Abstract
Background
Multi-detector computed tomography (MDCT) has been proposed as a tool for routine screening for coronary artery calcification (CAC) in asymptomatic individuals. As proposed, such screening could involve tens of millions of individuals, but detailed estimates of radiation doses and potential risk of radiation-induced cancer are not currently available. We estimated organ-specific radiation doses and associated cancer risks from CAC screening with MDCT, according to age, frequency and scan protocol.
Methods
Radiation doses to adult patients were calculated from a range of available protocols using Monte Carlo radiation transport. Radiation risk models, derived using data from Japanese atomic bomb survivors and medically-exposed cohorts, were used to estimate the excess lifetime risk of radiation-induced cancer.
Results
Radiation dose from a single CAC CT scan varied more than 10-fold (effective dose range=0.8 to 10.5 mSv) depending on the protocol. In general higher radiation doses were associated with higher x-ray tube current, higher tube potential, and spiral scanning with low pitch, and retrospective gating. The wide dose variation also resulted in wide variation in estimated radiation-induced cancer risk. Assuming screening every five years from age 45-75 for men and from age 55-75 for women, the estimated excess lifetime cancer risk using the median dose of 2.3 mSv (0.8-10.5 mSv) was 42 cases/100,000 for men (range 14-200) and 62 cases/100,000 for women (range 21-300).
Conclusions
These radiation risk estimates can be compared to potential benefits from screening, when such estimates are available. Doses and hence risks can be minimized by using optimized protocols.
INTRODUCTION
Computed tomography (CT) has been proposed as a tool for routine screening for coronary artery calcification (CAC) in asymptomatic individuals as part of a comprehensive risk assessment. A national survey in the US reported that about 27% of diagnostic radiologists already read CAC CT screening scans regularly, making it the most common type of CT screening currently undertaken in the US 1. The Screening for Heart Attack Prevention and Education (SHAPE) guidelines recommend screening of all asymptomatic men 45-75 years of age and asymptomatic women 55-75 years of age except those defined as very low risk 2. Such screening in the US could involve tens of millions of individuals. However, the benefits from such screening have not yet been demonstrated directly in randomized trials with cardiovascular events or mortality as an endpoint but it has been suggested that the use of CAC scoring can detect disease in asymptomatic people who would be at low risk when assessed by traditional risk factors 3. The potential risks also have to be considered along with the potential benefits from screening however and these include the risk of radiation-induced cancer.
It is impractical to estimate the risk of radiation-induced cancer from CT scans directly through an observational study, because this would require follow-up of hundreds of thousands of patients for their entire lifetime 4. The difficulty was also underscored in a recent study 5. The magnitude of the risks can be estimated indirectly by extrapolating risk models from existing long-term studies of the effects of radiation exposure, such as the Life Span Study of Japanese atomic bomb survivors 6-8.
In the present study, we review a number of protocols for CAC screening by multi-detector computed tomography (MDCT) to estimate organ-specific radiation doses. We then use the radiation risk models from the National Research Council's BEIR (Biological Effects of Ionizing Radiation) VII committee to estimate the potential radiation-induced cancer risk from screening according to age and gender.
METHODS
CT Protocols
We reviewed the literature for recent protocols for CAC measurement using MDCT. Unlike other cardiac imaging modalities such as nuclear medicine and echocardiography, where protocols are more standardized 9, 10, cardiac CT protocols still vary widely depending on institutions and scanners. There have been no agreed-upon standard scan protocols for CAC measurement by MDCT, and various protocols have been employed in previous studies 11-18. Here we considered CT scan protocols established by some national cardiac studies 19-23, and one international study on standardization in cardiac CT 24. In practice, most CT technologists are not believed to adjust the calcium scoring protocols much. They use a default protocols by just adjusting scan area. In addition, protocols currently used clinically at three different university hospitals (Columbia University/New York-Presbyterian Hospital, Cleveland Clinic, and Penn State University) were included. The protocols, including technical parameters, are summarized in Table 1.
Table 1.
CT scan protocols for CAC screening and protocol parameters
| Protocol Source (Reference) | Scanner | Scan Mode | ECG Synchronization | Tube Potential (kVp) | Gantry Rotation Time (s) | Tube Current-Time Product (mAs)a | Detector Configuration | Pitchb |
|---|---|---|---|---|---|---|---|---|
| Study 1 (22, 23) | LightSpeed QX/i | Axial | Retrospective | 120 | 0.8 | 320 (400) | 4×2.5 mm | 1 |
| LightSpeed Plus | Axial | Prospective | 120 | 0.5 | 104 (130) | 4×2.5 mm | 1 | |
| Volume Zoom | Axial | Prospective | 140 | 0.5/0.361 | 50 (63) | 4×2.5 mm | 1 | |
| Study 2 (20, 21) | LightSpeed Pro 16 | Axial | Prospective | 120 | 0.5 | 104 (130) | 4×2.5 mm | 1 |
| Sensation 16 | Axial | Prospective | 140 | 0.5 | 50 (63) | 12×1.5 mm | 1 | |
| Sensation 64 | Axial | Prospective | 120 | 0.33 | 50 (63) | 30×0.6 mm | 1 | |
| Aquilion 64 | Axial | Prospective | 135 | 0.33 | 48 (61) | 4×3 mm | 1 | |
| Study 3 (19) | LightSpeed 16 | Axial | Prospective | 120 | 0.5 | 106 (132) | 8×2.5 mm | 1 |
| Sensation 16 | Axial | Prospective | 120 | 0.42/0.37 | 70 (88) | 6×3 mm | 1 | |
| Study 4 (24) | LightSpeed Plus | Axial | Prospective | 120 | 0.5 | 70 (25, 145) | 4×2.5 mm | 1 |
| MX 8000 | Axial | Prospective | 120 | 0.5 | 30 (10, 65) | 4×2.5 mm | 1 | |
| Volume Zoom | Axial | Prospective | 120 | 0.5 | 55 (20, 135) | 4×2.5 mm | 1 | |
| Volume Zoom | Spiral | Retrospective | 120 | 0.5 | 50 (20, 115) | 4×2.5 mm | 0.375 | |
| Aquilion | Axial | Prospective | 120 | 0.5 | 45 (20, 90) | 4×3 mm | 1 | |
| Sensation 64 | Spiral | Retrospective | 120 | 0.33 | 70 (20, 145) | 64×0.6 mm | 0.2 | |
| Hospital 1c | Precedence 16P | Axial | Prospective | 120 | 0.5 | 70 | 8×3 mm | 1 |
| LightSpeed VCT | Axial | Prospective | 120 | 0.35 | 70-88 | 8×2.5 mm | 1 | |
| Hospital 2c | Brilliance 64 | Axial | Prospective | 120 | 0.4 | 50 (20, 120) | 40×0.625 mm | 1 |
| Definition | Axial | Prospective | 120 | 0.33 | 95 (45, 195) | 6×3 mm | 1 | |
| Hospital 3c | Definition | Axial | Prospective | 120 | 0.33 | 150 | 6×3 mm | 1 |
Abbreviations: ECG, electrocardiographic.
Some protocols provide different mAs by patient size. Numbers in parenthesis for Study 1, Study 2, and Study 3 protocols are mAs for those who weighed more than 100 kg. Numbers in parenthesis for Study 4 and Hospital 2 are mAs for small and large-size patients. Hospital 1 protocol for LightSpeed VCT scanner also provides range of mAs to adjust based on patient habitus.
Pitch is applied to spiral scan. Here it indicates pitch for spiral scan defined as ratio of the table feed per x-ray tube rotation to the x-ray beam width 42. For axial scan, it indicates ratio of the table increment between successive x-ray beam rotations to the x-ray beam width 41.
Protocols currently used clinically at three different university hospitals. Hospitals 1 – 3 indicate Columbia University/New York-Presbyterian Hospital, Cleveland Clinic, and Penn State University, respectively.
Radiation Dose Calculation
Radiation doses were calculated using the CT scan protocols and CT dosimetry programs, CTDosimetry version 0.99x and CT-Expo version 1.6 25, 26. The programs use organ dose databases generated based on Monte Carlo radiation transport modeling by the National Radiological Protection Board (NRPB) in the United Kingdom and the National Research Center for Environment and Health (GSF) in Germany 27-30.
According to the CT operational manuals for the Studies 1 – 3, each participant receives two scans for the purposes of increased reliability and quality control 19-21, 23. The double scan is performed for research purposes and is not typical clinical practice. Therefore, radiation dose calculation and risk estimation in this study was performed assuming only a single CT scan per participant. Studies and scanner specific CT setting parameters given in Table 1 were used for dose calculation with the additional assumption that the typical scan length is 12 cm 17. Some protocols provide a range of x-ray tube current-time products (mAs) for patients of different sizes. For these protocols, tube current-time products for medium-size patients were used for dose calculation. Organ-specific radiation doses and effective dose for each protocol were calculated. Effective dose is sum of weighted absorbed doses in all irradiated tissues and organs in the body and describes non-uniform dose in order of equivalent whole-body dose 31. This is one of the most frequently reported dosimetric quantities from CT scan. International Commission on Radiological Protection (ICRP) Publication 103 tissue weighting factors were used to calculate the effective dose 32.
Cancer Risk Estimation
We used the radiation risk models for sex- and organ-specific cancer incidence that were developed by the BEIR VII committee 33 combined with our organ-specific dose estimates to estimate the risk of radiation-induced cancer. Site-specific models were not available for some cancer sites such as esophagus, pancreas, skin, and kidney and so cancer risks at these sites were estimated using the radiation coefficients from the excess relative risk model for ‘other solid cancers’ in the BEIR VII report. For most cancer sites the committee's risk models were estimated using data from the Life Span Study of the Japanese atomic bomb survivors; the major exceptions were the risk models for breast cancer and thyroid cancer. The breast cancer model was based on an excess relative risk model from a pooled analysis of eight cohort studies including both atomic bomb survivors and medically-exposed individuals by Preston et al. 34. Thyroid cancer was based on a pooled analysis of seven studies by Ron et al. 35. Background cancer incidence rates for the general US population were estimated using site-specific cancer incidence rates for all races from the Surveillance, Epidemiology and End Results (SEER) Program cancer registries for 2000-2005 36.
After an initial lag period (assumed to be five years for solid cancers and two years for leukemia) the risk of radiation-induced cancer remains elevated for the remainder of the person's lifetime 37. Therefore, the total risk of radiation-induced cancer was calculated using life table methods as a cumulative lifetime risk with adjustment for competing causes of death made using all-cause mortality rates for the US population 38.
Potential radiation-induced cancer risks from CAC screening by MDCT were estimated according to age at screening and gender. The SHAPE guidelines recommend screening of all asymptomatic men 45-75 years of age and asymptomatic women 55-75 years of age except those defined as very low risk 2. While SHAPE suggests screening with either CAC or carotid intima-media thickness measurement, the former is more reproducible and predictive of future events 39 and it would be expected that most patients would therefore undergo CAC screening. There are approximately 50 million people in the US within the age-range recommended for CAC screening by the SHAPE guidelines. The SHAPE guide lines recommended reassessment within 5 years for those with a positive test for atherosclerosis and every 5-10 years for those with a negative test. Here we estimated cancer risks under two different scenarios. Firstly, we estimated radiation-induced cancer risk from a single CAC screening by MDCT at any age between 40 and 80 for each of the different protocols. Secondly, we estimated the total radiation-induced cancer risk in accordance with the SHAPE guidelines; repeated CT screening every five years from age 45-75 for men and from age 55-75 for women.
RESULTS
Radiation Dose
The estimated effective dose from a single CAC screening varied widely from 0.8 to 10.5 mSv across the CT protocols (Table 2). The median and mean values were 2.3 and 3.1 mSv, respectively. The organs/tissues that were estimated to receive measurable radiation doses, in approximate order of magnitude were: breast, lung, thymus, esophagus, bone surface, adrenals, bone marrow, liver, pancreas, skin, spleen, muscle, stomach, and kidney. The wide variation in radiation doses can be attributed to many factors, including different CT scanner models and different CT scan techniques. In general higher radiation doses were associated with higher x-ray tube current, higher tube potential, and spiral scanning with low pitch, and retrospective gating.
Table 2.
Organ-specific and effective dose estimates for each CAC CT screening protocol
| Scanner | Organ Dose (mGy) |
Effective Dosea (mSv) | ||||||
|---|---|---|---|---|---|---|---|---|
| Breasts | Lungs | Esophagus | Bone Marrow | Liver | Skin | |||
| Study 1 | LightSpeed QX/i | 36.0 | 28.0 | 16.0 | 5.6 | 5.3 | 4.1 | 10.5 |
| LightSpeed Plus | 12.0 | 9.4 | 5.4 | 1.9 | 1.7 | 1.4 | 3.5 | |
| Volume Zoom | 8.1 | 5.4 | 2.9 | 1.1 | 1.0 | 0.9 | 2.2 | |
| Study 2 | LightSpeed Pro 16 | 11.0 | 8.9 | 5.1 | 1.8 | 1.7 | 1.3 | 3.3 |
| Sensation 16 | 8.1 | 5.4 | 2.9 | 1.1 | 1.0 | 0.9 | 2.2 | |
| Sensation 64 | 3.8 | 3.1 | 1.8 | 0.6 | 0.6 | 0.4 | 1.1 | |
| Aquilion 64 | 8.5 | 6.5 | 3.8 | 1.3 | 1.2 | 1.0 | 2.5 | |
| Study 3 | LightSpeed 16 | 11.0 | 8.9 | 5.5 | 1.9 | 1.7 | 1.3 | 3.4 |
| Sensation 16 | 5.8 | 4.4 | 2.6 | 0.9 | 0.9 | 0.6 | 1.7 | |
| Study 4 | LightSpeed Plus | 7.9 | 6.2 | 3.5 | 1.2 | 1.2 | 0.9 | 2.3 |
| MX 8000 | 2.6 | 2.0 | 1.2 | 0.4 | 0.4 | 0.3 | 0.8 | |
| Volume Zoom (Ax) | 4.6 | 3.5 | 2.0 | 0.7 | 0.7 | 0.5 | 1.3 | |
| Volume Zoom (Sp) | 11.0 | 8.5 | 4.9 | 1.7 | 1.6 | 1.2 | 3.1 | |
| Aquilion | 7.4 | 4.7 | 2.6 | 0.9 | 0.8 | 0.9 | 1.9 | |
| Sensation 64 | 27.0 | 22.0 | 13.0 | 4.5 | 4.2 | 3.1 | 8.0 | |
| Hospital 1 | Precedence 16P | 5.7 | 4.4 | 2.5 | 0.9 | 0.8 | 0.6 | 1.7 |
| LightSpeed VCT | 7.7 | 6.4 | 3.9 | 1.2 | 1.2 | 0.9 | 2.3 | |
| Hospital 2 | Brilliance 64 | 4.1 | 3.1 | 1.8 | 0.6 | 0.6 | 0.5 | 1.2 |
| Definition | 9.0 | 5.5 | 1.2 | 0.9 | 2.1 | 1.0 | 2.3 | |
| Hospital 3 | Definition | 14.1 | 8.6 | 1.8 | 1.4 | 3.3 | 1.5 | 3.6 |
Effective dose based on ICRP Publication 103 tissue weighting factors 32
Cancer Risk
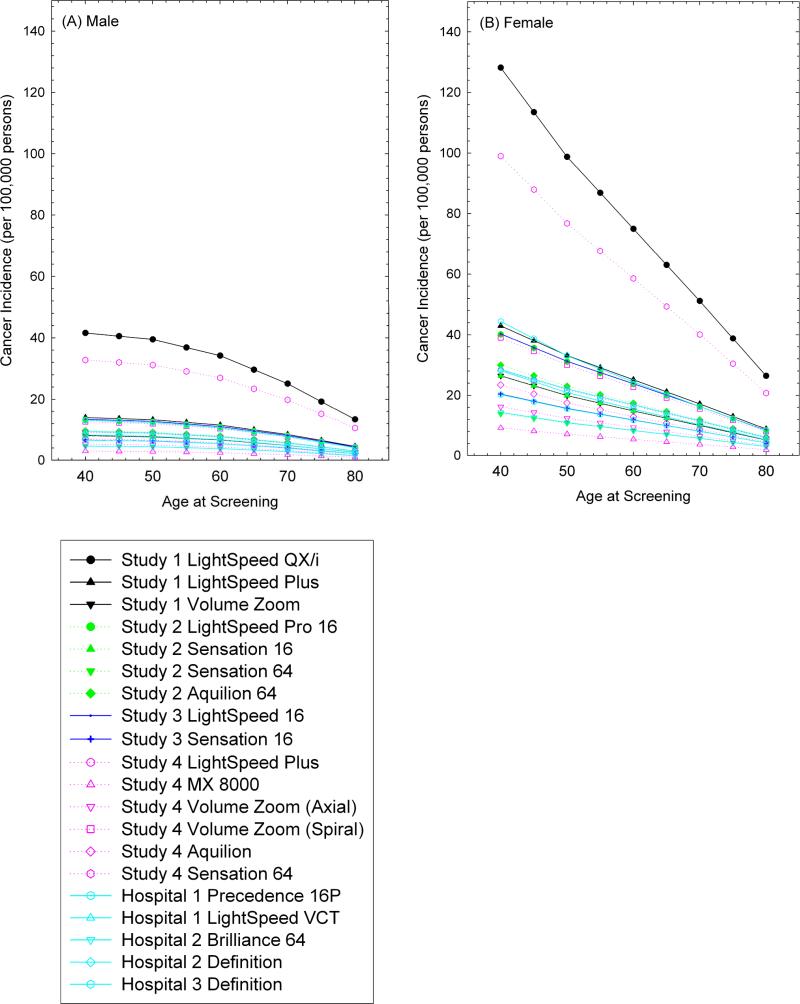
Figure 1 shows estimates of the excess lifetime risk of radiation-induced cancer from a single CAC CT according to age at screening. The estimated risks decreased with increasing age at screening for all protocols primarily because of the reduced life expectancy. For the median dose 2.3 mSv (0.8-10.5 mSv), a single screening at age of 40 was estimated to result in a lifetime excess cancer risk of 9 (3-42) and 28 (9-130) cancers per 100,000 persons for males and females, respectively. This decreased to 3 (1-13) (males) and 6 (2-26) (females) per 100,000 from screening at age of 80.
Figure 1.
Estimated lifetime risk of radiation-induced cancer (per 100,000 persons) from a single CT scan to assess CAC, by age at screening
The largest proportion of the total radiation-induced cancer incidence, 72% and 71% for males and females, respectively, was due to lung cancer, followed by breast cancer for females (20%), and then leukemia (12% and 4% for males and females, respectively) (Table 3). The estimated risk from all cancers combined was higher for females than for males because of the contribution from radiation-induced breast cancer and also because the risk of radiation-induced lung cancer was estimated to be about two-fold higher for females than for males.
Table 3.
Site-specific estimates of the lifetime risk of radiation-induced cancer from a single CAC CT screen at age 55.
| Cancer | Radiation-Induced Cancer Incidence (per 100,000 persons)a |
|
|---|---|---|
| Male | Female | |
| Breast | - | 4 (20%) |
| Lung | 6 (72%) | 14 (71%) |
| Leukemia | 1 (12%) | 1 (4%) |
| Other solid | 1 (15%) | 1 (6%) |
| Total | 8 (100%) | 20 (100%) |
Site-specific risks were estimated based on organ dose estimates associated with a median effective dose of 2.3 mSv.
Assuming screening every five years from age 45-75 for males and from age 55-75 for females, the cumulative radiation-induced cancer risks from the median dose of 2.3 mSv (0.8-10.5 mSv) were estimated to be 42 per 100,000 for males (14-200) and 62 per 100,000 for females (21-300).
COMMENT
We observed more than ten-fold variation in radiation doses from CAC screening with MDCT and therefore wide variation in the estimated radiation-induced cancer risk.
The range of estimated effective doses is similar to the range from a recent literature review (range 1.0-12 mSv, mean 3.0 mSv) 40. Many factors influence radiation dose from medical radiation sources. For CAC CT these include CT scanner model, scan mode, electrocardiographic (ECG) triggering or gating, x-ray tube potential (kVp), mAs, pitch 41, 42, and scan length. Even for the same CT scanner model, CT scan technique or parameter settings may vary between hospitals. For example, there are three different protocols in Table 1 for the Volume Zoom CT scanner. Despite the fact that the current-time products were similar for these three protocols (50-55 mAs) the estimated effective radiation doses varied from 1.3 mSv to 3.1 mSv. The higher estimates were primarily due to the use of higher tube potential or spiral scanners with lower pitch.
Slower gantry speed and retrospective gating were the main explanations for the higher radiation exposures in some of the protocols (eg Study 1 LightSpeed QX/i scanner). Because of the intrinsic slower gantry speed, two full gantry rotations are necessary to create an image during the desired phase of the cardiac cycle 22. In addition, retrospective gating results in the x-ray beam being turned on during the whole rotation time. Both features result in an extended exposure time of 1.6 sec per imaging level and thus a higher radiation dose, where as exposure times for the other protocols ranged from 0.2 to 0.5 sec. Low pitch and retrospective gating also yielded relatively high radiation doses (eg Study 4 Sensation 64 scanner and Volume Zoom scanner). Radiation dose is also inversely proportional to pitch and a low pitch factor, typically between 0.2 and 0.375, is used for a spiral scanning for cardiac CT.
Radiation dose increases rapidly with kVp (as a rule of thumb radiation dose is proportional to kVp 2.5 43). The Study 1 protocol for the Volume Zoom scanner uses 140 kVp while most other protocols we reviewed recommend 120 kVp (Table 1). The higher kVp resulted in an estimated effective dose for this protocol that was about 70% higher than the otherwise similar protocol for the same scanner (Study 4 - axial scan).
Radiation dose is linearly proportional to the tube current time product (mAs). Two of the university hospital protocols here use the same Siemens Definition scanner, but differ significantly in radiation dose due to the different mAs used for imaging (protocols from Hospital 2 and Hospital 3). The same mAs will result in higher radiation dose 44 and less image noise for smaller patients due to less radiation attenuation by less tissue. Some protocols suggest reducing mAs for small patients (Table 1), although in practice most CT technologists use a default protocol for calcium scoring, adjusting just the scan area to cover from the carina to below the cardiac apex.
The differences in dose observed between scanners and protocols highlights the importance of using scanner equipment that enables a low-dose scan to be performed, and of optimizing protocols for a specific scanner model. These considerations are even more important when the CT scan is being used for screening a population that will mostly involve healthy individuals. Moreover, the broader the population that is to be scanned (SHAPE proposes screening approximately 50 million Americans), the greater the potential impact in terms of attributable cancer from a small increase in radiation dose. Thus, it is essential to optimize calcium scoring protocols to minimize dose while maintaining adequate image quality to yield a reliable calcium score.
The wide variation in the protocols reviewed here also highlights the fact that there are still no agreed-upon standard protocols for CAC quantification by MDCT. While the International Consortium has recently recommended that protocols should be standardized, to date it has only published protocols for a limited number of scanners, including few current-generation scanners 24. Further efforts by professional societies are necessary to standardize protocols.
Diamond and Kaul have performed cost-effectiveness analyses of the SHAPE paradigm in comparison with other cardiovascular prevention approaches 45. They estimated that one-time screening of 50 million individuals would, assuming perfect statin adherence in patients with high coronary artery calcification, could prevent 24,000 deaths and 96,000 nonfatal cardiovascular events, at a net cost of $17 billion, equivalent to $32,000 per life-year equivalent saved in comparison to a standard prevention strategy based on National Cholesterol Education Program guidelines 46. Assuming that 50 million individuals in the US in the age group had a single screening with one of the protocols with the median radiation dose of 2.3 mSv (Hospital 1 second protocol), our estimates suggest that this could result in about 5,600 (range from 2,700-37,000 depending on CT protocol) individuals developing a radiation induced cancer in the future. Estimates of the radiation-induced cancers were not included in the cost-effectiveness calculations described above. These calculations also provide an informal indication of how the radiation risks might compare to the potential benefits under the best case scenario of 100% treatment compliance.
There are a number of sources of uncertainty in radiation risk estimates due to the lack of precision in the parameter estimates and uncertain assumptions, such as the form of the dose-response relationship at low doses. In the current paper the extrapolation was performed under the linear no-threshold assumption with a dose and dose-rate effectiveness factor of 1.5. However, there is also epidemiological and radiobiological evidence which supports both downwardly and upwardly curving slopes and these alternatives to the BEIR VII risk models would result in higher or lower risk estimates, respectively 5.
Another uncertainty involved in radiation risk assessment is the transfer of risk models estimated from the Japanese to other populations with different background cancer rates. The BEIR VII committee's approach to this uncertainty was to use a weighted average of two risk models with different underlying assumptions: the excess relative risk model which is based on the assumption that the risk from radiation exposure multiplies the background cancer risk in the population, and the absolute excess risk model which is based on the assumption that the risk from radiation exposure adds to the background cancer risk in the population. Although we have not calculated formal confidence bounds for our radiation risk estimates, the BEIR VII committee did conduct such calculations for the risk of all cancers combined and their results suggest that combination of the uncertainty in the dose and dose-rate effectiveness factor (DDREF), the transport of the risk models from the Japanese to the US population and in the radiation risk coefficients could mean that the projected cancer risks for each protocol could be higher or lower than our point estimates by a factor of two 33.
We reviewed protocols and estimated radiation doses and associated cancer risks from CAC screening with MDCT. These risks can be compared with estimates of the benefits from such screening once they are available, so as to design appropriate screening and prevention strategies for coronary artery disease.
There have been no widely agreed-upon standard protocols for CAC screening by MDCT. Radiation doses and relevant radiation-induced cancer risks vary depending on protocols up to an order of magnitude. Many technical factors influence radiation dose from CAC measurement with MDCT. Careful optimization of these factors may reduce radiation exposure without detriment to the clinical purpose of the screening exam. Further efforts by professional societies are necessary to standardize protocols in order to decrease unnecessary radiation exposure and minimize cancer risk.
Contributor Information
Kwang Pyo Kim, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD; Department of Nuclear Engineering, Kyung Hee University, Republic of Korea.
Andrew J. Einstein, Department of Medicine, Division of Cardiology and Department of Radiology, Columbia University College of Physicians and Surgeons, New York, NY.
Amy Berrington de Gonzalez, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD.
REFERENCES
- 1.Burger IM, Kass NE, Sunshine JH, Siegelman SS. The use of CT for screening: a national survey of radiologists’ activities and attitudes. Radiology. 2008;248(1):160–168. doi: 10.1148/radiol.2481071369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naghavi M, Falk E, Hecht HS, et al. From vulnerable plaque to vulnerable patient - Part III: Executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) task force report. American Journal of Cardiology. 2006;98(2A):2H–15H. doi: 10.1016/j.amjcard.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Waugh N, Black C, Walker S, McIntyre L, Cummins E, Hillis G. The effectiveness and cost-effectiveness of computed tomography screening for coronary artery disease: systematic review. Health Technology Assessment. 2006;10(39) doi: 10.3310/hta10390. [DOI] [PubMed] [Google Scholar]
- 4.Land CE. Statistical limitations in relation to sample-size. Environmental Health Perspectives. 1981;42:15–21. doi: 10.1289/ehp.814215. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner DJ, Doll R, Goodhead DT, et al. Cancer risks attributable to low doses of ionizing radiation: Assessing what we really know. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(24):13761–13766. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berrington de Gonzalez AB, Kim KP, Samet JM. Radiation-induced cancer risk from annual computed tomography for patients with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine. 2007;176(10):970–973. doi: 10.1164/rccm.200704-591OC. [DOI] [PubMed] [Google Scholar]
- 7.Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. Journal of the American Medical Association. 2007;298(3):317–323. doi: 10.1001/jama.298.3.317. [DOI] [PubMed] [Google Scholar]
- 8.Berrington de Gonzalez A, Darby S. Risk of cancer from diagnostic X-rays: estimates for the UK and 14 other countries. Lancet. 2004;363(9406):345–351. doi: 10.1016/S0140-6736(04)15433-0. [DOI] [PubMed] [Google Scholar]
- 9.Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS. Stress protocols and tracers. J Nucl Cardiol. 2006;13(6):e80–90. doi: 10.1016/j.nuclcard.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Pellikka PA, Nagueh SF, Elhendy AA, Kuchl CA, Sawada SG. American society of echocardiography recommendations for performance, interpretation, and application of stress echocardiography. Journal of the American Society of Echocardiography. 2007;20(9):1021–1041. doi: 10.1016/j.echo.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Trabold T, Buchgeister M, Kuttner A, et al. Estimation of radiation exposure in 16-detector row computed tomography of the heart with retrospective ECG-gating. Rofo-Fortschritte Auf Dem Gebiet Der Rontgenstrahlen Und Der Bildgebenden Verfahren. 2003;175(8):1051–1055. doi: 10.1055/s-2003-40926. [DOI] [PubMed] [Google Scholar]
- 12.Poll LW, Cohnen M, Brachten S, Ewen K, Modder U. Dose reduction in multi-slice CT of the heart by use of ECG-controlled tube current modulation (“ECG pulsing”): Phantom measurements. Rofo-Fortschritte Auf Dem Gebiet Der Rontgenstrahlen Und Der Bildgebenden Verfahren. 2002;174(12):1500–1505. doi: 10.1055/s-2002-35945. [DOI] [PubMed] [Google Scholar]
- 13.Morin RL, Gerber TC, McCollough CH. Radiation dose in computed tomography of the heart. Circulation. 2003;107(6):917–922. doi: 10.1161/01.cir.0000048965.56529.c2. [DOI] [PubMed] [Google Scholar]
- 14.Mollet NR, Cademartiri F, van Mieghem CAG, et al. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112(15):2318–2323. doi: 10.1161/CIRCULATIONAHA.105.533471. [DOI] [PubMed] [Google Scholar]
- 15.Hunold P, Vogt FM, Schmermund A, et al. Radiation exposure during cardiac CT: Effective doses at multi-detector row CT and electron-beam CT. Radiology. 2003;226(1):145–152. doi: 10.1148/radiol.2261011365. [DOI] [PubMed] [Google Scholar]
- 16.Flohr TG, Schoepf UJ, Kuettner A, et al. Advances in cardiac imaging with 16-section CT systems. Academic Radiology. 2003;10(4):386–401. doi: 10.1016/s1076-6332(03)80027-2. [DOI] [PubMed] [Google Scholar]
- 17.McCollough CH. Patient dose in cardiac computed tomography. Herz. 2003;28(1):1–6. doi: 10.1007/s00059-003-2447-2. [DOI] [PubMed] [Google Scholar]
- 18.Coles DR, Smail MA, Negus IS, et al. Comparison of radiation doses from multislice computed tomography coronary angiography and conventional diagnostic angiography. Journal of the American College of Cardiology. 2006;47(9):1840–1845. doi: 10.1016/j.jacc.2005.11.078. [DOI] [PubMed] [Google Scholar]
- 19.Carr JJ. CARDIA (cardiovascular risk development in young adults) - CT scan site manual of operations: Year 20 CT exam. 2005.
- 20.MESA Multiethnic study of atherosclerosis - Computed tomography manual of operations exam II: Multi-Ethnic Study of Atherosclerosis. 2003.
- 21.MESA Multiethnic study of atherosclerosis - Computed tomography manual of operations exam III: Multi-Ethnic Study of Atherosclerosis. 2005.
- 22.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: Standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 23.CARDIA Cardiovascular risk development in young adults - Computed tomography manual of operations: cardiovascular risk development in young adults. 2000.
- 24.McCollough CH, Ulzheimer S, Halliburton SS, et al. Coronary artery calcium: A multi-institutional, multimanufacturer international standard for quantification at cardiac CT. Radiology. 2007;243(2):527–538. doi: 10.1148/radiol.2432050808. [DOI] [PubMed] [Google Scholar]
- 25.Stamm G, Nagel HD. CT-Expo V1.6 - A tool for dose evalulation in computed tomography. V1.6 ed. 2007.
- 26.ImPACT . ImPACT CT patient dosimetry calculator - Version 0.99x. Imaging Performance Assessment of CT Scanners; London, UK: 2006. [Google Scholar]
- 27.GSF . In: The calculation of dose from external photon exposures using reference human phantoms and Monte Carlo methods, Part VI: Organ doses from computed tomographic examinations. Zankl M, Panzer W, Drexler G, editors. Gesellschaft fur Strahlenund Umweltforschung mbH; Neuherberg: 1991. [Google Scholar]
- 28.GSF . In: Tomographic anthropomorphic models. Part II: Organ doses from computed tomographic examinations in paediatric radiology. GSF-Bericht 30/93. Zankl M, Panzer W, Drexler G, editors. Gesellschaft fur Strahlen- und Umweltforschung mbH; Neuherberg: 1993. [Google Scholar]
- 29.NRPB . In: Survey of CT practice in the UK. Part 3 - Normalized organ doses calculated using Monte Carlo techniques. Jones DG, Shrimpton PC, editors. National Radiological Protection Board; Chilton, UK: 1991. [Google Scholar]
- 30.NRPB . In: Normalized organ doses for x-ray computed tomography calculated using Monte Carlo techniques. Jones DG, Shrimpton PC, editors. National Radiological Protection Board; Chilton, UK: 1993. [Google Scholar]
- 31.ICRP 1990 recommendations of the international commission on radiological protection: International Commission on Radiological Protection. 1991.
- 32.ICRP . The 2007 recommendations of the International Commission on Radiological Protection. International Commission on Radiological Protection; New York, NY: 2007. [Google Scholar]
- 33.NAS . Health risks from exposure to low levels of ionizing radiation: BEIR VII phase 2. National Academy of Sciences; Washington D.C.: 2005. [PubMed] [Google Scholar]
- 34.Preston DL, Mattsson A, Holmberg E, Shore R, Hildreth NG, Boice JD. Radiation effects on breast cancer risk: A pooled analysis of eight cohorts. Radiation Research. 2002;158(2):220–235. doi: 10.1667/0033-7587(2002)158[0220:reobcr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 35.Ron E, Lubin JH, Shore RE, et al. Thyroid-cancer after exposure to external radiation - a pooled analysis of 7 studies. Radiation Research. 1995;141(3):259–277. [PubMed] [Google Scholar]
- 36.NCI . Surveillance, Epidemiology and End Results 2008 vol. National Cancer Institute; 2008. [Google Scholar]
- 37.Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958-1998. Radiation Research. 2007;168(1):1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 38.CDC . National vital statistics reports: United States life tables 1999. Center for Disease Control and Prevention; Hyattsville, MD: 2002. [Google Scholar]
- 39.Folsom AR, Kronmal RA, Detrano RC, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med. 2008;168(12):1333–9. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mettler FA, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: A catalog. Radiology. 2008;248(1):254–263. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 41.CRCPD . Nationwide evaluation of x-ray trends (NEXT): Tabulation and graphical summary of 2000 survey of computed tomography. Conference of Radiation Control Program Directors; Frankfort, KY: 2007. [Google Scholar]
- 42.IEC . Medical Electrical Equipment. Part 2-44: Particular requirements for the safety of x-ray equipment for computed tomography. International Electrotechnical Commission Central Office; Geneva, Switzerland: 2002. [Google Scholar]
- 43.Brix G, Nagel HD, Stamm G, et al. Radiation exposure in multi-slice versus single-slice spiral CT: results of a nationwide survey. European Radiology. 2003;13(8):1979–1991. doi: 10.1007/s00330-003-1883-y. [DOI] [PubMed] [Google Scholar]
- 44.Lee C, Lodwick D, Williams JL, Bolch WE. Hybrid computational phantoms of the 15-year male and female adolescent: Applications to CT organ dosimetry for patients of variable morphometry. Medical Physics. 2008;35(6):2366–2382. doi: 10.1118/1.2912178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diamond GA, Kaul S. The things to come of SHAPE: Cost and effectiveness of cardiovascular prevention. American Journal of Cardiology. 2007;99(7):1013–1015. doi: 10.1016/j.amjcard.2006.10.070. [DOI] [PubMed] [Google Scholar]
- 46.NIH . Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. National Institutes of Health; Bethesda, MD: 2002. [PubMed] [Google Scholar]