University, Columbus, OH). PANC-1 and HEK 293 cells were grown in DMEM supplemented with 10% fetal bovine serum. WRO 82-1 and ARO 81-1 cells provided by Dr. Guy Juillard (UCLA, CA) were maintained as previously described (13).
PANC-1 cell lines stably over-expressing siRNA against human TLR3, TLR4, or a scrambled control sequence (negative) were made by transfection with either psiRNA-hTLR3, psiRNA-hTLR4, or psiRNA-Scrambled using LF2000 Reagent (Invitrogen). Two days post-transfection, Zeocin (100 µg/ml) was added to select for plasmid containing cells; and stable transfectants isolated following 2 weeks in Zeocin (100 µg/ml). Clones were analyzed for siRNA expression using standard RT-PCR methods. The rate of cell proliferation was measured using the BrdU Cell Proliferation Assay Kit (Chemicon International).
Real-Time PCR
Total RNA was isolated using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. DNA was removed from total RNA during total RNA isolation, using the RNase-Free DNase set (Qiagen). Human TLR3, Wnt5a, CXCL10, IFNβ, and IL-6 expression levels were measured by qRT2-PCR with gene specific primers designed using the Plexor primer design software (Promega), and the Plexor One-Step qRT-PCR System (Promega) according to the manufacturer’s instructions. For accuracy, human GAPDH was co-amplified with TLR3, Wnt5a, CXCL10, IFNβ, and IL-6 each in separate multiplex reactions. Human GAPDH primers were obtained from BIOSEARCH Technologies. The delta Ct method of real-time PCR analysis was used for comparison of values.
RT-PCR
Total RNA was isolated using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. DNA was removed from total RNA using the DNA-free Kit (Ambion) according to the manufacturer’s instructions. A total of 1 µg of RNA was then used to synthesize cDNA using the Advantage RT-for-PCR Kit (BD Biosciences) according to the manufacturer’s protocol. A total of 50 ng of cDNA was subsequently used for PCR of TLR-3, β-Actin, and Wnt5a, and 250 ng of cDNA was used for IFN-β and CXCL10. These primers and reaction conditions used have been previously described (13).
Luciferase Assays and Plasmids
Plasmids were constructed and luciferase assays were performed as described (13, 16).
Western Blot Analysis
Nuclear proteins were isolated using the NE-PER Nuclear and Cytoplasmic Isolation kit (Pierce) according to the manufacturer’s instructions. Thirty (30) µg of protein was resolved on denaturing gels using the Nu-PAGE System (Invitrogen) unless otherwise noted. All proteins were transferred to nitrocellulose membranes and subsequent antibody binding was revealed using ECL Plus reagents (Amersham Parmacia Biotech).
ELISA Analysis
Cells were treated with either 0.5 mM C10 or equal amounts of DMSO for 24 hours. Cell supernatants were collected and IL-6 levels determined using a human IL-6 ELISA.
Quantification of Cell Growth
Cells were evenly seeded and grown on sterile 96-well plates. Cells were then treated with 0.5 mM C10 or equal amounts of DMSO for 24 hours. Cell growth was then quantified using the MTT-based In Vitro Toxicology Assay Kit (Sigma-Aldrich).
Scratch Assays
Scratch assays were performed as described (7). Briefly, confluent cells were scratched using a sterile pipette tip, treated or not with C10, and analyzed by digital photography for ability to heal the scratch.
Animals
All nude (NU/NU, Strain Code: 088) and SCID (C.B-17/Icr-Prkdc<scid>/Crl, Strain Code: 236) mice, 5 weeks of age, were obtained from Charles River Laboratories. All animals were housed in a clean animal facility and allowed free access to standard rodent chow (Harlan Teklad) and water ad libitum. The animal studies were approved by the Institutional Animal Care and Use Committee of Ohio University.
Mouse Tumor Studies
Nude or SCID mice were injected subcutaneously near the dorsal neck region with 500,000 PANC-1 or 1×106 M93-047 cells/mouse. At the time of injection and once daily thereafter, one group of mice was injected i/p with 10% DMSO (control solvent), another with 1 and/or 3mg/kg C10, and a final group either “mock” injected (i.e. received only mechanical puncturing i/p via the syringe) or untreated. The pancreatic cancer experiment was carried out for 50 days in total, whereas the melanoma experiments were carried out for 2 weeks.
Statistics
All experiments were replicated at least three times on different groups of cells. All data are expressed as mean ± standard deviation. Data in Figure 1C, Figure 2–Figure 4, Figure 6B & C were evaluated for statistical significance using one-way ANOVA, and statistical significance for comparison of means of different groups was calculated using the Tukey-Kramer multiple comparison post-hoc analysis using NCSS software. The differences were considered significant at P values indicated in the figure legends.
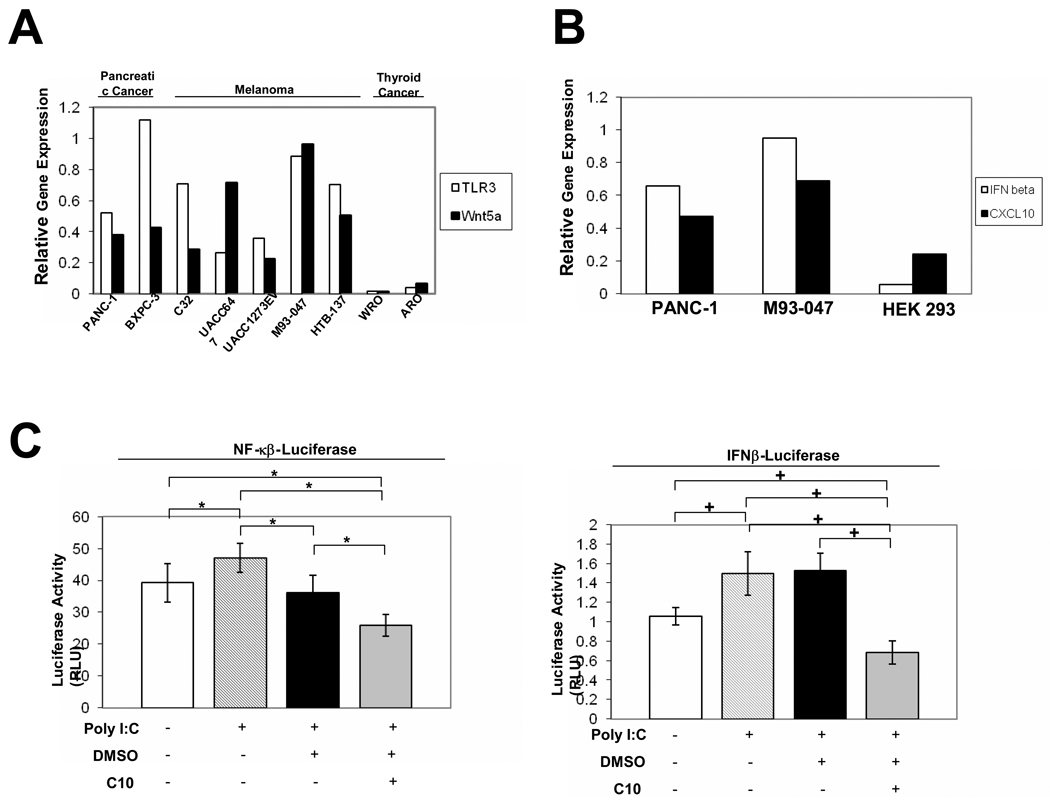
Fig. 1. TLR3, Wnt5a, IFN-β and CXCL10 are constitutively expressed and TLR3 is functional in cells derived from human pancreatic and melanoma tumors.
(A) The relative mRNA levels of TLR3 and Wnt5a in PANC-1, BXPC-3 pancreatic cancer, and in C32, UACC647, UACC1273EV, M93-047, and HTB-137 melanoma cells, and ARO and WRO, thyroid cancer cells as determined by semi-quantitative RT-PCR. (B) The mRNA levels of IFN-β and CXCL10 in PANC-1, M93-047, and HEK 293 cells as determined by semi-quantitative RT-PCR. (A & B) Bands from RT-PCR were quantitated using ImageGauge 3.12 software and data are represented as relative gene expression levels which were standardized against the internal control gene β-Actin. (C) Treatment with poly I:C activates NF-κB and IFN-β gene expression in PANC-1 cells. PANC-1 cells were transiently transfected with 100 ng of luciferase reporter plasmid pNF-kB Luc or pIFN-β Luc and 2 ng of internal control plasmid phRL-Tk. 24 hours post-transfection, cells were incubated with poly I:C (100 µg/ml) or poly I:C in addition to 0.25% DMSO, or 0.5 mM C10 for 6 H. Error bars represent standard deviations. The * represents P<0.05 and + represents P<0.001 significant differences between groups as indicated.
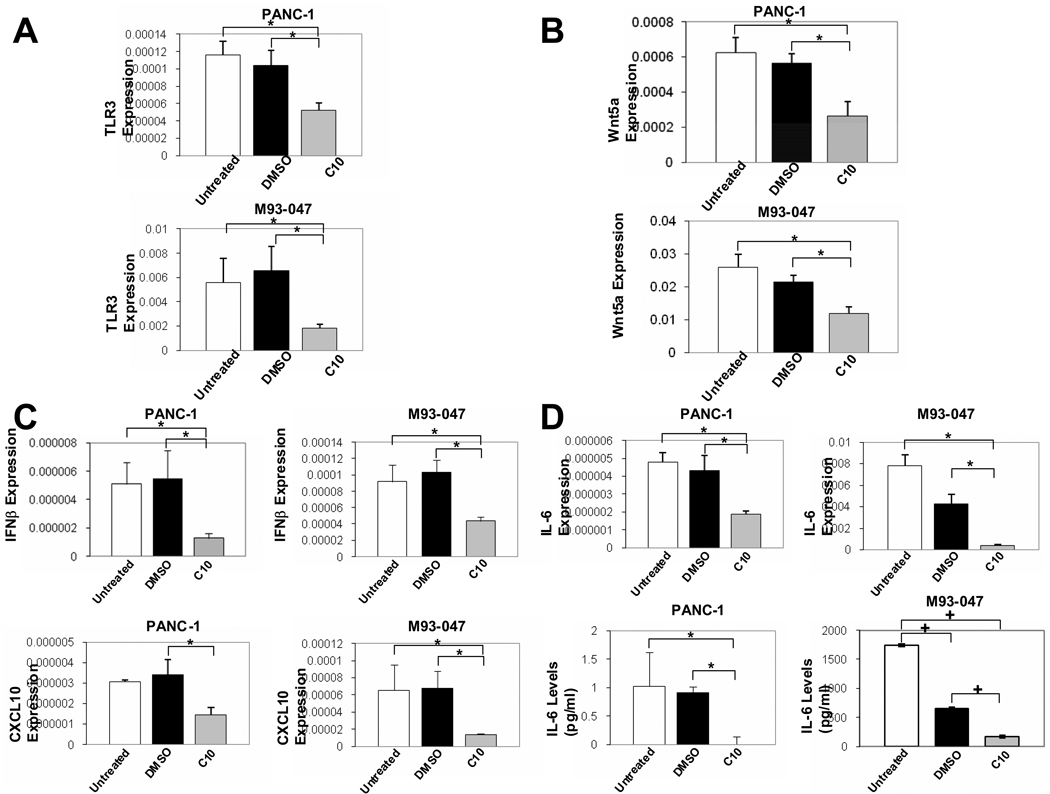
Fig. 2. C10 decreases constitutive TLR3, Wnt5a, CXCL10, IFNβ, and IL-6 RNA as well as IL-6 protein levels in human pancreatic cancer and melanoma cells.
PANC-1 and M93-047 cells were treated with 0.25% DMSO or 0.5 mM C10 for 24H. Real-time PCR was performed to evaluate TLR3, Wnt5a, CXCL10, IFN-β and IL-6 RNA levels (A–D) and IL-6-specific ELISA was performed to evaluate IL-6 protein levels (D). The * and + show significant differences between groups as indicated (*P<0.05 and + P<0.00000001).
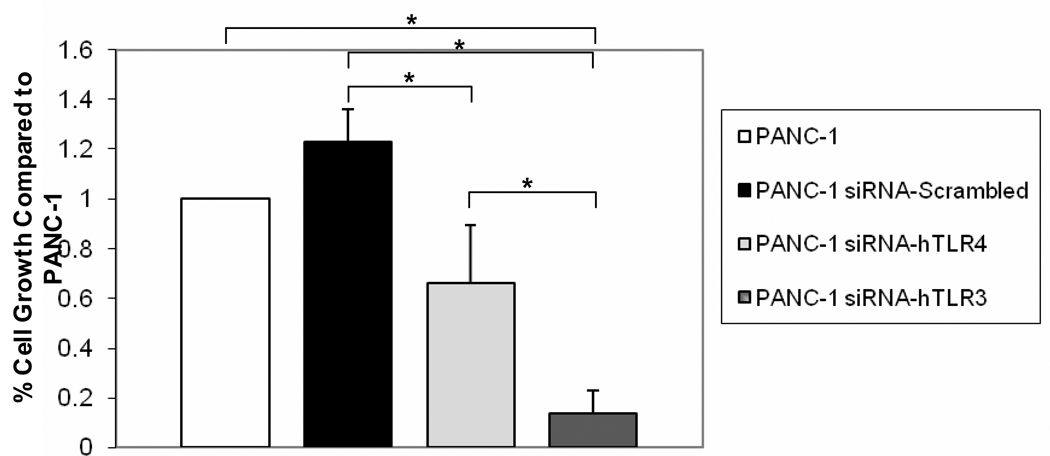
Fig. 4. TLR3 siRNA inhibits PANC-1 cell growth.
PANC-1 cells were stably transfected with siRNA against human TLR3, TLR4, or a control “scrambled” sequence. Cell growth rates for PANC-1 cells expressing siRNA against human TLR3, TLR4, and scrambled sequences were then compared to the growth rate of PANC-1 cells. Data are represented as % Cell Growth compared to control PANC-1 cells. Error bars represent standard deviations and the * shows significant differences between groups as indicated (P = 0.02).
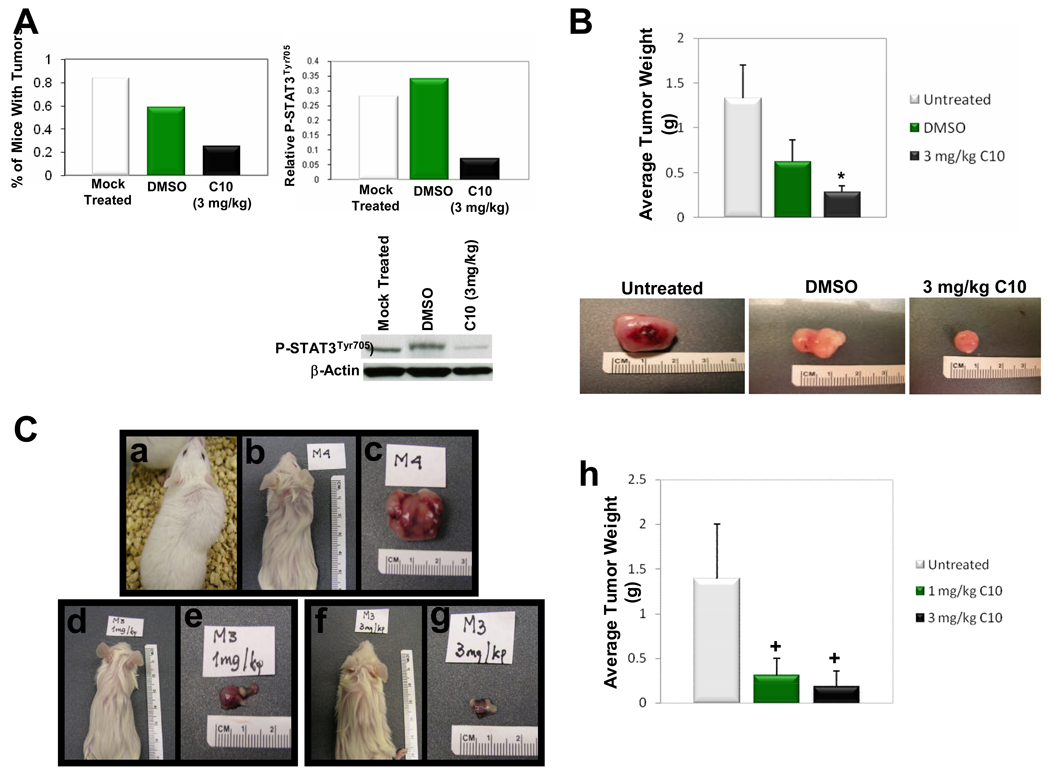
Fig. 6. C10 inhibits tumor growth in a nude mouse model of pancreatic cancer and in nude and SCID mouse models of human melanoma.
Each mouse in the pancreatic cancer nude mouse model was injected with 500,000 PANC-1 cells, and each nude and SCID mouse in the melanoma experiments received 1×106 melanoma cells. Cells were subcutaneously placed behind the neck. Mice were then either left untreated or treated with 10% DMSO, C10, or “mock” injected for 50 days in the pancreatic cancer experiment or 2 weeks in the melanoma experiments as indicated. “Mock” injection = insertion of a comparable syringe needle. (A) C10 largely prevented tumor formation and decreased phospho-STAT3Tyr705 levels in C10-treated animals. Bands from Western analysis were quantitated using ImageGauge 3.12 software and data are represented as relative gene expression levels which were standardized against the internal control gene β-Actin. N=12 mice per group. (B) Nude mice were injected with human melanoma cells as described above and treated as indicated. Data are represented as average tumor weight, and representative tumors from each group are also depicted. (C) SCID mice injected with human melanoma cells at 2 weeks post-injection. No abnormal changes in coat texture and/or appearance was observed in any mice as particularly illustrated by the mouse in panel (a) which has a large control tumor yet was active and was not cachectic. Mice displayed in (b), (d), and (f) have coats saturated with alcohol prior to tumor excision to allow better visualization of tumors. Panels (c), (e), and (g), are tumors excised from mice in panels (b), (d), and (f), respectively. Panel (h) presents average tumor mass per group of SCID mice. Error bars represent standard deviations and the * and + show significant differences between Untreated and C10-treated groups (*P <0.05 and +P <0.01).
Results
TLR3 and Wnt5a RNA Levels are Constitutively Expressed in Human Pancreatic Cancer and Melanoma Cell Lines
Wnt5a is expressed in a variety of human cancers (6–9). We analyzed expression of TLR3 and Wnt5a RNA in two human pancreatic cancer and five different human melanoma cell lines (Fig. 1A). RT-PCR revealed the expression of TLR3 and Wnt5a in 100% of the cell lines analyzed (Fig. 1A). The RT-PCR was confirmed by real-time PCR (data not shown). That these were high constitutive TLR3 and Wnt5a levels was confirmed by studies with C10 which decreased these levels significantly in all cases (see below), and by comparison to two purported thyroid cancer cell lines which do not express TLR3 or Wnt5a (Fig. 1A) (13).
Despite being expressed constitutively in all pancreatic and malignant melanoma cell lines tested, the levels of TLR3 and Wnt5a were not proportionately increased, but, rather, differed in their ratios in each tumor cell line (Fig. 1A). We had recognized and discussed this possibility in our previous report (13). In the following experiments, we characterize the functional significance of high constitutive TLR3 and its relationship to high Wnt5 using PANC1 and M93-047 as index cell lines; however, all cell lines were evaluated with similar results except where noted.
TLR3 is Functional in Human Pancreatic Cancer and Melanoma Cell Lines
Along with the high constitutive levels of TLR3, we could detect constitutive levels of two hallmark TLR3 signaling products, IFN-β and CXCL10. This is illustrated with the PANC-1 and M93-047 cells (Fig. 2C). This suggested that the TLR3 signaling system might be constitutively activated in these cells. That C10 decreased levels of IFN-β and CXCL10 together with TLR3 and Wnt5a support this suggestion (see below).
To further explore function, we questioned whether extracellular polyinosine-polycytidylic acid [poly (I:C)], which mimics the dsRNA ligand that binds to TLR3(24, 30–34), could activate both the NF-κB and the IRF-3/type I IFN (IFN-β) pathways in PANC-1 and M93-047 cells, as in thyrocytes and immune cells (13, 16, 24, 30–34). When poly I:C was incubated with pNF-κB-Luciferase-transfected cells and in IFN-β-Luciferase-transfected cells (Fig. 1C), it caused a statistically significant increase in NF-κB Luciferase activity by comparison to non-treated cells (Fig. 1C) or cells transfected with control plasmid alone (data not shown). Poly I:C incubation more significantly increased IFN-β promoter activity in PANC1 cells by comparison to non-treated cells (Fig. 1C) or cells transfected with control plasmid alone (data not shown). Similar results were seen in another pancreatic cell line (BXPC3) (data not shown); however, transfection difficulties prevented similar studies being completed in the human melanoma cell lines. The data in Fig. 1 & Fig. 2, which showed high constitutive levels of IFN-β and CXCL10 as well as TLR3, was, nevertheless, an indication of potential TLR3 functionality in the melanoma cell lines.
Phenylmethimazole (C10), can inhibit dsRNA signaling by its ability to inhibit the TLR3-mediated IRF-3/IFN-β pathway and can inhibit STAT activation in FRTL-5 thyrocytes (16). FRTL-5 thyrocytes do not express Wnt5a (29) suggesting the primary action of C10 is on TLR signaling. We took advantage of this C10 activity to explore the functional role of TLR3 in pancreatic cancer and malignant melanoma cells as well as the relationship between TLR3 and Wnt5a.
C10 inhibits TLR3 Expression and Signaling in Human Cancer Cells
Using (i) poly I:C-induced NF-κB activation in PANC-1 cells transfected with the NF-κB-Luciferase construct or (ii) poly I:C-induced promoter activity in IFN-β-Luciferase-transfected PANC-1 cells, we could show C10 significantly inhibited both by comparison to control solvent (DMSO)-treated cells (Fig. 1C).
Our previous studies showed that C10 decreased high constitutive TLR3 in the purported PTC cells and high levels of TLR3 induced by dsRNA transfection of normal thyrocytes(13, 16). We thus evaluated the effect of C10 on the high, constitutive levels of TLR3 RNA in the human pancreatic cancer cell line, PANC-1, and the human melanoma cell line M93-047. As was the case in PTC cells, we observed significant decreases in TLR3 RNA levels following C10 treatment in PANC-1 and M93-047 cells (Fig. 2A).
Also in agreement with our previous observations (13), C10 significantly decreased expression of Wnt5a RNA levels in PANC-1 and M93-047 cell lines (Fig. 2B), again exposing a potential functional relationship between the high constitutive Wnt5a and TLR3.
C10 treatment of PANC-1 and M93-047 cells also significantly decreased the products (24, 35–37) of the TLR3 signal, IFN-β and CXCL10 (Fig. 2C). In PANC-1 cells, CXCL10 expression was statistically different when comparing the solvent (DMSO) control and C10 treatment groups, despite the fact that no “statistical” difference was found between the untreated control group and the C10 treatment group (Fig. 2C). IL-6 is another TLR3 signaling product important for the growth and migration of cancer cells (24, 38, 39). Constitutive expression of IL-6 was significantly reduced by C10 in PANC-1 and M93-047 cells as determined by both real-time PCR (Fig. 2D) and by ELISA (Fig. 2D) analyses. C10 affect on constitutive IL-6 expression was dose-dependent; inhibition relative to control was 17.7% at 0.125 mM and 62.7% at 0.50 mM. In M93-047 cells, solvent (DMSO) treatment also decreased constitutive IL-6 protein levels compared to untreated cells; however, compared to DMSO, C10 significantly further reduced constitutive IL-6 levels. This phenomenon was not seen in PANC-1 cells (Fig. 2D).
The data in Fig. 1 & Fig. 2 support the conclusion that the constitutive levels of TLR3 are abnormally high in the pancreatic carcinoma and malignant melanoma cells as evident in the index cells, PANC-1 and M93-047 melanoma cells. In addition, as is the case in the purported PTC cells, TLR3 expression levels and TLR3 signaling can be effectively lowered by C10.
C10 Inhibits Pancreatic Cancer and Melanoma Growth and Migration and Down-Regulates Potential Downstream Signals of Growth Modulated by TLR3
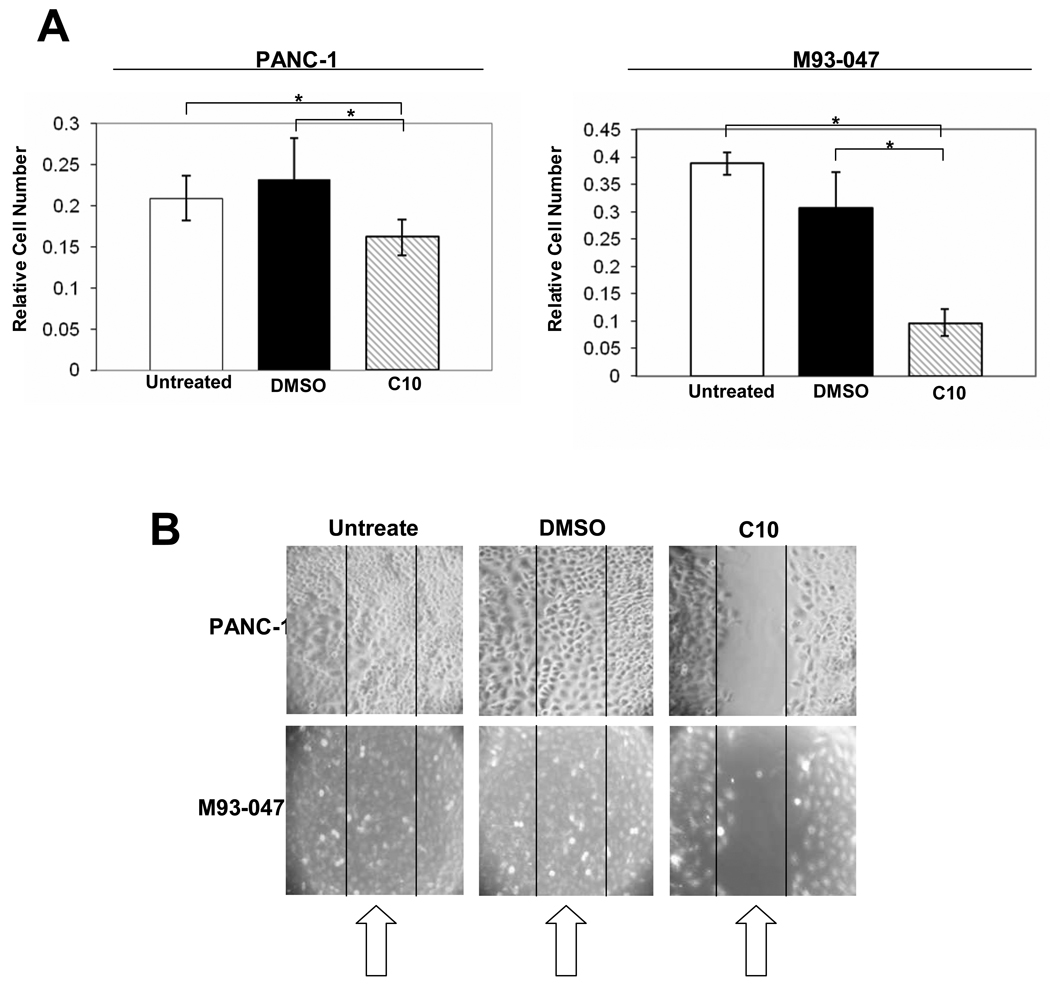
In addition to its effect on constitutive TLR3 and Wnt5a RNA levels and function, 0.5 mM C10 treatment of PANC-1 and M93-047 cells led to significant inhibition of cell growth (Fig. 3A). Furthermore, treatment of PANC-1 and M93-047 cells with 0.5 mM C10 led to significant inhibition of motility/migration as measured using scratch assays (Fig. 3B). In a scratch assay, migration to cover a scratched area on the plate surface is measured; retention of a visible scratch line as in Fig. 3B (+C10) is evidence of inhibition of cell migration (7). Effects in both assays were evident with 0.1 and 0.25 mM C10 treatment and maximal at 0.5 mM (data not shown); thus the 0.5 mM concentration was used in all previous and following in vitro experiments.
Fig. 3. C10 inhibits proliferation and migration of human pancreatic cancer and melanoma cells.
Cell proliferation (A) and migration (B) were measured, following a 24 hour treatment with either 0.25% DMSO or 0.5 mM C10. Error bars represent standard deviations and the * shows significant differences between groups as indicated (P<0.00001).
To ensure that TLR3 was driving cell growth, we transfected cells with TLR3 siRNA. Compared to the control “scrambled” siRNA PANC-1 cell line and normal PANC-1 cell line, PANC-1 cells expressing the TLR3 siRNA grew significantly slower than control cells as measured by BrdU incorporation (Fig. 4). Interestingly, PANC-1 cells expressing the TLR4 siRNA also grew significantly slower than “scrambled” siRNA PANC-1 cells, however they did not grow significantly slower than normal PANC-1 cells (Fig. 4). In addition, PANC-1 cells expressing the TLR3 siRNA grew significantly slower than the PANC-1 cells expressing TLR4 siRNA (Fig. 4), suggesting that TLR3 signaling is more important for proliferation of PANC-1 cells than TLR4 signaling. Compared to control cells transfected with scrambled siRNA, TLR3 siRNA and TLR4 siRNA inhibited STAT3 phosphorylation together with, and apparently proportional to, their effects on growth (data not shown).
Activated STAT3 (P-STAT3) is known to mediate uncontrolled cell growth in many types of human cancers; and IL-6, a pro-inflammatory cytokine produced as a result of active TLR signaling, is known to activate STAT3 [reviewed in (28)]. STAT3 is phosphorylated on multiple residues for activation; phosphorylation of both its tyrosine 705 and serine 727 residues are necessary for maximal transcriptional activity (40–43). Based on our findings that C10 significantly reduces TLR3 gene expression and TLR3 signaling and with the knowledge that IL-6 is a product of the TLR signaling pathway, we asked if C10 inhibited PANC-1 and M93-047 constitutive STAT3 and IL-6-induced activation.
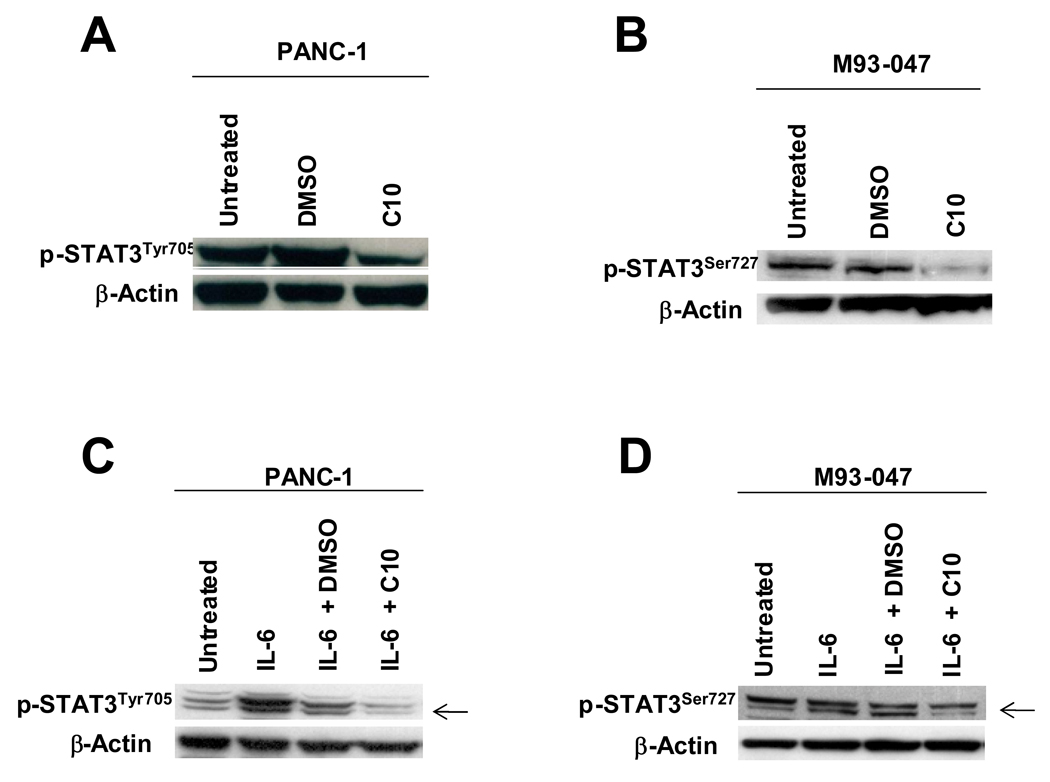
Constitutive STAT3 phosphorylation was detected in both cell lines. We observed large decreases in phosphorylated STAT3Tyr705 in PANC-1 and STAT3Ser727 in M93-047 cells with 0.5 mM C10 treatment (Fig. 5); likewise, C10 treatment produced a large decrease in IL-6-induced phosphorylated STAT3Tyr705 protein levels in PANC-1 cells and in phosphorylated STAT3 (S727) protein levels in M93-047 cells (Fig. 5). C10 inhibition of IL-6-induced phosphorylated STAT3 protein levels was dose dependent (data not shown).
Fig. 5. C10 inhibits constitutive and IL-6-induced STAT3 phosphorylation in human pancreatic cancer and melanoma cells.
In (A & B), PANC-1 and M93-047 cells were treated with 0.25% DMSO or 0.5 mM C10 for 6H. Nuclear protein was then extracted and constitutive phospho-STAT3Tyr705 and phospho-STAT3Ser727 were analyzed using Western analysis. In (C& D), PANC-1 and M93-047 cells were pre-treated for 1 hour with or without 0.25% DMSO or 0.5 mM C10 and then treated or not for 10 minutes with 20 ng/ml human IL-6. Nuclear protein was then extracted and phospho-STAT3Tyr705 and phospho-STAT3Ser727 evaluated by Western analysis.
Interestingly, C10 did not affect STAT3Ser727 phosphorylation in PANC-1 nor STAT3Tyr705 in M93-047 cell lines reflecting individual variations of STAT3 phosphorylation among different cancers. Phosphorylation of STAT3 at serine 727 and tyrosine 705 is mediated by a variety of kinase signaling pathways in response to various stimuli. STATs are known to be phosphorylated at tyrosine residue 705 by JAKs, and at serine residue 727 by members of the MAPK and JNK family of serine kinases. Rho GTPases (RhoA, Cdc42, and Rac1) can efficiently modulate STAT3 transcriptional activity by inducing the simultaneous phosphorylation of tyrosine 705 and serine 727. It is unclear at this time why C10 differentially regulates STAT3 phosphorylation of tyrosine and serine residues in PANC-1 and M93-047 cells. Of note, separate studies conducted in cells purported to be PTC cells, but now recognized to be melanoma and colon cancer cells, revealed significant inhibition of STAT3 phosphorylation at both serine 727 and tyrosine 705, which correlated with inhibition of cell growth and migration (13). Preliminary studies, revealed no effects of C10 on AKT and MAPK activation (data not shown). Additional studies are in progress to investigate the mechanism of this differential regulation of STAT3 phosphorylation by C10 in these tumor cell lines.
Despite C10 inhibition of phosphorylation on different sites on the STAT3 molecule, inhibition of either should result in similar downregulation of STAT3 transcriptional activity as noted in the literature (40–43). Of note, despite the significant reduction in constitutive IL-6 protein levels by DMSO in M93-047 cells (Fig. 2D), DMSO had no effect on STAT3 phosphorylation, growth or migration of these cells (Fig. 3 & Fig. 5). DMSO has been reported to affect protein turnover (44); this was not evaluated here.
C10 Inhibits Tumor Growth and STAT3 Phosphorylation In Vivo
As an initial validation of the relevance of C10’s ability to inhibit cancer cell growth in vivo in tumors which pathologically express TLR3 and down-stream signaling, we evaluated the effect of C10 on human pancreatic and melanoma tumor growth in multiple mouse models. Both PANC-1 and M93-047 cells were injected subcutaneously with C10, at doses shown to be effective when given prenterally in other animal models wherein TLR signaling is important for disease expression (colitis, toxic shock, etc) (45, 46). As can be seen C10 was able to considerably inhibit the number of tumors in human pancreatic implants as only three of twelve animals even exhibited tumors (Fig. 6A). Consistent with the effects of C10 in PANC-1 cells in vitro, it also significantly reduced phosphorylated STAT3Tyr705 levels in the pancreatic tumors in these mice (Fig. 6A). In the melanoma models (Fig. 6B & C) tumor number was not decreased; however, the size of the tumors was significantly decreased. C10 had no effect on coat texture, food intake, nor did it cause systemic toxicity [Fig. 6C, Panel (a) and legend]. Given daily for as long as 3 months, there has been no evidence of thyroid function changes, changes in TSH levels, liver or kidney toxicity with C10 treatment.
Discussion
In previous work in which we studied cells purported to be papillary thyroid carcinomas (but now known to be melanoma cells) we demonstrated that high constitutive levels of TLR3 and Wnt5a are coordinately expressed and regulated, functional and linked to cell growth and migration (13). We also found that phenylmethimazole (C10), which decreases TLR3 levels and signaling in FRTL-5 thyrocytes [cells which do not express Wnt5a (16, 29)], reduces Wnt5a as well. This report offers several novel observations based on the hypothesis that this might not be a unique phenomenon but typical of many tumor cells reported to have high Wnt5a levels in vivo and in vitro. First, we show that pancreatic cancer and melanoma cells derived from human tumors also coordinately express high constitutive levels of functional TLR3 and Wnt5a. Second, we show for the first time that the high constitutive levels of both TLR3 and Wnt5a in human pancreatic cancer and melanoma cells are returned toward normal levels by treatment with C10, which acts on TLR3 signaling in the presence or absence of Wnt5a (13, 16, 29). Third, C10 decreased Wnt5a and TLR3 levels are associated with decreased STAT3 activation as well as pancreatic cancer and melanoma cell growth and motility in vitro. Last, C10 inhibits tumor growth in vivo in a mouse model of human pancreatic cancer and mouse models of malignant melanoma.
A role for TLR3 in cancer progression has been described and has been shown to signal via intermediates such as PKC and STAT3 in multiple cell types (13, 47). The relationship between IL-6, STAT3 activation, PKC, and Wnt5a levels appears to be more cell specific. In cardiac myocytes and thyrocytes, IL-6 activated STAT3 can increase Wnt5a (13, 48). In contrast, IL-6 increases in melanoma cells have smaller effects on STAT3 activation, due to high levels of SOCS3 expression, yet Wnt5a requires STAT3 to effect its downstream signal transduction (49). Both PKC and STAT3 have been shown to increase, and be increased by, Wnt5a (13, 48, 49). Our previous work in the purported papillary thyroid cancer cells (13) led us to generate a speculative model in which an inciting event can cause carcinoma cells to increase TLR3. TLR3 signal generation via TRIF/TICAM-1/IRF-3 increases IFN-β which can act as an autocrine/paracrine factor to further increase TLR3 (16). TLR3-induction of both the NF-κB and IFN-β signal pathways contribute to increases in IL-6 (31, 50) and the subsequent activation of STAT3, which then increases Wnt5a. C10 inhibits the TLR3 activated IRF-3 signaling, IRF-3-dependent increase in IFN-β, and the TLR3-induced increase in STAT3 phosphorylation. Phosphorylated STAT3 appears critical to both the increase in Wnt5a as well as the growth and motility of the cells; cytokines and chemokines resultant from over-expressed TLR3 signaling are important in this process. The data presented herein supports this speculative model and hypotheses, suggesting that TLR3 and consequently Wnt5a signaling are important mediators of cell growth and migration in a subset of human cancers.
It has long been recognized that Wnt5a is over-expressed in many types of human cancers including, but not limited to, malignant melanoma, breast, and prostate cancer (6–8), however its role in these cancers is not yet known. Interestingly, the progression of these cancers is thought to be controlled by the activation of STAT3 (7, 13, 28, 49). We show here that C10 is able to inhibit (i) constitutive TLR3 expression and signaling, (ii) activation of STAT3, (iii) growth and migration of human pancreatic cancer and melanoma cells in culture, and (iv) pancreatic cancer and human melanoma tumor growth in mouse models. Taken together, this data is highly suggestive that TLR3 signaling is important for regulating cell growth, Wnt5a expression, and cell motility in a variety of human cancers and that TLR3 and Wnt5a signaling systems are interrelated. These data open new doorways for studies of cancers which appear to involve TLR3/Wnt5a signaling, and raise the possibility that C10 may have potential efficacy as an anti-cancer agent in tumors which express high constitutive levels of TLR3 and Wnt5a.
Translational Relevance
Pancreatic cancer and malignant melanoma have poor prognoses because of their highly invasive nature, metastases before discovery, and limited response to chemo- or immune-therapeutic intervention. This report describes a novel therapeutic approach applicable to both pancreatic cancer and malignant melanoma. We show that (i) TLR3, like Wnt5a, is constitutively expressed in human pancreatic cancer and malignant melanoma cells, (ii) phenylmethimazole (C10), an inhibitor of TLR3 signaling, can decrease constitutive TLR3 and Wnt5a expression and signaling, suggesting they are interrelated signal systems, (iii) C10 significantly inhibits the growth of human pancreatic cancer and melanoma tumor cells in vitro, and (iv) C10 significantly inhibits the growth of pancreatic cancer and malignant melanoma in vivo in nude and SCID mouse models, in association with inhibition of STAT3 activation. Inhibitors of abnormally expressed, TLR3-induced, innate immune signaling in non-immune cells, by agents like C10, may be useful therapeutics in pancreatic cancer and malignant melanoma.
Acknowledgments
This work was supported, in part, by funds from the Intramural Research Program of the National Institute on Aging and from an STTR grant from the National Institutes of Allergy and Infectious Diseases, NIH, Baltimore, MD to the Interthyr Corporation and Ohio University.
References
- 1.Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11(24):3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 2.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis--a look outside the nucleus. Science. 2000;287(5458):1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 3.Chan SK, Struhl G. Evidence that Armadillo transduces wingless by mediating nuclear export or cytosolic activation of Pangolin. Cell. 2002;111(2):265–280. doi: 10.1016/s0092-8674(02)01037-1. [DOI] [PubMed] [Google Scholar]
- 4.Bienz M, Clevers H. Armadillo/beta-catenin signals in the nucleus--proof beyond a reasonable doubt? Nat Cell Biol. 2003;5(3):179–182. doi: 10.1038/ncb0303-179. [DOI] [PubMed] [Google Scholar]
- 5.Kuhl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000;16(7):279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- 6.Iozzo RV, Eichstetter I, Danielson KG. Aberrant expression of the growth factor Wnt-5A in human malignancy. Cancer Res. 1995;55(16):3495–3499. [PubMed] [Google Scholar]
- 7.Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, et al. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1(3):279–288. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 8.Saitoh T, Katoh M. Expression and regulation of WNT5A and WNT5B in human cancer: up-regulation of WNT5A by TNFalpha in MKN45 cells and up-regulation of WNT5B by beta-estradiol in MCF-7 cells. Int J Mol Med. 2002;10(3):345–349. [PubMed] [Google Scholar]
- 9.Pham K, Milovanovic T, Barr RJ, Truong T, Holcombe RF. Wnt ligand expression in malignant melanoma: pilot study indicating correlation with histopathological features. Mol Pathol. 2003;56(5):280–285. doi: 10.1136/mp.56.5.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu H, Julius MA, Giarre M, Zheng Z, Brown AM, Kitajewski J. Transformation by Wnt family proteins correlates with regulation of beta-catenin. Cell Growth Differ. 1997;8(12):1349–1358. [PubMed] [Google Scholar]
- 11.Wong GT, Gavin BJ, McMahon AP. Differential transformation of mammary epithelial cells by Wnt genes. Mol Cell Biol. 1994;14(9):6278–6286. doi: 10.1128/mcb.14.9.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olson DJ, Papkoff J. Regulated expression of Wnt family members during proliferation of C57mg mammary cells. Cell Growth Differ. 1994;5(2):197–206. [PubMed] [Google Scholar]
- 13.McCall KD, Harii N, Lewis CJ, Malgor R, Kim WB, Saji M, Kohn AD, Moon RT, Kohn LD. High Basal Levels of Functional Toll-Like Receptor 3 (TLR3) and Non-Cannonical Wnt5a Are Expressed in Papillary Thyroid Cancer (PTC) and Are Coordinately Decreased by Phenylmethimazole Together with Cell Proliferation and Migration. Endocrinology. 2007;148(9):4226–4237. doi: 10.1210/en.2007-0459. [DOI] [PubMed] [Google Scholar]
- 14.Underhill DM. Toll-like receptors: networking for success. Eur J Immunol. 2003;33(7):1767–1775. doi: 10.1002/eji.200324037. [DOI] [PubMed] [Google Scholar]
- 15.Anders HJ, Banas B, Schlondorff D. Signaling danger: toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol. 2004;15(4):854–867. doi: 10.1097/01.asn.0000121781.89599.16. [DOI] [PubMed] [Google Scholar]
- 16.Harii N, Lewis C, Vasko V, McCall K, Benavides-Peralta U, Sun X, et al. Thyrocytes express a functional toll-like receptor 3(TLR3): Overexpression can be induced by viral infection, reversed by Phenylmethimazole, and is associated with Hashimoto's autoimmune thyroiditis. Molecular Endocrinology. 2005;19(5):1231–1250. doi: 10.1210/me.2004-0100. [DOI] [PubMed] [Google Scholar]
- 17.Tsan MF. Toll-like receptors, inflammation and cancer. Semin Cancer Biol. 2005 doi: 10.1016/j.semcancer.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Rudofsky G, Jr, Reismann P, Witte S, Humpert PM, Isermann B, Chavakis T, et al. Asp299Gly and Thr399Ile genotypes of the TLR4 gene are associated with a reduced prevalence of diabetic neuropathy in patients with type 2 diabetes. Diabetes Care. 2004;27(1):179–183. doi: 10.2337/diacare.27.1.179. [DOI] [PubMed] [Google Scholar]
- 19.Kolek MJ, Carlquist JF, Muhlestein JB, Whiting BM, Horne BD, Bair TL, et al. Toll-like receptor 4 gene Asp299Gly polymorphism is associated with reductions in vascular inflammation, angiographic coronary artery disease, and clinical diabetes. Am Heart J. 2004;148(6):1034–1040. doi: 10.1016/j.ahj.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 20.Toiyama Y, Araki T, Yoshiyama S, Hiro J, Miki C, Kusunoki M. The expression patterns of Toll-like receptors in the ileal pouch mucosa of postoperative ulcerative colitis patients. Surg Today. 2006;36(3):287–290. doi: 10.1007/s00595-005-3144-y. [DOI] [PubMed] [Google Scholar]
- 21.Baumgarten G, Knuefermann P, Schuhmacher G, Vervolgyi V, von Rappard J, Dreiner U, et al. Toll-like receptor 4, nitric oxide, and myocardial depression in endotoxemia. Shock. 2006;25(1):43–49. doi: 10.1097/01.shk.0000196498.57306.a6. [DOI] [PubMed] [Google Scholar]
- 22.Baumgarten G, Knuefermann P, Wrigge H, Putensen C, Stapel H, Fink K, et al. Role of Toll-like receptor 4 for the pathogenesis of acute lung injury in Gram-negative sepsis. Eur J Anaesthesiol. 2006;23(12):1041–1048. doi: 10.1017/S0265021506001098. [DOI] [PubMed] [Google Scholar]
- 23.Stoll LL, Denning GM, Weintraub NL. Endotoxin, TLR4 signaling and vascular inflammation: potential therapeutic targets in cardiovascular disease. Curr Pharm Des. 2006;12(32):4229–4245. doi: 10.2174/138161206778743501. [DOI] [PubMed] [Google Scholar]
- 24.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 25.Berstein LM. Clinical usage of hypolipidemic and antidiabetic drugs in the prevention and treatment of cancer. Cancer Lett. 2005;224(2):203–212. doi: 10.1016/j.canlet.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Otake S, Takeda H, Suzuki Y, Fukui T, Watanabe S, Ishihama K, et al. Association of visceral fat accumulation and plasma adiponectin with colorectal adenoma: evidence for participation of insulin resistance. Clin Cancer Res. 2005;11(10):3642–3646. doi: 10.1158/1078-0432.CCR-04-1868. [DOI] [PubMed] [Google Scholar]
- 27.von Hafe P, Pina F, Perez A, Tavares M, Barros H. Visceral fat accumulation as a risk factor for prostate cancer. Obes Res. 2004;12(12):1930–1935. doi: 10.1038/oby.2004.242. [DOI] [PubMed] [Google Scholar]
- 28.Calo V, Migliavacca M, Bazan V, Macaluso M, Buscemi M, Gebbia N, et al. STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol. 2003;197(2):157–168. doi: 10.1002/jcp.10364. [DOI] [PubMed] [Google Scholar]
- 29.Kim W, Lewis C, McCall K, Malgor R, Kohn A, Moon R, et al. Overexpression of Wnt-1 in Thyrocytes Enhances Cellular Growth But Suppresses Transcription of the Thyroperoxidase Gene via Different Signaling Mechanisms. Journal of Endocrinology. 2007;192:1–15. doi: 10.1677/JOE-06-0025. [DOI] [PubMed] [Google Scholar]
- 30.Takeda K, Akira S. Toll receptors and pathogen resistance. Cell Microbiol. 2003;5(3):143–153. doi: 10.1046/j.1462-5822.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 31.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413(6857):732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 32.Oshiumi H, Matsumoto M, Funami K, Akazawa T, Seya T. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat Immunol. 2003;4(2):161–167. doi: 10.1038/ni886. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, et al. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol. 2002;169(12):6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 34.Miettinen M, Sareneva T, Julkunen I, Matikainen S. IFNs activate toll-like receptor gene expression in viral infections. Genes Immun. 2001;2(6):349–355. doi: 10.1038/sj.gene.6363791. [DOI] [PubMed] [Google Scholar]
- 35.Cheng G, Nazar AS, Shin HS, Vanguri P, Shin ML. IP-10 gene transcription by virus in astrocytes requires cooperation of ISRE with adjacent kappaB site but not IRF-1 or viral transcription. J Interferon Cytokine Res. 1998;18(11):987–997. doi: 10.1089/jir.1998.18.987. [DOI] [PubMed] [Google Scholar]
- 36.Majumder S, Zhou LZ, Chaturvedi P, Babcock G, Aras S, Ransohoff RM. Regulation of human IP-10 gene expression in astrocytoma cells by inflammatory cytokines. J Neurosci Res. 1998;54(2):169–180. doi: 10.1002/(SICI)1097-4547(19981015)54:2<169::AID-JNR5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 37.Ohmori Y, Hamilton TA. The interferon-stimulated response element and a kappa B site mediate synergistic induction of murine IP-10 gene transcription by IFN-gamma and TNF-alpha. J Immunol. 1995;154(10):5235–5244. [PubMed] [Google Scholar]
- 38.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4(4):221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 39.Garcia M, Fernandez-Garcia NI, Rivas V, Carretero M, Escamez MJ, Gonzalez-Martin A, et al. Inhibition of xenografted human melanoma growth and prevention of metastasis development by dual antiangiogenic/antitumor activities of pigment epithelium-derived factor. Cancer Res. 2004;64(16):5632–5642. doi: 10.1158/0008-5472.CAN-04-0230. [DOI] [PubMed] [Google Scholar]
- 40.Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82(2):241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 41.Gartsbein M, Alt A, Hashimoto K, Nakajima K, Kuroki T, Tennenbaum T. The role of protein kinase C {delta} activation and STAT3 Ser727 phosphorylation in insulin-induced keratinocyte proliferation. J Cell Sci. 2006;119(Pt 3):470–481. doi: 10.1242/jcs.02744. [DOI] [PubMed] [Google Scholar]
- 42.Barboza JA, Wang S, Schaefer TS. Generation and characterization of a constitutively active Stat3 protein. Mol Biol Rep. 2004;31(1):13–21. doi: 10.1023/b:mole.0000013503.16301.82. [DOI] [PubMed] [Google Scholar]
- 43.Yeh YT, Ou-Yang F, Chen IF, Yang SF, Wang YY, Chuang HY, et al. STAT3 ser727 phosphorylation and its association with negative estrogen receptor status in breast infiltrating ductal carcinoma. Int J Cancer. 2006;118(12):2943–2947. doi: 10.1002/ijc.21771. [DOI] [PubMed] [Google Scholar]
- 44.Zanger R, Novak R. Posttranslational elevation of cytochrome P450 3A Levels and Activity by Dimethyl Sulfoxide. Archives of Biochemistry and Biophysics. 1998;353(1):1–9. doi: 10.1006/abbi.1997.0571. [DOI] [PubMed] [Google Scholar]
- 45.Kohn LD, Goetz D, Benavides-Peralta U, Gonzalez-Murguiondo M, Harii N, Lewis C, Napolitano G, Dagia N, inventors. 10/801,986. Compositions and methods for treatment of colitis patent. 2004
- 46.Kohn LD, Harii N, Benavides-Peralta U, Gonzalez-Murguiondo MA, Lewis CJ, Napolitano G, et al., inventors. 11/130,922. Use of Phenylmethimazoles, Methimazole derivatives, and tautomeric cyclic thiones for the treatment of autoimmune/inflammatory diseases associated with Toll-Like receptor overexpression patent. 2005
- 47.Paone A, Starace D, Galli R, Padula F, De Cesaris P, Filippini A, et al. Toll-like receptor 3 triggers apoptosis of human prostate cancer cells through a PKC-alpha-dependent mechanism. Carcinogenesis. 2008;29(7):1334–1342. doi: 10.1093/carcin/bgn149. [DOI] [PubMed] [Google Scholar]
- 48.Fujio Y, Matsuda T, Oshima Y, Maeda M, Mohri T, Ito T, et al. Signals through gp130 upregulate Wnt5a and contribute to cell adhesion in cardiac myocytes. FEBS Lett. 2004;573(1–3):202–206. doi: 10.1016/j.febslet.2004.07.082. [DOI] [PubMed] [Google Scholar]
- 49.Dissanayake SK, Olkhanud PB, O'Connell MP, Carter A, French AD, Camilli TC, et al. Wnt5A regulates expression of tumor-associated antigens in melanoma via changes in signal transducers and activators of transcription 3 phosphorylation. Cancer Res. 2008;68(24):10205–10214. doi: 10.1158/0008-5472.CAN-08-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300(5625):1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]