Abstract
The simplest regeneration experiments involve the ablation of a single cell type. While methods exist to ablate the melanocytes of the larval zebrafish,1,2 no convenient method exists to ablate melanocytes in adult zebrafish. Here, we show that the copper chelator neocuproine (NCP) causes fragmentation and disappearance of melanin in adult zebrafish melanocytes. Adult melanocytes expressing eGFP under the control of a melanocyte-specific promoter also lose eGFP fluorescence in the presence of NCP. We conclude that NCP causes melanocyte death. This death is independent of p53 and melanin, but can be suppressed by the addition of exogenous copper. NCP is ineffective at ablating larval melanocytes. This now provides a tool for addressing questions about stem cells and the maintenance of the adult pigment pattern in zebrafish.
Introduction
Understanding adult stem cells and their regulatory mechanisms is aided by tools that manipulate or ablate the tissue the stem cell monitors. We are interested in the regulation of the melanocyte pattern and precursors or stem cells that may sustain it at various stages of the life cycle in zebrafish. We have previously developed laser protocols1 or drugs2 that allow us to specifically ablate larval zebrafish melanocytes that subsequently regenerate. Amputation of adult caudal fins is followed by fin regeneration with concomitant regeneration of the fin melanocyte stripes. In each case, we have inferred the existence of melanocyte stem cells (MSCs) that support the melanocyte pattern. A mammalian MSC has also been identified in the hair follicle.3
Genetic analysis of melanocyte regeneration after chemical ablation in larvae4 or in the regenerating caudal fin5 has provided several insights into mechanisms that regulate the MSC, including identifying differences between ontogenetic and regenerative development. However, each of these systems also has limitations for the study of stem cell regulation. For instance, experiments on larval melanocyte regeneration must be completed before the onset of metamorphosis, approximately 14 days postfertilization (dpf), to ensure that the new melanocytes are regenerative and not part of the wave of new melanocytes that develop upon metamorphosis. Thus, regeneration experiments in the larvae are currently limited to two rounds of ablation and regeneration. Moreover, many of the mutations that affect the adult pigment pattern in zebra-fish have little or no effect on the embryonic or larval melanocyte. The ability to reliably ablate melanocytes from the adult body stripes would both allow for multiple rounds of melanocyte regeneration and also allow us to exploit the richness of mutations that affect adult pattern6–10 in studying the mechanisms that regulate the MSC.
The small molecule 4-(4-morpholinobutylthio) phenol (MoTP) that we previously described2 that ablates larval melanocytes is a prodrug that is converted by the melanin synthesizing enzyme tyrosinase into a cytotoxic phenolic compound. The high specificity of ablation of melanocytes is explained by the fact that only developing or newly pigmented melanocytes express sufficiently high levels of this enzyme to produce cytotoxic levels of the phenolic product. One limitation of MoTP for melanocyte ablation is that it fails to ablate mature melanocytes that no longer express high levels of tyrosinase2 (this study). Thus, embryonic melanocytes become largely refractile to ablation by MoTP after approximately 6 dpf, and most adult melanocytes are also resistant to MoTP-mediated ablation. The laser protocol described for ablation of embryonic melanocytes,1 which utilizes the intense flux of dermatology tattoo removal lasers, is effective in ablating melanocytes from the adult pigment stripes, but is less specific than in the embryo. Laser treatment of the adult body stripe also results in the ablation of the yellow xanthophores as well as causing some collateral tissue damage (O'Reilly-Pol, unpublished data). Identification of a small molecule or drug that specifically ablates mature melanocytes, particularly in the adult body stripes, and has no effect on xanthophores is now required to fill this gap in our ability to ablate melanocytes and study the potential of MSCs to regenerate the adult melanocyte population.
In this study, we describe the identification of a drug, neocuproine (NCP), that specifically ablates the melanocytes of adult zebrafish. We have previously shown that NCP, a copper chelator, prevents tyrosinase function and melanin synthesis in the zebrafish embryo.11 In adult zebrafish, we show that the melanocytes exhibit the same sequelae of death (contraction and fragmentation) as observed in larval melanocyte ablation, as well as in other adult teleosts.1,12,13 This effect of NCP is suppressed by exogenous copper, suggesting that it acts through copper depletion to ablate melanocytes. We also show through the use of albino fish that melanin is not necessary for this ablation. NCP now provides us with a tool to specifically ablate melanocytes in the adult to allow us to explore the regulation of the MSC in the adult zebrafish.
Results
Knowing that NCP, a copper chelator, affects copper-dependent processes, including melanin synthesis, in the zebrafish embryo,11 we wanted to see the effects of disrupting copper homeostasis in adult zebrafish. Adult zebrafish were treated with 750 nM NCP, a concentration below the thresh-old necessary for blocking melanin synthesis in the embryo. We found that by 10 days of treatment, fish were largely devoid of melanin in their body stripes and fins (Fig. 1B). When fish were returned to fresh water, the pigment pattern was reconstituted within 4 weeks after drug washout (not shown).
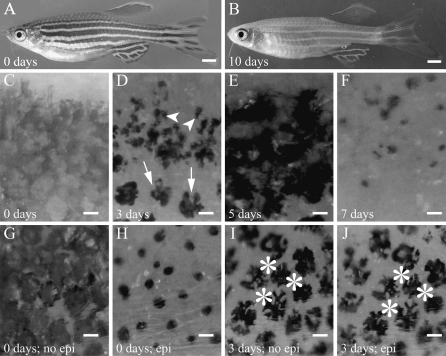
FIG. 1.
Progression of melanocyte morphology during exposure to NCP. (A) An untreated, fully pigmented fish. (B) A fish after 10 days of NCP treatment. It is almost entirely devoid of melanin in the body stripes and fins. (C–J) Close-ups of melanocyte stripes after treatment with NCP. Melanosomes are fully relaxed, and there are many pigmented processes before treatment with NCP (C). After 3 days, the melanin has contracted to result in lobular melanin masses (D, arrows). Some melanocytes have begun to fragment (D, arrowheads). After 5 days of NCP, melanin appears as if acellular (E). After 7 days, most of the melanin has been cleared, leaving scattered granules (F). Fish untreated with NCP prior (G) and after exposure (H) to epinephrine. Note the contracted melanosomes in (H) compared to (G) and that these contracted melanosomes are regular and round in appearance. Lobular melanin masses after 3 days in NCP look similar before (I) and after exposure (J) to epinephrine. Asterisks in (I) and (J) mark the same melanocytes. Note that there is no change in these melanocytes after epinephrine treatment. Scale bars in (A, B) are 1 mm and in (C–J) are 100 μm.
We wanted to better understand the depigmentation process, so we observed the morphology of melanocytes undergoing treatment with NCP. Melanocytes start out with their melanin (melanosomes) fully dispersed and have many pigmented dendritic processes (Fig. 1C). By 3 days of treatment in 750 nM NCP, all melanocytes have lost pigmented processes, and the melanin is more contracted overall (Fig. 1D). Some of the melanocytes have discreet puncta of melanin. After 5 days of treatment, the melanin appears hazy, as if it is no longer cellular (Fig. 1E). At 7 days, most of the melanin is gone, leaving only scattered dark puncta (Fig. 1F). After 10 days, nearly all the melanin has been cleared, although there are areas that appear resistant to NCP such as some scale melanocytes and the distal tips of the fins. We suggest that this sequence of events reflects the death of melanocytes followed by mechanisms to clear the detritus (see Discussion section).
The observed phenotypes at 3 days intrigued us. Melanosomes contract when exposed to epinephrine, with the melanosomes moving along microtubule networks toward the center of the cell14 (compare Fig. 1G and H). Melanin is partially contracted after 3 days of NCP treatment (compare Fig. 1G and I). However, the shapes are different. Epinephrine treatment results in round aggregations of melanin, whereas NCP treatment results in irregularly shaped or lobular melanin aggregates (compare Fig. 1H and J). Moreover, after 3 days of NCP treatment melanin did not noticeably contract in response to epinephrine (compare asterisked melanocytes in Fig. 1I and J). This suggests that the lobular melanin is not an intermediate stage of melanosome contraction.
Loss of tyrp1:eGFP expression from melanocytes in NCP-treated fish
Next, we used the previously described construct of fugu tyrp1 promoter driving eGFP15 to make eight mosaic adults with eGFP expression in clones of melanocytes. Tyrosinase-related protein 1 (Tyrp1) is expressed exclusively in melanocytes in both fish15 and mammals.16 After 1 day in 750 nM NCP, we found that eGFP expression was no longer detectable in a majority of melanocytes (182/194 cells). Some of these cells retain a small amount of eGFP. However, the eGFP no longer surrounds the melanin mass as it did before NCP treatment (Fig. 2B, arrowhead). A few cells (12/194, Fig. 2B, arrow) retain all of the eGFP expression, suggesting that they are resistant to NCP. We note that these melanocytes appear smaller than the melanocytes that lost eGFP expression after NCP treatment. Thus, the resistant melanocyte may be younger developmentally and may need to mature to become NCP sensitive (discussed below in sections “Effective periods of drug exposure for melanocyte ablation” and “NCP only ablates melanocytes in mature tissue”).
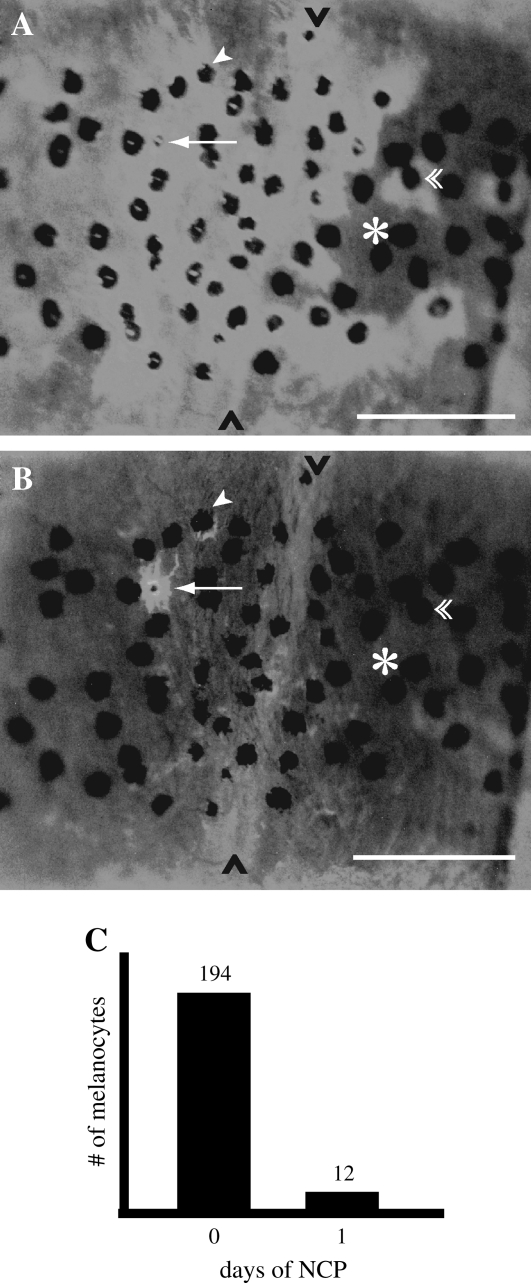
FIG. 2.
Loss of tyrp1:eGFP from mosaic adults after treatment with 750 nM NCP. (A) 0 day. (B) 1 day. The arrow indicates one melanocyte that fully retained eGFP after treatment. The arrowhead indicates one melanocyte that partially retained eGFP after treatment. The eGFP no longer surrounds the melanin, and is not in dendritic processes (compare with the arrow). The double arrowhead indicates a melanocyte that lost all eGFP expression (compare with arrow and arrowhead). The asterisk indicates melanocytes that were initially negative for eGFP. The black carats mark the boundary of the myotome, which has mild autofluorescence. (C) Quantification of eGFP+ melanocytes in eight mosaic fish before and after NCP treatment. Scale bars are 200 μm.
We also note that two of the mosaic adults contained xanthophore clones adjacent to melanocyte clones. Xanthophores are a related, but different, type of dermal pigment cell that does not contain melanin. Although stable transgenic lines for the tyrp1:eGFP construct do not express eGFP in their xanthophores, we occasionally observe mosaic expression in xanthophores of injected animals. These mosaic animals provide an opportunity to ask whether NCP affects transgene expression in another cell type. In these fish, the eGFP-expressing xanthophores maintained eGFP expression after neighboring eGFP-expressing melanocytes had extinguished their expression and fragmented (not shown).
To further explore the loss of eGFP, we treated transgenic adult expressing tyrp1:eGFP with NCP. In the proximal caudal fin, approximately 98% (1174/1195, n = 4) of the melanocytes lost eGFP expression after 1 day of NCP treatment. The minority of melanocytes that continued expressing eGFP at 24 h persisted their eGFP expression through 4 days of drug treatment (not shown).
Melanocyte specific loss of eGFP
To determine the specificity of NCP for melanocytes, we utilized the transgenic line j999a. This line contains eGFP under the control of the fugu kit promoter. Expression, however, is mosaic, resulting in eGFP expression in many cells not known to express kit, including skin and muscle. As above, treating these fish with NCP for 1 day ablates eGFP expression in melanocytes. In contrast, eGFP expression persists unperturbed in all skin and muscle clones of NCP-treated fish (not shown).
Brief exposure to NCP
As tyrp1:eGFP expression was extinguished after 1 day, we next wanted to see if the melanocytes were capable of recovering from this brief exposure to NCP. We treated fish with NCP for 1 day, and then washed them into fresh water. To our surprise, not only did melanocytes progress as if they were still in drug, but also the events were accelerated. After 2 days of washout, they more closely resembled fish that had been in NCP continuously for 7 days (compare Fig. 3C to 1D and F). It is unclear why removal of NCP speeds progression of the melanocyte ablation, but this may reveal a role for copper in the clearing of melanocyte detritus. Nevertheless, this result reveals that adult melanocytes have committed to death within 24 h of NCP exposure.
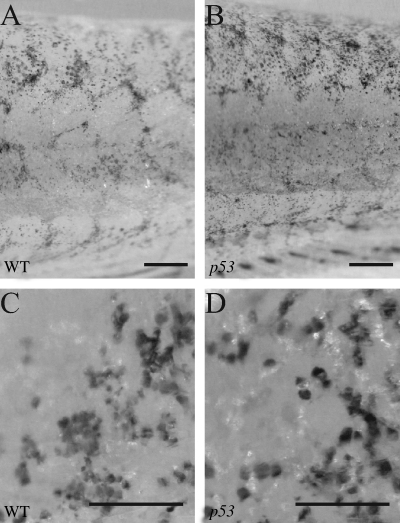
FIG. 3.
Melanocyte loss in p53 mutants. Fish were treated for 1 day in 750 nM NCP and washed into fresh water for 2 days. WT (A, C) and p53 mutants (B, D) are equally affected by the NCP treatment. The melanocytes have already fragmented and begun to be cleared (C, D). Scale bars in (A, B) are 500 μm and in (C, D) are 100 μm.
Independence of NCP-induced death from p53
We wanted to know if the observed melanocyte death went through classic apoptotic pathways or a different mode of cell death (i.e., nonclassical apoptosis or necrosis). As many of the ways to assess apoptosis (TUNEL staining, acridine orange staining, etc.) are difficult to perform in the melanocyte, as the melanin prevents label detection, we instead used fish with a homozygous mutation in p53.17 This mutant is defective for apoptosis.17 Wild-type (WT) and p53 mutant fish were treated for 1 day with NCP. At 2 days after NCP washout, WT (Fig. 3A, C) and p53 mutants (Fig. 3B, D) are indistinguishable: both genotypes retain only scattered puncta of melanin (Fig. 3C, D). This indicates that melanocyte ablation after NCP treatment is not via p53-mediated apoptosis.
Melanin is not required for NCP-induced melanocyte ablation
We wondered whether melanin was a necessary component of the NCP-mediated ablation, as xanthophores, a pigment cell lacking melanin, were unaffected by NCP. We used the albino mutant, which has unpigmented melanocytes. To test this notion, we crossed the tyrp1:eGFP transgene into albino stocks to allow us to observe melanocytes (Fig. 4A). When we treated these fish with NCP, we found that eGFP expression was largely gone by 24 h (Fig. 4B), and completely absent from albino fish by 5 days of NCP treatment (Fig. 4C). This shows that NCP does not require melanin or functional melanin synthesis to cause melanocyte ablation.
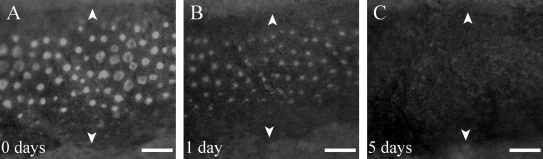
FIG. 4.
Loss of tyrp1:eGFP from albino transgenics after treatment with 750 nM NCP. (A) 0 day. (B) 1 day. Note that most of the eGFP signal is lost [compare with (A), but some faint eGFP is evident]. (C) 5 days. All the eGFP signal is lost. Arrowheads mark the melanocyte/xanthophore stripe boundary. Scale bars are 200 μm.
Role of copper during NCP-induced melanocyte ablation
We next wanted to know what the effective concentration of NCP was for melanocyte ablation. We discovered that concentrations greater than 1 μM NCP were highly toxic to fish at 7 days (Table 1), placing these doses outside of the effective range. About 750 nM NCP caused approximately 20% fish lethality, with negligible death at all lower concentrations of NCP (Table 1).
Table 1.
Concentration Response to NCP and Suppression of NCP by Exogenous Copper
| Treatment | Affecteda | Half-affectedb | Unaffected | Dead |
|---|---|---|---|---|
| 10 μM NCP | 0 | 0 | 0 | 10 |
| 3 μM NCP | 2 | 0 | 0 | 8 |
| 1 μM NCP | 47 | 0 | 0 | 34 |
| 750 nM NCP | 78 | 0 | 0 | 22 |
| 500 nM NCP | 62 | 0 | 0 | 1 |
| 300 nM NCP | 84 | 23 | 1 | 6 |
| 100 nM NCP | 41 | 38 | 34 | 2 |
| 30 nM NCP | 0 | 4 | 61 | 0 |
| 10 nM NCP | 0 | 0 | 20 | 0 |
| 750 nM NCP + 500 nM CuCl2 | 0 | 0 | 0 | 30 |
| 300 nM NCP + 1 μM CuCl2 | 1 | 6 | 105 | 1 |
| 100 nm NCP + 1 μM CuCl2 | 0 | 0 | 104 | 1 |
Affected fish are missing most or all of their melanocytes of their body stripe.
Halfaffected fish are missing ∼25–50% of the melanocytes in their body stripes.
To assess the effects of NCP on induced melanocyte ablation, we examined fish 7 days after treatment, putting fish into one of three categories based on the pigmentation: unaffected, half-affected, or completely affected. Half-affected fish lose approximately one quarter to half of their melanocytes from the middle stripe. Typically, depletion was strongest in the caudal half and proceeded rostral in completely affected fish. At concentrations over 500 nM, all fish are completely affected by 7 days of NCP treatment. Similarly, at 300 nM, most fish are also completely affected. However, at 100 nM the treated fish are distributed among the three categories. At 30 nM, a large majority of the fish is unaffected, with no effect seen at 10 nM (Table 1).Thus, we conclude that 500–1000 nM NCP produces the most effective dose for melanocyte ablation, although there is some fish lethality observed at the higher end of this range.
To test the role of copper during NCP-induced melanocyte ablation, we added 1 μM of CuCl2 to fish treated with intermediate concentrations (100 or 300 nM) of NCP. At these concentrations of NCP, copper is in excess (approximately 20-or 7-fold excess Cu2+). In each case, the excess copper suppressed the NCP-induced melanocyte death (Table 1), thus tending to suggest that NCP acts to ablate melanocytes by depleting the available copper. We note, however, that exogenous copper did increase fish lethality at higher concentrations of NCP (Table 1), suggesting that in other cells in the fish NCP may exert its lethal effects by inappropriately mobilizing copper.
Effects of other copper chelators
We asked whether other copper chelators could induce melanocyte ablation. We tested bathocuproine sulfate (BCS), an analog of NCP, which, unlike NCP, is cell impermeable, and N-phenylthiourea (PTU), a widely used drug that inhibits melanization of melanocytes and shares little structural similarity to NCP. Exposure of fish to these drugs (750 nM BCS and 100 μM PTU) caused no evidence of depigmentation or melanocyte ablation after 7 days in the drug.
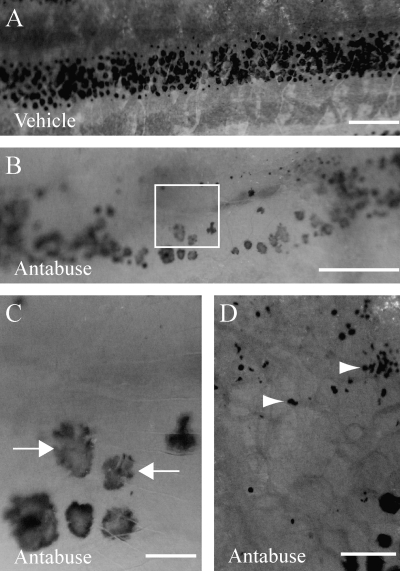
In contrast, the copper chelator antabuse caused weak depigmentation (Fig. 5). Antabuse, like PTU, is structurally dissimilar to NCP. Antabuse has been shown to prevent melanin synthesis in larval zebrafish.11 After antabuse treatment (1 μM), we found occasional fish (6/14) with gaps (Fig. 5B) in the stripe. These gaps are smaller than the half-affected fish described in our dosage testing of NCP (above), and some contained lobular melanocytes (Fig. 5C) or melanocyte detritus (Fig. 5D). Gaps and the presence of melanocyte detritus are typically never observed in untreated fish.
FIG. 5.
Partial loss of melanocytes after antabuse treatment. (A) Vehicle treatment. (B) 10 μM antabuse. Note the large gap in the stripe that is typically not observed in untreated fish. (C) A close-up of the box in (B) showing lobular melanocytes (arrows) (D). A close-up of a similar gap to (B), except with melanocyte detritus (arrowheads). Scale bars in (A, B) are 500 μm and in (C, D) are 100 μm.
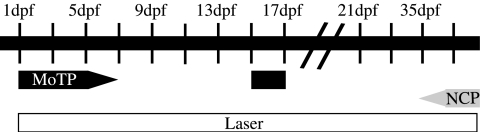
Effective periods of drug exposure for melanocyte ablation
Now possessing drugs that have been shown to ablate melanocytes in larval (MoTP) and adult (NCP) stages, we wanted to further define when each of these drugs was melanotoxic (summarized in Fig. 6). We treated our transgenic line expressing tyrp1:eGFP with NCP or MoTP and scored them for melanocyte morphology (fragmentation, as above) and loss of eGFP fluorescence as metrics for melanotoxicity.
FIG. 6.
Timeline for effective melanocyte ablation. Fish were treated for 2-day periods with either 5 μM NCP or 10 μg/mL MoTP at 1, 3, 5, 7, 9, 11, 13, and 15 dpf and assessed for melanocyte ablation. Fish were treated for 7-day periods with 20 μg/mL MoTP at >180 dpf or 750 nM NCP at 21, 28, 35 dpf, and >180 dpf. The effectiveness of the laser protocol represents a variety of experiments on larval1 and adult (O'Reilly-Pol, unpublished data) fish. Effective periods for each method are designated by the bar under the timeline (black for MoTP, shaded for NCP, and open for laser).
To confirm that NCP only prevented pigmentation in embryonic melanocytes, we treated embryos from the tyrp1:eGFP transgenic line for 3 days with MoTP, PTU, and NCP before the cells were melanized. All three treatments resulted in unpigmented embryos, but PTU and NCP contained eGFP-positive, melanin-negative melanocytes, showing that for these copper chelators the melanocytes were not ablated. In contrast, MoTP had no eGFP signal, consistent with the previous finding that MoTP kills melanoblasts as well as differentiated larval melanocytes2 (not shown).
We find that MoTP no longer affects melanocytes after 6–7 dpf. This is consistent with our previous finding that MoTP requires conversion by tyrosinase into a cytotoxin, and that tyrosinase expression decreases after 5 dpf.2 We find that MoTP ablates melanocytes once again at 15 dpf (not shown), which is approximately the onset of metamorphosis when adult melanocytes begin to differentiate and thus should require high tyrosinase activity for pigmentation. In adult fish (>6 months), MoTP shows no discernible effect on melanocytes, even at concentrations higher than the larval threshold or when applied for 7 days.
To explore the effect of NCP on melanocyte survival in larval zebrafish, we used NCP at 5 μM. This is the minimum dose required to block melanin synthesis in the embryo,11 and is 10-fold greater than what is necessary in the adult to ablate melanocytes. However, even at this high concentration, we did not observe any morphological changes or loss of eGFP expression in the melanocytes exposed to NCP for any 2-day period between 3 and 15 dpf. Similarly, we observed no depigmentation when larval fish were treated with 750 nM NCP for 7 days. We first observed NCP-induced melanocyte ablation in fish once they had reached 5 weeks of age. This suggests that there is some physiological change in the adult that occurs as it matures that makes it susceptible to ablation by NCP.
NCP only ablates melanocytes in mature tissue
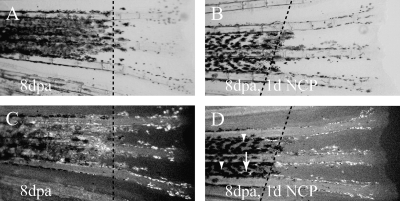
The observation that NCP only ablated melanocytes in older fish led us to ask whether this result reflected a global change in fish physiology resulting in NCP sensitivity. Alternatively, the change in sensitivity could reflect the age of the melanocyte or its immediately surrounding tissue. We reasoned that the regeneration of melanocytes in regenerating fins would allow us to test between these models. Accordingly, we amputated fins in the tyrp1:eGFP line and allowed them to regenerate for 7 days before treatment with NCP. This allowed significant development of new melanocytes in the fin regenerate (Fig. 7A, B). The regenerative and ontogenetic melanocytes both expressed eGFP (Fig. 7C). After 1 day of NCP treatment, eGFP expression is lost in the older, ontogenetic melanocytes, but not in the younger, regenerative melanocytes (Fig. 7D). This result rules out the model that global changes in the adult physiology confer NCP sensitivity. Instead, this result tends to suggest that the melanocytes or the tissue surrounding it must mature to become sensitive to NCP.
FIG. 7.
NCP does not ablate newly regenerated melanocytes. (A) Fish fin carrying transgenic tyrp1:eGFP 8 days postamputation (dpa) under white light. The dashed line represents the amputation plane. (B) A fish fin carrying transgenic tyrp1:eGFP 8 dpa and 1 day of NCP treatment under white light. The dashed line is the amputation plane. (C) The same fin as in (A), under fluorescent light. Note GFP is on both sides of the amputation plane in all melanocytes. (D) The same fin as (B), under fluorescent light. Most of the melanocytes proximal (to the left) of the amputation plane have lost GFP expression (arrowheads), although some retain GFP expression (arrow). All of the melanocytes distal (to the right) of the amputation plane express GFP.
Discussion
We first considered the model that NCP was revealing normal turnover of melanin or melanocytes in the adult. This seems unlikely because we never see these lobular or fragmented melanin clusters that we observe in NCP-treated fish (Fig. 1D) in untreated fish. Additionally, another drug that blocks melanin synthesis, PTU, has been often used in adult zebrafish without any suggestion of revealing melanocyte turnover.5,18 Also, we have kept fish in PTU for up to 5 weeks without affecting pigmented melanocytes (O'Reilly-Pol, unpublished data). Thus, we tend to reject the model that NCP, by blocking melanin synthesis, reveals normal turnover of melanin or melanocyte death in adults. This led us to explore models of NCP inducing melanin turnover (Turnover Model) or melanocyte death (Death Model).
These two models of induced turnover of melanin and melanocytes make testable predictions about the depigmentation of fish. Under the Turnover Model, other cellular components should persist in the presence of NCP. We would expect that a melanocyte expressing eGFP under the control of a melanocyte-specific promoter, such as the tyrp1 or kit promoters used, would continue to express eGFP. They do not (Fig. 2), as the Death Model predicts. The continued eGFP expression in multiple cell types during NCP treatment shows specificity for NCP to quench eGFP in the melanocyte. The use of two different promoters tends to rule out loss of eGFP expression from an interaction between NCP and the tyrp1 promoter. In the amputation experiment, eGFP expression is preserved in the regenerative melanocytes, further establishing that NCP does not generally interact with eGFP or the tyrp1 promoter. The Turnover Model predicts that melanocytes should gradually lose their pigmentation (fade) in the presence of NCP while maintaining their overall size, shape, and identity. In contrast, the Death Model predicts that as melanocytes die, they will fragment as previously decribed,1,12,13 which they do (Fig. 1C–F). Weaker support comes from the loss of melanocyte sensitivity to epinephrine (Fig. 1G–I) and commitment to depigmentation after brief exposure to NCP (Fig. 3). Thus, we favor the Death Model. It does remain formally possible that NCP is not inducing melanocyte death but instead exerts multiple effects by beginning a cascade with continuing effects that results in depigmentation, loss of epinephrine sensitivity, and quenching of eGFP only in melanocytes.
Once we established to our satisfaction that NCP caused melanocyte death, we wondered if this death was classic apoptosis. We could not do this in many of the standard ways. We have been unable to accurately determine melanocyte nuclei in whole mount fish fins or dissected skin. Thus, we were prevented from gauging the presence of pycnotic or TUNEL-stained nuclei to assess apoptosis. However, we found identical NCP-induced melanocyte ablation in mutants homozygous for a p53 mutation, suggesting that whatever type of cell death was occurring, it was not p53-mediated apoptosis.
We also explored the role of copper in NCP-induced melanocyte ablation. In the first model, NCP depletes copper from the cell, revealing essential melanocyte-specific, copper-dependent protein activity. In a second model, NCP may inappropriately mobilize copper in the cell causing damage from the redox activity of copper. A third model is an off-target, possibly copper-independent, effect of NCP. These models can be partially distinguished by adding exogenous copper. If the first model is correct, the addition of excess copper should suppress the effect. This test was used in larval zebrafish to show that NCP affected melanin synthesis through copper chelation.11 Alternatively, for the second model, adding exogenous copper should worsen the phenotype. This is the conclusion favored in studies of NCP in HepG2 cells19 and cultured rat cortical astrocytes20 where copper increases the toxicity of NCP. The third model would probably be unaffected by copper. However, binding of copper may change the structure of NCP, and thus also the off-target properties of NCP, so the exact effect cannot be predicted.
Our results that copper suppresses the melanotoxic effects of NCP (Table 1)tend to argue against the second model that purports inappropriate copper mobilization. As we find that a structurally dissimilar copper chelator, antabuse, is also melanotoxic in adults, we tend to argue against our third model for off-target effects. Thus, we favor the first model (copper depletion) for NCP's mode of action, and that copper is required for the survival of adult melanocytes.
Conclusion
We show that NCP, a copper chelator, ablates adult, but not larval, melanocytes. This melanotoxicity reveals a specific role for copper in the continuing survival of melanocytes. Death of these melanocytes occurs in a manner that is independent of p53-mediated apoptosis, and does not require the presence of melanin. Only mature adult melanocytes are affected. This drug now provides a tool for ablating adult melanocytes and exploration of the role of MSCs in the maintenance of the adult pattern.
Materials and Methods
Fish stocks and husbandry
Standard fish husbandry protocols were followed.21 WT fish stocks were largely AB. j999a Is a transgenic line containing a 7.7 kb promoter fragment from upstream of the fugu kit gene cloned into the tol2 transposon vector and driving eGFP, inserted into the genome as previously described.22 Melanophilinaj120 mutants were used in the generation of transgenic and mosaic fish (see “tyrp1:eGFP” section below) as the melanosomes are concentrated in the center of the cell, thus allowing visualization of eGFP in most of the cytoplasm.14 Albinob4 and p53zdf1 were obtained from the Zebrafish International Resource Center (NIH P40 Grant RR012546).
Drug treatments
MoTP was custom synthesized by Gateway Chemical Technology (St. Louis, MO). PTU (catalog number P7629), epinephrine (cat. E4250), NCP (cat. N1501), bathocuproine sulfate (BCS) (cat. B1125), and antabuse (cat. T1132) were obtained form Sigma-Aldrich (St. Louis, MO). All drugs were changed every 2–3 days. NCP was used at 750 nM, except when noted as otherwise. Epinephrine was used at 1 mg/mL. NCP and antabuse stock solutions were made in DMSO, and diluted to a final concentration in less than 0.1% DMSO.
As an internal control to demonstrate the effectiveness of copper chelation by NCP, PTU, BCS, and antabuse, a simultaneous partial amputation of the caudal fin was performed. In each case, the concentration of drug used was sufficient to block most or all melanin synthesis in the regenerating fin. Treatment with vehicle (0.1% DMSO) allowed full melanin synthesis.
tyrp1:eGFP
The tol2 transposon construct used to generate tyrp1:eGFP mosaic and transgenic fish was previously described.15 The methods for generating mosaic fish and stable transgenics have also been described.22 Quantification of melanocyte loss was performed by counting along the middle stripe of the caudal fin for a length of three fin ray segments in the proximal portion of the fin.
Photography
All pictures were taken with a ProgRes C14 camera with accompanying software (Jenoptik Laser. Optik. Systeme., Jena, Germany) on a Nikon SMZ 1500 stereomicroscope (Tokyo, Japan). An X-cite 120 Fluorescent Illumination System (Exfo Life Sciences, Mississauga, Ontario, Canada) was used for fluorescent excitation of eGFP. In some cases (Fig. 1A–F), fish were euthanized and mounted in 1% agar on 10 cm Petri dishes. They were then photographed through the bottom of the plate to reduce glare. All other pictures were of live, unmounted fish taken with incidental light. Pictures were edited with Photoshop CS2 (Adobe Systems, San Jose, CA).
Acknowledgments
We thank Erik Madsen for his intellectual contributions, Ahu Turkoz for technical assistance, and Chao-Tsung Yang for generating the j999a (fugu kit:eGFP) transgenic line. We also thank Jian Zou and Xiangyun Wei for the tyrp1:eGFP construct. This work was funded by National Institutes of Health Grant GM56988 to S.L.J.
Disclosure Statement
No competing financial interests exist.
References
- 1.Yang C. Sengelmann R. Johnson S. Larval melanocyte regeneration following laser ablation in zebrafish. J Invest Dermatol. 2004;123:924–929. doi: 10.1111/j.0022-202X.2004.23475.x. [DOI] [PubMed] [Google Scholar]
- 2.Yang CT. Johnson SL. Small molecule-induced ablation and subsequent regeneration of larval zebrafish melanocytes. Development. 2006;133:3563–3573. doi: 10.1242/dev.02533. [DOI] [PubMed] [Google Scholar]
- 3.Osawa M. Egawa G. Mak SS. Moriyama M. Freter R. Yonetani S, et al. Molecular characterization of melanocyte stem cells in their niche. Development. 2005;132:5589–5599. doi: 10.1242/dev.02161. [DOI] [PubMed] [Google Scholar]
- 4.Yang CT. Hindes AE. Hultman KA. Johnson SL. Mutations in gfpt1 and skiv2l2 cause distinct stage-specific defects in larval melanocyte regeneration in zebrafish. PLoS Genet. 2007;3:e88. doi: 10.1371/journal.pgen.0030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rawls JF. Johnson SL. Zebrafish kit mutation reveals primary and secondary regulation of melanocyte development during fin stripe regeneration. Development. 2000;127:3715–3724. doi: 10.1242/dev.127.17.3715. [DOI] [PubMed] [Google Scholar]
- 6.Budi EH. Patterson LB. Parichy DM. Embryonic requirements for ErbB signaling in neural crest development and adult pigment pattern formation. Development. 2008;135:2603–2614. doi: 10.1242/dev.019299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parichy DM. Turner JM. Zebrafish puma mutant decouples pigment pattern and somatic metamorphosis. Dev Biol. 2003;256:242–257. doi: 10.1016/s0012-1606(03)00015-0. [DOI] [PubMed] [Google Scholar]
- 8.Johnson SL. Africa D. Walker C. Weston JA. Genetic control of adult pigment stripe development in zebrafish. Dev Biol. 1995;167:27–33. doi: 10.1006/dbio.1995.1004. [DOI] [PubMed] [Google Scholar]
- 9.Lopes SS. Yang X. Muller J. Carney TJ. McAdow AR. Rauch GJ, et al. Leukocyte tyrosine kinase functions in pigment cell development. PLoS Genet. 2008;4:e1000026. doi: 10.1371/journal.pgen.1000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parichy DM. Ransom DG. Paw B. Zon LI. Johnson SL. An orthologue of the kit-related gene fms is required for development of neural crest-derived xanthophores and a subpopulation of adult melanocytes in the zebrafish, Danio rerio. Development. 2000;127:3031–3044. doi: 10.1242/dev.127.14.3031. [DOI] [PubMed] [Google Scholar]
- 11.Mendelsohn BA. Yin C. Johnson SL. Wilm TP. Solnica-Krezel L. Gitlin JD. Atp7a determines a hierarchy of copper metabolism essential for notochord development. Cell Metab. 2006;4:155–162. doi: 10.1016/j.cmet.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Parichy DM. Rawls JF. Pratt SJ. Whitfield TT. Johnson SL. Zebrafish sparse corresponds to an orthologue of c-kit and is required for the morphogenesis of a subpopulation of melanocytes, but is not essential for hematopoiesis or primordial germ cell development. Development. 1999;126:3425–3436. doi: 10.1242/dev.126.15.3425. [DOI] [PubMed] [Google Scholar]
- 13.Sugimoto M. Uchida N. Hatayama M. Apoptosis in skin pigment cells of the medaka, Oryzias latipes (Teleostei), during long-term chromatic adaptation: the role of sympathetic innervation. Cell Tissue Res. 2000;301:205–216. doi: 10.1007/s004410000226. [DOI] [PubMed] [Google Scholar]
- 14.Sheets L. Ransom DG. Mellgren EM. Johnson SL. Schnapp BJ. Zebrafish melanophilin facilitates melanosome dispersion by regulating dynein. Curr Biol. 2007;17:1721–1734. doi: 10.1016/j.cub.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou J. Beermann F. Wang J. Kawakami K. Wei X. The Fugu tyrp1 promoter directs specific GFP expression in zebrafish: tools to study the RPE and the neural crest-derived melanophores. Pigm Cell Res. 2006;19:615–627. doi: 10.1111/j.1600-0749.2006.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson IJ. Chambers D. Rinchik EM. Bennett DC. Characterization of TRP-1 mRNA levels in dominant and recessive mutations at the mouse brown (b) locus. Genetics. 1990;126:451–459. doi: 10.1093/genetics/126.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berghmans S. Murphey RD. Wienholds E. Neuberg D. Kutok JL. Fletcher CD, et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc Natl Acad Sci USA. 2005;102:407–412. doi: 10.1073/pnas.0406252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawls JF. Johnson SL. Requirements for the kit receptor tyrosine kinase during regeneration of zebrafish fin melanocytes. Development. 2001;128:1943–1949. doi: 10.1242/dev.128.11.1943. [DOI] [PubMed] [Google Scholar]
- 19.Tsang SY. Tam SC. Bremner I. Burkitt MJ. Research communication copper-1,10-phenanthroline induces inter-nucleosomal DNA fragmentation in HepG2 cells, resulting from direct oxidation by the hydroxyl radical. Biochem J. 1996;317(Pt 1):13–16. doi: 10.1042/bj3170013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen S. Lin J. Liu S. Liang Y. Lin-Shiau S. Apoptosis of cultured astrocytes induced by the copper and neocuproine complex through oxidative stress and JNK activation. Toxicol Sci. 2008;102:138–149. doi: 10.1093/toxsci/kfm292. [DOI] [PubMed] [Google Scholar]
- 21.Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) Eugene: University of Oregon Press; 1995. [Google Scholar]
- 22.Kawakami K. Takeda H. Kawakami N. Kobayashi M. Matsuda N. Mishina M. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev Cell. 2004;7:133–144. doi: 10.1016/j.devcel.2004.06.005. [DOI] [PubMed] [Google Scholar]