Abstract
We found that increasing ghrelin levels, through subcutaneous injections or calorie restriction, produced anxiolytic- and antidepressant-like responses in the elevated plus maze and forced swim test. Moreover, chronic social defeat stress, a rodent model of depression, persistently increased ghrelin levels, whereas growth hormone secretagogue receptor (Ghsr) null mice showed increased deleterious effects of chronic defeat. Together, these findings demonstrate a previously unknown function for ghrelin in defending against depressive-like symptoms of chronic stress.
Chronic stress induces changes in mood, feeding and metabolism by a poorly understood neurobiological mechanism. Recent studies have suggested that key metabolic signals may interact with CNS circuits to regulate reward and mood1. To further explore these links, we investigated the potential role of ghrelin, an important feeding peptide, in the development of depressive symptoms. Ghrelin is a hormone synthesized predominantly by specialized gastrointestinal endocrine cells and is released during periods of negative energy balance2. In response to energy insufficiency, ghrelin induces a potent feeding response via activation of the growth hormone secretagogue receptor (GHSR, ghrelin receptor)2,3.
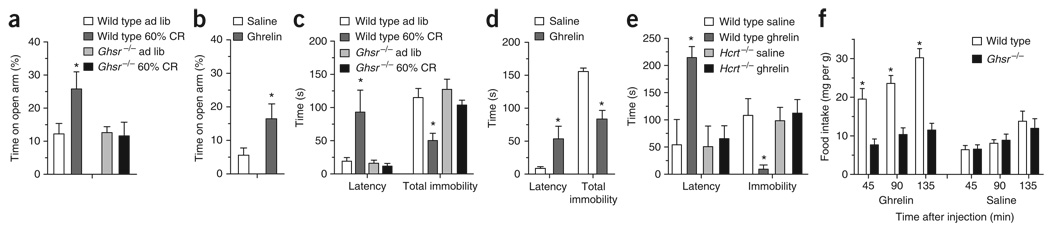
To determine whether ghrelin can affect mood symptoms, we physiologically increased ghrelin levels by restricting the food intake of mice with a diet containing 60% of normal calories for ten days (60% calorie restriction; Fig. 1). This resulted in a fourfold increase in circulating levels of acylated ghrelin (calorie restricted wild-type mice: 7.93 ± 1.59 pg mL−1, n = 6; wild-type mice fed ad libitum: 1.98 ± 0.37 pg mL−1, n = 5; P < 0.01). Calorie-restricted wild-type mice showed robust anxiolytic- and antidepressant-like behavior in the elevated plus maze (EPM) and forced swim test (FST), respectively, as compared with wild-type mice fed ad libitum (controls; Fig. 1a,c). In contrast, genetic blockade of ghrelin signaling in Ghsr−/− mice negated these calorie restriction–associated anxiolytic- and antidepressant-like effects. Further analyses demonstrated that the observed differences between the two genotypes cannot be attributed to differences in sensorimotor coordination, general locomotor activity or body weight (Supplementary Figs. 1–3 online).
Figure 1.
Anxiolytic- and antidepressant-like effects of ghrelin signaling. (a–d) Calorie restriction (CR) induced an anxiolytic-like effect in the EPM (*P < 0.02, a) and an antidepressant-like effect in the FST in wild-type, but not Ghsr−/−, mice (latency to immobility, *P < 0.002; total immobility, *P < 0.02; c). Wild type ad lib indicates wild-type mice fed ad libitum. Administration of ghrelin (2 µg per g of body weight subcutaneously), but not saline, produced an anxiolytic-like effect in the EPM (*P < 0.05; b) and an antidepressant-like effect in the FST in wild-type mice 45 min after injection (latency to immobility, *P < 0.04; total immobility, *P < 0.0002; d). (e) The ghrelin-induced antidepressant-like effect that we observed in wild-type mice in the FST was absent in orexin-deficient (Hcrt−/−) mice (n = 5 for both wild-type groups, n = 6 for the saline-treated Hcrt−/− group, n = 7 for the ghrelin-treated Hcrt−/− group). (f) Food intake responses of Ghsr−/− and wild-type littermates following subcutaneous administration of ghrelin (2 µg per g) or saline in a crossover fashion at 10–11 weeks of age and again 1 month later. Statistically significant differences between food intake of ghrelin-injected wild-type mice and that of similarly treated Ghsr−/− littermates and littermates treated with saline are indicated (*P < 0.001, n = 6 per group). Data are mean ± s.e.m. See Supplementary Methods online for detailed methods.
We used a pharmacologic approach to extend our food-restriction results. We subcutaneously injected C57BL6/J mice with a dose of ghrelin that induces potent feeding (Fig. 1f) and tested them in the EPM and FST 45 min later. Mice receiving ghrelin demonstrated significantly less anxiety- and depression-like symptoms in these tests compared with saline-injected controls (Fig. 1b,d).
Next, we determined whether ghrelin signaling regulates depressive symptoms in a mouse model of chronic stress. We used the chronic social defeat stress (CSDS) procedure, which subjects mice to ten daily bouts of social defeat by aggressive CD1 male mice1,4 (Fig. 2). Mice subjected to CSDS showed lasting behavioral deficits, including social avoidance (Supplementary Fig. 4 online), which can be reversed by chronic, but not acute, antidepressant treatment4. Following CSDS, defeated C57BL6/J mice had significantly elevated levels of ghrelin that persisted for at least 4 weeks after the last defeat (P < 0.02; Fig. 2a). This finding is consistent with previous studies that have demonstrated increases in gastric ghrelin mRNA or total plasma ghrelin after acute stress5,6.
Figure 2.
Ghrelin signaling regulates social isolation after CSDS. (a) Acylated ghrelin was persistently elevated after CSDS in wild-type C57BL6/J mice (significant effect of treatment, *P < 0.02; post hoc analysis showed no significant effect of day in the control mice; n = 5 in the control group and 10 in the CSDS group). (c) Ghsr−/− mice showed increased social avoidance after CSDS (*P < 0.05). (b,d) Although there is no effect on body weight in any group either during or shortly after CSDS (b), increased food intake was induced during the 10 d of CSDS and maintained for at least 3 d following CSDS in wild-type, but not Ghsr−/−, mice (during CSDS (days 1–10), *P < 0.002; after CSDS (days 11–13), *P < 0.002; n = 6 in each control group, 9 in the wild-type defeated group and 10 in the Ghsr−/− defeated group; d). Data are mean ± s.e.m.
We then tested Ghsr−/− mice and their wild-type littermates in CSDS to determine the role of these elevated ghrelin levels in the development of depressive symptoms. Ghsr−/− mice showed significantly greater social avoidance than wild-type littermates, thus indicating an exacerbation of the depressive-like symptoms normally induced by CSDS (P < 0.002; Fig. 2c). Moreover, although there was no difference in body weight following CSDS between the two genotypes (Fig. 2b), food intake was significantly elevated in wild-type, but not Ghsr−/−, mice (Fig. 2d). These findings suggest that activation of ghrelin signaling pathways in response to chronic stress may be a homeostatic adaptation that helps an individual cope with stress, but at the expense of increased caloric intake.
Ghrelin’s antidepressant- and anxiolytic-like actions might involve the engagement of neurons in the ventral tegmental area or hippocampus, both of which express GHSRs, experience ghrelin-induced modulation of synapse formation and are important sites of mood regulation4,7,8. Ghrelin’s antidepressant actions also may include direct and/or indirect activation of orexin-containing neurons in the lateral hypothalamic area, as we have recently shown that orexin neurons are required for the antidepressant-like effect of calorie restriction1. Consistent with this hypothesis, ghrelin can induce c-Fos in orexin neurons and can stimulate the activity of isolated orexin neurons9,10. We found that ghrelin’s antidepressant-like effects in the FST were blocked in mice lacking orexin (Fig. 1e).
These data reveal a unique and previously unrecognized function for ghrelin in the regulation of mood symptoms. Our data suggest that chronic stress, such as repeated social defeat, can elevate ghrelin levels. Although unclear, stress may act to increase circulating ghrelin via direct stimulation of ghrelin cells by catecholamines following activation of the sympathetic nervous system11. The ghrelin response then helps the animal cope with the stress by generating anxiolytic- and antidepressant-like behavioral adaptations. These results may also be relevant in the psychopathology of conditions with known alterations of ghrelin, such as anorexia nervosa12.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank C.E. Lee, M. Choi and M. Perello. This work was supported by grants from the US National Institutes of Health (K08DK068069-01A2, R01DK71320, P01DK56116, RL1DK081185, P50MH66172 and ADA 1-06-JF-59), a Foundation for Prader-Willi Research Grant, a NARSAD Young Investigator Award and a University of Texas Southwestern Disease-Oriented Clinical Scholars Award. M.Y. is a Howard Hughes Medical Institute investigator.
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
- 1.Lutter M, et al. J. Neurosci. 2008;28:3071–3075. doi: 10.1523/JNEUROSCI.5584-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zigman JM, Elmquist JK. Endocrinology. 2003;144:3749–3756. doi: 10.1210/en.2003-0241. [DOI] [PubMed] [Google Scholar]
- 3.Zigman JM, et al. J. Clin. Invest. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishnan V, et al. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Asakawa A, et al. Neuroendocrinology. 2001;74:143–147. doi: 10.1159/000054680. [DOI] [PubMed] [Google Scholar]
- 6.Kristenssson E, et al. Regul. Pept. 2006;134:114–117. doi: 10.1016/j.regpep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Abizaid A, et al. J. Clin. Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diano S, et al. Nat. Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Endocrinology. 2002;143:155–162. doi: 10.1210/endo.143.1.8561. [DOI] [PubMed] [Google Scholar]
- 10.Yamanaka A, et al. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 11.Mundinger TO, Cummings DE, Taborsky GJ., Jr Endocrinology. 2006;147:2893–2901. doi: 10.1210/en.2005-1182. [DOI] [PubMed] [Google Scholar]
- 12.Jimerson DC, Wolfe BE. CNS Spectr. 2004;9:516–522. doi: 10.1017/s1092852900009603. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.