Abstract
This is a targeted review of the critical immaturities limiting psychophysical luminance contrast detection in human infants. Three-month-old infants are 50 times less sensitive to contrast than adults are. Rod experiments suggest that early-stage immaturities, like the short length of infant rod outer segments, have only a modest direct effect on infant visual performance. Infant contrast sensitivity may resemble adult extrafoveal sensitivity, because the foveal cones of the neonate are immature and may not generate strong enough responses to mediate visual performance. This use of the extrafoveal retina reduces the high-spatial-frequency end of the infant contrast sensitivity function, contributing to poor infant resolution acuity. The remaining difference between infant and adult contrast sensitivity functions may be a simple overall reduction in infant sensitivity. The maximum of the infant contrast sensitivity function increases proportionately with age, and may be numerically near the infant's age in weeks. Contrast discrimination experiments indicate that the critical immaturity that limits infant contrast sensitivity is a mid-level phenomenon, occurring before the site of the contrast gain control. For example, the infant ascending visual pathway might be limited by large amounts of intrinsic noise. These results suggest that there is little effect of inattentiveness to the psychophysical task by ostensibly alert infant patients or subjects. The clinician or researcher can interpret behavioral measurements of infant visual performance with confidence.
Keywords: Infant, visual development, contrast sensitivity, contrast discrimination, critical immaturity
Critical Immaturities in Infant Vision
Infants do not see as well as adults do: infant psychophysical sensitivity to light, color, and contrast is far below the comparable values in adults, especially between one and four months, where the most data are available. Infant visual acuity is poor at birth1, 2, and does not reach the adult value until at least 3 years3-5. Vernier acuity6, 7 and stereopsis8-11 cannot be measured until at least age 3 months. At the same time, infants are maturing in other ways, and the infant visual nervous system has several well-known immaturities, the most famous of which is that infant photoreceptors are immature both in their morphology and in their distribution across the retina12, 13. The classic question is, which of these immaturities actually limit infant visual performance? We define the “critical immaturities” to be those differences between infants and adults that are actually responsible for the poor visual performance of infants14.
The perception of contrast is fundamental to vision. We have argued elsewhere that an underlying inability of infants to perceive contrast is the critical immaturity limiting several aspects of infant visual performance. In many experiments, infant color contrast threshold is predicted from infant luminance contrast threshold15, 16, infant stereopsis is no worse than expected, given how hard it is for infants to see the stereo targets even at 100% contrast11, and infant vernier acuity is not worse than adult vernier acuity, when contrast discrimination is used as a benchmark7. But, what limits infant contrast sensitivity and contrast discrimination?
Here, we define three groups of immaturities that could reduce infant sensitivity to contrast (Fig. 1). The most distal (“early-stage”) limitations operate before the site of light adaptation. These might include a smaller numerical aperture of the infant eye and smaller amounts of photopigment in the photoreceptor outer segments. The most proximal (“late-stage”) limitations operate at the highest levels of visual processing, after the site of contrast adaptation (the “contrast gain control”). These might include infant inattentiveness to the psychophysical task,a such as forced-choice preferential looking (FPL)17 and acuity card preferential looking18, which were used in the experiments reviewed here. Between these, “middle-stage” limitations could occur after the site of visual light adaptation, but before the site of the contrast gain control. These limitations might include higher levels of intrinsic noise in infant sensory neurons. Which of the three sites critically limit infant responsiveness to luminance contrast?
Figure 1.
Schematic overview of the infant visual system. The experiments reviewed in this paper rule out early-stage immaturities (e.g., immaturities in the photoreceptor outer segments) and late-stage immaturities (e.g., inattentivness to the experimental task) as explanations for infant insensitivity to contrast. Thus, the critical immaturity limiting infant visual performance lies at the middle-stage section of this diagram. An example of a middle-stage immaturity is the possibly larger amount of noise in the infant ascending visual pathway.
Absolute and Increment Thresholds
Let us begin with the detection of a flash of light presented to the dark-adapted eye. The absolute threshold of the 2-month-old infant is about 1.76 log units higher (58 times less sensitive) than the adult value19, 20, see Fig. 8.4 of ref. 21), with the measured absolute threshold depending on the size and duration of the target stimulus22, 23. It is tempting to suppose that the high absolute threshold of infants is an early-stage effect, because infant rod outer segments are shorter than those of adults13, and probably contain less rhodopsin. However, the anatomical evidence suggests that infants should be less sensitive by no more than 0.46 log units (less than a factor of 2.9), depending on what assumptions are made about the source and effects of intrinsic noise21 (see Fig. 11.2 in ref.24). In the dark, the numerical aperture of the infant's eye is the same as the adult's20. These small effects cannot easily account for the difference between the adult and 2-month-old infant absolute threshold values, suggesting that at least some of the insensitivity must be due to middle-stage or late-stage immaturities.
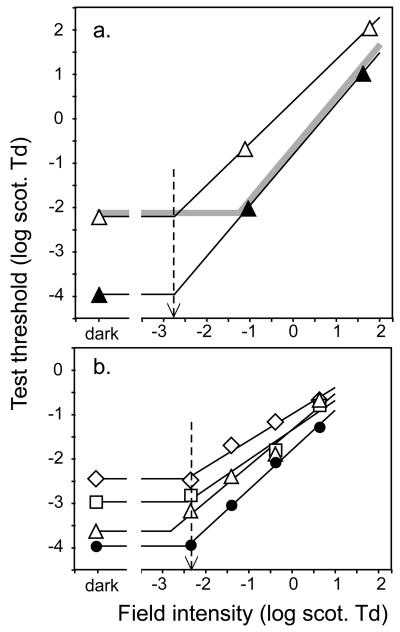
Converging evidence on this point comes from studies of rod increment thresholds (Fig. 2). An overall early-stage immaturity will reduce the visual effectiveness of the target stimulus (the “test sensitivity”), but it will also reduce the adapting effectiveness of the background (the “field sensitivity”) equally, because it occurs before the site of light adaptation. The light-adapted increment threshold will be little affected by an early-stage immaturity, because the sensitivity that is lost by the reduced test sensitivity is recouped by reduced desensitizing light adaptation. In fact, if Weber's law is obeyed, no change in increment threshold is predicted at high luminance (gray curve, Fig. 2). In contrast, a middle-stage or late-stage immaturity, both of which occur after the site of light adaptation, will not reduce the effectiveness of the adapting signal, so they will reduce infant test sensitivity but not field sensitivity. Test sensitivity is measured at the absolute threshold, where there is no adapting background field to worry about. Field sensitivity is most straightforwardly estimated as the background intensity where a line drawn through the increment threshold function intersects the dark-adapted threshold value (black lines and dashed arrows in Fig. 2). There is room for disagreement about the size of the contribution of the early-stage component, because the estimated field sensitivity value depends on the slope of the line drawn through the increment threshold function20, 25, 26. However, all studies agree that there is an important contribution from a middle- or late-stage component, which cannot be explained by the known rod immaturities, because studies agree that infant field sensitivity is nearer the adult value than infant test sensitivity is. Rod increment threshold data cannot determine where in the visual pathway (beyond the site of light adaptation) this middle- or late-stage immaturity occurs.
Figure 2.
Rod increment threshold functions on infants and adults. Horizontal lines: absolute threshold of each data set, which measures test sensitivity; sloping lines: regression lines fitted to the increment threshold data; dashed arrows: the measured field sensitivity, where the two solid lines cross. Gray curve: infant data predicted by an early-stage immaturity. A, 7-week-olds (white triangles) and adults (black circles), from ref. 20. B, 4-week-olds (diamonds), 10-week-olds (squares), 18-week-olds (triangles), and adults (black circles), from ref. 25.
We are not aware of any measurements of the infant photopic increment threshold function. Whereas infant contrast sensitivity (the infant Weber fraction) is considerably reduced compared to the adult value, we do not know whether Weber's law holds for infants under photopic conditions, so we cannot say for sure whether an early-stage immaturity affects infant cone vision, and if so, what its magnitude is. This is an important gap in our knowledge of infant visual development.
Infant Photopic Sensitivity to Contrast
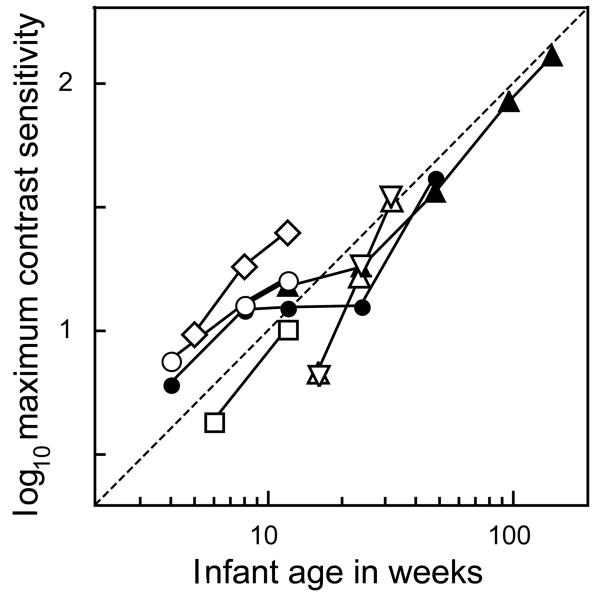
Infant photopic contrast sensitivity is much worse than that of adults, but its exact value varies across experiments. The data depend on whether the stimuli are spatial sine-waves or not, whether they are static or time-varying, whether the mean luminance is high or low, and what testing method is used. Here, we restrict our attention to static stimuli and psychophysical data (Fig. 3). There is a consensus that the maximum contrast sensitivity measured using static sine-waves is about 15 (range: 10–25) at age 3 months, about 1/50 of the adult value. From the limited data available, maximum contrast sensitivity appears to improve linearly with age, and may be numerically close to the infant's age in weeks (see Ref. 27 for a similar analysis of infant visual acuity). Further research will be needed to confirm or refute this mnemonic generalization.
Figure 3.
Maximum infant contrast sensitivity, measured using static sinewave stimuli. White symbols, FPL data: diamonds, ref. 36; circles, ref. 37; squares, ref. 38; upright triangles, ref. 39; inverted triangles, ref. 40. Black symbols: acuity-card-style preferential looking data: circles, ref. 41; triangles, ref. 42. The dotted fiducial line of unit slope suggests that infant contrast sensitivity is numerically near the subject's age in weeks, and improves approximately proportionately to age in weeks.
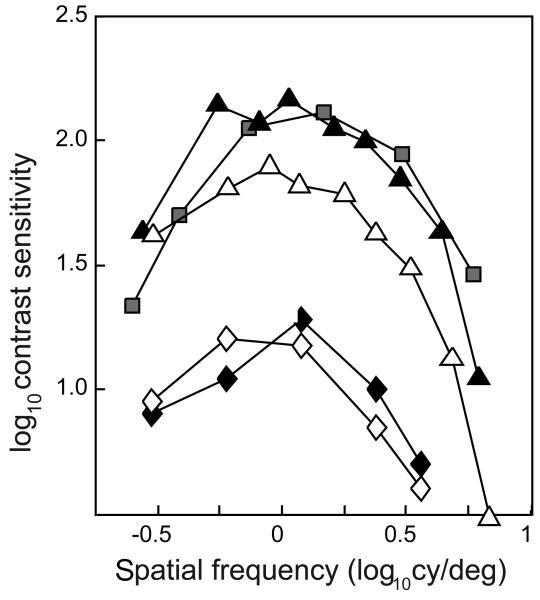
The infant contrast sensitivity function (CSF) is not merely reduced relative to the canonical CSF of adults: it is also shifted to lower spatial frequencies. This suggests that infant visual acuity might be affected by an early-stage immaturity: infant foveal cones are stubby, with short outer segments and inner segments that seem unsuitable as waveguides12, 13. Others have argued that the immature foveal cones directly cause the high spatial frequency loss28, but we think it more likely that the immature fovea forces infants to use their more mature extrafoveal vision for most visual tasks29. This issue can be examined by comparing the free-fixating infant CSF30 to the CSF of extrafoveally-tested adults31, 32. When the adult CSF is measured at approximately the eccentricity of the grating stimuli relative to the center of the preferential looking apparatus (about 7.5 deg), infant and adult CSFs are similar in shape, and can be more-or-less well superimposed by a vertical shift (relative to a logarithmic Y-axis) of about 0.75 log units (Fig. 4). The fit cannot be perfect in general, if only because infants have different CSFs depending on their age. The details of this equivalence between free-fixating infant performance and extrafoveal adult performance will be difficult to disentangle, because it is difficult to know what part of the retina infants of each age actually use in a preferential looking experiment. However, the changes in contrast sensitivity with age are generally analogous to the changes in contrast sensitivity with eccentricity within the visual field.
Figure 4.
Contrast sensitivity functions on free-fixating 3-month-olds and extrafoveally-fixating adults. Black diamonds, monocular data30, white diamonds, binocular data30. Black triangles, adult temporal monocular data (eccentricity=7.5 deg)31, white triangles, adult nasal data (eccentricity=10 deg)31. Squares, adult data using an annular target spanning 8—16 deg. in the visual periphery32.
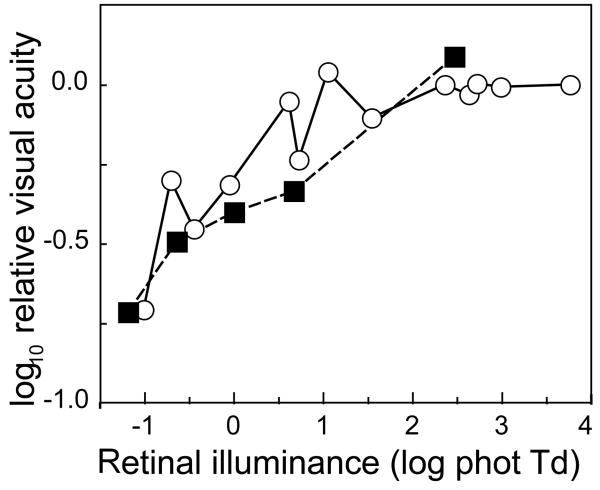
We can further examine the analogy between the free-fixating infant and the extrafoveal adult by determining whether the relative contributions of the rods and the cones to visual acuity are similar. Fig. 5 shows infant and extrafoveal adult visual acuity as a function of luminance, including scotopic, mesopic, and photopic luminance levels, taken from ref. 29. The data have been translated vertically to determine whether a good fit is possible. The agreement between the infant and adult data sets is imperfect, but we think that it is good enough to support a general analogy between infant vision and extrafoveal adult vision, in conjunction with the overall reduced contrast sensitivity in the infant. The remaining differences might be related to the distribution of the cones across the extrafoveal retina of the infant.
Figure 5.
Visual resolution acuity data from ref. 29 at scotopic, mesopic, and photopic retinal illuminances. White circles: free-fixating infant data (from Fig. 3);29 black squares: extrafoveal adult data (from Fig. 2).29 Adult and infant data have been scaled vertically for good agreement.
Infant Contrast Increment Thresholds
Where is the critical immaturity that is responsible for the high contrast threshold (low contrast sensitivity) of infants? Is it before the site of the contrast adaptation gain control, which elevates the rising part of the contrast increment threshold function, making it an early- or middle-stage immaturity? Or is it after the contrast gain control, making it a late-stage immaturity? We can examine this question using the contrast increment threshold function, where the subject discriminates between a higher-contrast stimulus (adapting contrast plus increment) and a lower-contrast stimulus (just the adapting contrast). The logic of this approach is analogous to the luminance increment threshold experiments described above, except that the adaptation site is the contrast gain control. On one hand, an early- or middle-stage immaturity will reduce the visual signals from the adapting contrast and the contrast increment by the same amount. In that case, infant sensitivity to the contrast increment will be counteracted by insensitivity to the desensitizing effects of the adapting contrast, and there will be little effect of immaturity on the contrast increment threshold at high contrast. On the other hand, a late-stage immaturity will leave the visual signal from the adapting contrast intact, and contrast discrimination thresholds should begin to rise at the same standard contrast in infants as they do in adults (gray curves in Fig. 6; see ref. 33 for a discussion of the dipper shapes of two of the curves, and ref. 34 for a similar argument for adults).
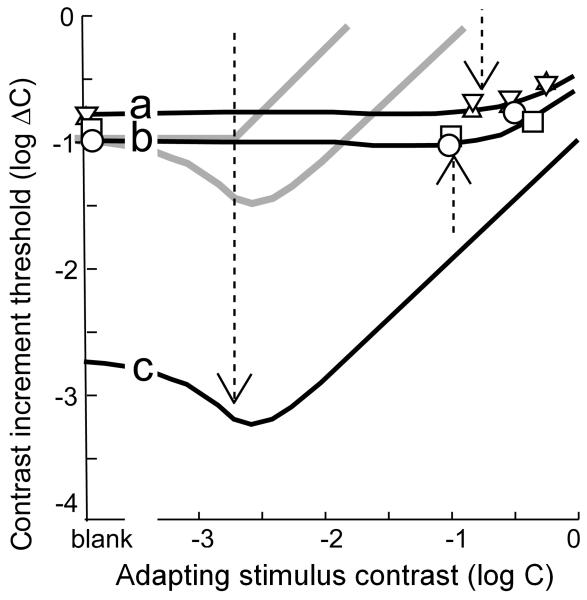
Figure 6.
Contrast increment threshold functions. Triangles, infant FPL data from ref. 35. Circles and squares, infant OKN data from ref. 33. Curves were fitted in ref. 33 to infant (a, b) and adult (c) data. Dashed arrows: the X-axis value at which the adapting stimulus contrast is equal to the contrast detection threshold (the Y-axis value at “blank”). Gray curves: infant functions predicted by a late-stage immaturity.
Infant contrast increment threshold functions, measured using FPL35 and OKN33, refute the late-stage hypothesis (Fig. 6). Infant contrast increment threshold functions do not change from their simple detection values until long after the adapting contrast reaches its own detection threshold (dashed arrows). Whereas infant contrast detection threshold is about 50 times higher than the adult value, infant contrast increment thresholds are higher by a factor of only 3 to 433. Thus, the critical immaturity that limits infant contrast detection and discrimination is not primarily a high-level phenomenon. Combining the results of the luminance increment threshold and the contrast increment threshold experiments: early-stage and late-stage immaturities, if they exist, are not critical; infant contrast perception is critically limited by immaturities in the middle-level part of the model in Fig. 1.
CONCLUSIONS
The field of infant visual sensation is reaching maturity. We now have data on infant performance on most of the major visual sensory processes, many of which are discussed in this issue. Over her exciting career, Velma Dobson has watched this field from its inception, and has contributed to most of these topics, thus moving us along to a better understanding of infant visual development. On the firm footing she helped establish, Velma has pushed the field to tackle clinical problems relating to the developing visual system.
Inasmuch as infant insensitivity to contrast may be the critical immaturity that limits other aspects of infant visual performance, such as color vision, vernier acuity, and stereopsis, this review suggests that these aspects of infant visual performance are also critically limited by middle-level immaturities, such as large amounts of noise in the infant ascending visual pathway. Infant inattentiveness to the psychophysical task probably has only a limited influence on infant visual performance, assuming the data were collected when the subject or patient is alert and active. Psychophysical methods like FPL and acuity card preferential looking can probably be used without worrying about whether ostensibly attentive infants are participating fully in the task required of them. This is good news for those who use behavioral methods to test the visual function of infants: if you think that your patient is awake and doing the task, he/she probably is, and you can interpret your measurements with confidence.
ACKNOWLEDGMENTS
Supported by a grant from the National Eye Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, MD (R21EY018321).
Footnotes
We presume that the infant patient or subject is generally alert and to all appearances participating fully in the testing session. It is obviously possible to ruin a clinical measurement or a data set by testing a subject who is too fussy, too sleepy, or too distracted to generate reliable results, and we presume that the careful tester has retained only data that are not contaminated in this obvious way.
REFERENCES
- 1.Brown AM, Yamamoto M. Visual acuity in newborn and preterm infants measured with grating acuity cards. Am J Ophthalmol. 1986;102:245–53. doi: 10.1016/0002-9394(86)90153-4. [DOI] [PubMed] [Google Scholar]
- 2.Dobson V, Schwartz TL, Sandstrom DJ, Michel L. Binocular visual acuity of neonates: the acuity card procedure. Dev Med Child Neurol. 1987;29:199–206. doi: 10.1111/j.1469-8749.1987.tb02136.x. [DOI] [PubMed] [Google Scholar]
- 3.Mayer DL, Dobson V. Visual acuity development in infants and young children, as assessed by operant preferential looking. Vision Res. 1982;22:1141–51. doi: 10.1016/0042-6989(82)90079-7. [DOI] [PubMed] [Google Scholar]
- 4.Dobson V, Teller DY. Visual acuity in human infants: a review and comparison of behavioral and electrophysiological studies. Vision Res. 1978;18:1469–83. doi: 10.1016/0042-6989(78)90001-9. [DOI] [PubMed] [Google Scholar]
- 5.Boothe RG, Dobson V, Teller DY. Postnatal development of vision in human and nonhuman primates. Annu Rev Neurosci. 1985;8:495–545. doi: 10.1146/annurev.ne.08.030185.002431. [DOI] [PubMed] [Google Scholar]
- 6.Shimojo S, Held R. Vernier acuity is less than grating acuity in 2- and 3-month-olds. Vision Res. 1987;27:77–86. doi: 10.1016/0042-6989(87)90144-1. [DOI] [PubMed] [Google Scholar]
- 7.Brown AM, Adusumilli V, Lindsey DT. Detection of vernier and contrast-modulated stimuli with equal Fourier energy spectra by infants and adults. J Vis. 2005;5:230–43. doi: 10.1167/5.3.7. [DOI] [PubMed] [Google Scholar]
- 8.Held R, Birch E, Gwiazda J. Stereoacuity of human infants. Proc Natl Acad Sci U S A. 1980;77:5572–4. doi: 10.1073/pnas.77.9.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birch EE. Stereopsis in infants and its developmental relation to visual acuity. In: Simons K, editor. Early Visual Development: Normal and Abnormal. Oxford University Press; New York: 1993. pp. 224–36. [Google Scholar]
- 10.Dobson V, Sebris SL. Longitudinal study of acuity and stereopsis in infants with or at-risk for esotropia. Invest Ophthalmol Vis Sci. 1989;30:1146–58. [PubMed] [Google Scholar]
- 11.Brown AM, Lindsey DT, Satgunam P, Miracle JA. Critical immaturities limiting infant binocular stereopsis. Invest Ophthalmol Vis Sci. 2007;48:1424–34. doi: 10.1167/iovs.06-0718. [DOI] [PubMed] [Google Scholar]
- 12.Abramov I, Gordon J, Hendrickson A, Hainline L, Dobson V, LaBossiere E. The retina of the newborn human infant. Science. 1982;217:265–7. doi: 10.1126/science.6178160. [DOI] [PubMed] [Google Scholar]
- 13.Hendrickson AE. Morphological development of the primate retina. In: Simons K, editor. Early Visual Development: Normal and Abnormal. Oxford University Press; New York: 1993. pp. 287–95. [Google Scholar]
- 14.Brown AM. Development of visual sensitivity to light and color vision in human infants: a critical review. Vision Res. 1990;30:1159–88. doi: 10.1016/0042-6989(90)90173-i. [DOI] [PubMed] [Google Scholar]
- 15.Allen D, Banks MS, Norcia AM. Does chromatic sensitivity develop more slowly than luminance sensitivity? Vision Res. 1993;33:2553–62. doi: 10.1016/0042-6989(93)90134-i. [DOI] [PubMed] [Google Scholar]
- 16.Brown AM, Lindsey DT, McSweeney EM, Walters MM. Infant luminance and chromatic contrast sensitivity: optokinetic nystagmus data on 3-month-olds. Vision Res. 1995;35:3145–60. doi: 10.1016/0042-6989(95)00017-t. [DOI] [PubMed] [Google Scholar]
- 17.Teller DY. The forced-choice preferential looking procedure: a psychophyiscal technique for use with human infants. Infant Behav Devel. 1979;2:135–53. [Google Scholar]
- 18.McDonald MA, Dobson V, Sebris SL, Baitch L, Varner D, Teller DY. The acuity card procedure: a rapid test of infant acuity. Invest Ophthalmol Vis Sci. 1985;26:1158–62. [PubMed] [Google Scholar]
- 19.Powers MK, Schneck M, Teller DY. Spectral sensitivity of human infants at absolute visual threshold. Vision Res. 1981;21:1005–16. doi: 10.1016/0042-6989(81)90004-3. [DOI] [PubMed] [Google Scholar]
- 20.Brown AM. Scotopic sensitivity of the two-month-old human infant. Vision Res. 1986;26:707–10. doi: 10.1016/0042-6989(86)90084-2. [DOI] [PubMed] [Google Scholar]
- 21.Hansen RM, Fulton AB. Development of scotopic retinal sensitivity. In: Simons K, editor. Early Visual Development: Normal and Abnormal. Oxford University Press; New York: 1993. pp. 130–42. [Google Scholar]
- 22.Hamer RD, Schneck ME. Spatial summation in dark-adapted human infants. Vision Res. 1984;24:77–85. doi: 10.1016/0042-6989(84)90146-9. [DOI] [PubMed] [Google Scholar]
- 23.Brown AM. Saturation of rod-initiated signals in 2-month-old human infants. J Opt Soc Am (A) 1988;5:2145–58. doi: 10.1364/josaa.5.002145. [DOI] [PubMed] [Google Scholar]
- 24.Brown AM. Intrinsic noise and infant visual performance. In: Simons K, editor. Early Visual Development: Normal and Abnormal. Oxford University Press; New York: 1993. pp. 178–95. [Google Scholar]
- 25.Hansen RM, Fulton AB, Harris SJ. Background adaptation in human infants. Vision Res. 1986;26:771–9. doi: 10.1016/0042-6989(86)90092-1. [DOI] [PubMed] [Google Scholar]
- 26.Dannemiller JL, Banks MS. The development of light adaptation in human infants. Vision Res. 1983;23:599–609. doi: 10.1016/0042-6989(83)90065-2. [DOI] [PubMed] [Google Scholar]
- 27.Teller DY. The development of visual acuity in human and monkey infants. Trends Neurosci. 1981;4:21–4. [Google Scholar]
- 28.Banks MS, Bennett PJ. Optical and photoreceptor immaturities limit the spatial and chromatic vision of human neonates. J Opt Soc Am (A) 1988;5:2059–79. doi: 10.1364/josaa.5.002059. [DOI] [PubMed] [Google Scholar]
- 29.Brown AM, Dobson V, Maier J. Visual acuity of human infants at scotopic, mesopic and photopic luminances. Vision Res. 1987;27:1845–58. doi: 10.1016/0042-6989(87)90113-1. [DOI] [PubMed] [Google Scholar]
- 30.Drover JR, Earle AE, Courage ML, Adams RJ. Improving the effectiveness of the infant contrast sensitivity card procedure. Optom Vis Sci. 2002;79:52–9. doi: 10.1097/00006324-200201000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Rovamo J, Virsu V. An estimation and application of the human cortical magnification factor. Exp Brain Res. 1979;37:495–510. doi: 10.1007/BF00236819. [DOI] [PubMed] [Google Scholar]
- 32.Kelly DH. Retinal inhomogeneity. I. Spatiotemporal contrast sensitivity. J Opt Soc Am A. 1984;1:107–13. doi: 10.1364/josaa.1.000107. [DOI] [PubMed] [Google Scholar]
- 33.Brown AM. Intrinsic contrast noise and infant visual contrast discrimination. Vision Res. 1994;34:1947–64. doi: 10.1016/0042-6989(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 34.Huang L, Dobkins KR. Attentional effects on contrast discrimination in humans: evidence for both contrast gain and response gain. Vision Res. 2005;45:1201–12. doi: 10.1016/j.visres.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 35.Stephens BR, Banks MS. Contrast discrimination in human infants. J Exp Psychol Hum Percept Perform. 1987;13:558–65. doi: 10.1037//0096-1523.13.4.558. [DOI] [PubMed] [Google Scholar]
- 36.Atkinson J, Braddick O, Moar K. Development of contrast sensitivity over the first 3 months of life in the human infant. Vision Res. 1977;17:1037–44. doi: 10.1016/0042-6989(77)90007-4. [DOI] [PubMed] [Google Scholar]
- 37.Banks MS, Salapatek P. Acuity and contrast sensitivity in 1-, 2-, and 3-month-old human infants. Invest Ophthalmol Vis Sci. 1978;17:361–5. [PubMed] [Google Scholar]
- 38.Banks MS, Stephens BR, Hartmann EE. The development of basic mechanisms of pattern vision: spatial frequency channels. J Exp Child Psychol. 1985;40:501–27. doi: 10.1016/0022-0965(85)90080-3. [DOI] [PubMed] [Google Scholar]
- 39.Peterzell DH, Werner JS, Kaplan PS. Individual differences in contrast sensitivity functions: the first four months of life in humans. Vision Res. 1993;33:381–96. doi: 10.1016/0042-6989(93)90093-c. [DOI] [PubMed] [Google Scholar]
- 40.Peterzell DH, Teller DY. Individual differences in contrast sensitivity functions: the lowest spatial frequency channels. Vision Res. 1996;36:3077–85. doi: 10.1016/0042-6989(96)00061-2. [DOI] [PubMed] [Google Scholar]
- 41.Adams RJ, Mercer ME, Courage ML. A new technique to measure contrast sensitivity in human infants. Optom Vis Sci. 1992;69:440–6. doi: 10.1097/00006324-199206000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Adams RJ, Courage ML. Using a single test to measure human contrast sensitivity from early childhood to maturity. Vision Res. 2002;42:1205–10. doi: 10.1016/s0042-6989(02)00038-x. [DOI] [PubMed] [Google Scholar]